Influence of ABO Locus on PFA-100 Collagen-ADP Closure Time Is Not Totally Dependent on the Von Willebrand Factor. Results of a GWAS on GAIT-2 Project Phenotypes
Abstract
1. Introduction
2. Results and Discussion
2.1. Genetic Correlations of the PFA-100 Phenotypes
2.2. GWAS of the PFA-100 Phenotypes
2.3. Genetic Influence of ABO Locus on PFA-100 Phenotypes
3. Methods
3.1. Enrollment of Individuals and Families
3.2. Blood Collection, Laboratory Analyses and DNA Preparation
3.3. Genotyping Filtering and Imputation
3.4. Statistical Correlation Analyses
3.5. GWAS of PFA-100 Phenotypes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paniccia, R.; Priora, R.; Liotta, A.A.; Abbate, R. Platelet Function Tests: A Comparative Review. Vasc Health Risk Manag. 2015, 11, 133–148. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4340464/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Harrison, P. The role of PFA-100 testing in the investigation and management of haemostatic defects in children and adults. Br. J. Haematol. 2005, 130, 3–10. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365-2141.2005.05511.x (accessed on 17 May 2019). [CrossRef] [PubMed]
- Favaloro, E.J. Clinical utility of the PFA-100. Semin. Thromb. Hemost. 2008, 34, 709–733. Available online: https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-0029-1145254.pdf (accessed on 21 June 2019). [CrossRef] [PubMed]
- Ardillon, L.; Ternisien, C.; Fouassier, M.; Sigaud, M.; Lefrançois, A.; Pacault, M.; Ribeyrol, O.; Fressinaud, E.; Boisseau, P.; Trossaërt, M. Platelet function analyser (PFA-100) results and von Willebrand factor deficiency: A 16-year ‘real-world’ experience. Haemophilia 2015, 5, 646–652. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/hae.12653 (accessed on 17 May 2019). [CrossRef] [PubMed]
- Favaloro, E.J. Clinical utility of closure times using the platelet function analyzer-100/200. Am. J. Hematol. 2017, 92, 398–404. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/ajh.24620 (accessed on 17 May 2019). [CrossRef] [PubMed]
- Fuchs, I.; Frossard, M.; Spiel, A.; Riedmüller, E.; Laggner, A.N.; Jilma, B. Platelet function in patients with acute coronary syndrome (ACS) predicts recurrent ACS. J. Thromb. Haemost. 2006, 4, 2547–2552. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1538-7836.2006.02239.x (accessed on 17 May 2019). [CrossRef] [PubMed]
- Reny, J.L.; De Moerloose, P.; Dauzat, M.; Fontana, P. Use of the PFA-100 closure time to predict cardiovascular events in aspirin-treated cardiovascular patients: A systematic review and meta-analysis. J. Thromb. Haemost. 2008, 6, 444–450. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1538-7836.2008.02897.x (accessed on 17 May 2019). [CrossRef]
- Vázquez-Santiago, M.; Vilalta, N.; Cuevas, B.; Murillo, J.; Llobet, D.; Macho, R.; Pujol-Moix, N.; Carrasco, M.; Mateo, J.; Fontcuberta, J.; et al. Short closure time values in PFA-100® are related to venous thrombotic risk. Results from the RETROVE Study. Thromb. Res. 2018, 169, 57–63. Available online: https://www.thrombosisresearch.com/article/S0049-3848(18)30420-1/fulltext (accessed on 17 May 2019). [CrossRef]
- Nazarian, S.M.; Thompson, J.B.; Gluckman, T.J.; Laws, K.; Jani, J.T.; Kickler, T.S.; Rade, J.J. Clinical and laboratory factors associated with shear-dependent platelet hyper-reactivity in patients on chronic aspirin therapy. Thromb. Res. 2010, 126, 379–383. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2975867/ (accessed on 17 May 2019). [CrossRef][Green Version]
- Hayward, C.P.; Harrison, P.; Cattaneo, M.; Ortel, T.L.; Rao, A.K. Platelet Physiology Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J. Thromb. Haemost. 2006, 4, 312–319. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1538-7836.2006.01771.x (accessed on 17 May 2019). [CrossRef]
- Vázquez-Santiago, M.; Ziyatdinov, A.; Pujol-Moix, N.; Brunel, H.; Morera, A.; Soria, J.M.; Souto, J.C. Age and gender effects on 15 platelet phenotypes in a Spanish population. Comput. Biol. Med. 2016, 69, 226–233. Available online: https://www.sciencedirect.com/science/article/pii/S0010482515004163?via%3Dihub (accessed on 17 May 2019). [CrossRef] [PubMed]
- Lippi, G.; Franchini, M. Laboratory screening for abnormalities of primary hemostasis: what’s next? Clin. Chem. 2001, 47, 2071. Available online: http://clinchem.aaccjnls.org/content/47/11/2071.2.long (accessed on 17 May 2019). [PubMed]
- Moeller, A.; Weippert-Kretschmer, M.; Prinz, H.; Kretschmer, V. Influence of ABO blood groups on primary hemostasis. Transfusion 2001, 41, 56–60. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1537-2995.2001.41010056.x?sid=nlm%3Apubmed (accessed on 17 May 2019). [CrossRef] [PubMed]
- Favaloro, E.J.; Thom, J.; Patterson, D.; Just, S.; Baccala, M.; Dixon, T.; Meiring, M.; Koutts, J.; Rowell, J.; Baker, R. Potential supplementary utility of combined PFA-100 and functional von Willebrand factor testing for the laboratory assessment of desmopressin and factor concentrate therapy in von Willebrand disease. Blood Coagul. Fibrinolysis 2009, 20, 475–483. Available online: https://journals.lww.com/bloodcoagulation/Abstract/2009/09000/Potential_supplementary_utility_of_combined.17.aspx (accessed on 21 June 2019). [CrossRef] [PubMed]
- Souto, J.C.; Almasy, L.; Borrell, M.; Blanco-Vaca, F.; Mateo, J.; Soria, J.M.; Coll, I.; Felices, R.; Stone, W.; Fontcuberta, J.; et al. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: The GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am. J. Hum. Genet. 2000, 67, 1452–1459. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1287922/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Pujol-Moix, N.; Vázquez-Santiago, M.; Morera, A.; Ziyatdinov, A.; Remacha, A.; Nomdedeu, J.F.; Fontcuberta, J.; Soria, J.M.; Souto, J.C. Genetic determinants of platelet large-cell ratio, immature platelet fraction, and other platelet-related phenotypes. Thromb. Res. 2015, 136, 361–366. Available online: https://www.thrombosisresearch.com/article/S0049-3848(15)30031-1/fulltext (accessed on 17 May 2019). [CrossRef]
- Smith, N.L.; Chen, M.H.; Dehghan, A.; Strachan, D.P.; Basu, S.; Soranzo, N.; Hayward, C.; Rudan, I.; Sabater-Lleal, M.; Bis, J.C.; et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation 2010, 121, 1382–1392. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2861278/ (accessed on 17 May 2019). [CrossRef]
- Sabater-Lleal, M.; Huffman, J.E.; de Vries, P.S.; Marten, J.; Mastrangelo, M.A.; Song, C.; Pankratz, N.; Ward-Caviness, C.K.; Yanek, L.R.; Trompet, S.; et al. Genome-Wide Association Transethnic Meta-Analyses Identifies Novel Associations Regulating Coagulation Factor VIII and von Willebrand Factor Plasma Levels. Circulation 2019, 5, 620–635. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6438386/ (accessed on 17 May 2019). [CrossRef]
- Bunimov, N.; Fuller, N.; Hayward, C.P. Genetic loci associated with platelet traits and platelet disorders. Semin. Thromb. Hemost. 2013, 39, 291–305. Available online: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0033-1334466 (accessed on 17 May 2019). [CrossRef]
- Chen, M.H.; Yanek, L.R.; Backman, J.D.; Eicher, J.D.; Huffman, J.E.; Ben-Shlomo, Y.; Beswick, A.D.; Yerges-Armstrong, L.M.; Shuldiner, A.R.; O’Connell, J.R.; et al. Exome-chip meta-analysis identifies association between variation in ANKRD26 and platelet aggregation. Platelets 2017, 28, 1–10. Available online: https://www.tandfonline.com/doi/abs/10.1080/09537104.2017.1384538?journalCode=iplt20 (accessed on 17 May 2019). [CrossRef]
- Stierlin, F.B.; Molica, F.; Reny, J.L.; Kwak, B.R.; Fontana, P. Pannexin1 Single Nucleotide Polymorphism and platelet reactivity in a cohort of cardiovascular patients. Cell Commun. Adhes. 2017, 23, 11–15. Available online: https://www.tandfonline.com/doi/full/10.1080/15419061.2017.1282469 (accessed on 17 May 2019). [CrossRef] [PubMed]
- Sokol, J.; Skerenova, M.; Ivankova, J.; Simurda, T.; Stasko, J. Association of genetic variability in selected genes in patients with deep vein thrombosis and platelet hyperaggregability. Clin. Appl. Thromb. Hemost. 2018, 24, 1027–1032. Available online: https://journals.sagepub.com/doi/full/10.1177/1076029618779136?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed (accessed on 17 May 2019). [CrossRef] [PubMed]
- Johnson, A.D.; Yanek, L.R.; Chen, M.H.; Faraday, N.; Larson, M.G.; Tofler, G.; Lin, S.J.; Kraja, A.T.; Province, M.A.; Yang, Q.; et al. Genome-wide metaanalyses identifies seven loci associatedwith platelet aggregation in response to agonists. Nat. Genet. 2010, 42, 608–613. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057573/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Qayyum, R.; Becker, L.C.; Becker, D.M.; Faraday, N.; Yanek, L.R.; Leal, S.M.; Shaw, C.; Mathias, R.; Suktitipat, B.; Bray, P.F. Genome-wide association study of platelet aggregation in African Americans. BMC Genet. 2015, 6, 58. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4448541/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Heit, J.A.; Armasu, S.M.; Asmann, Y.W.; Cunningham, J.M.; Matsumoto, M.E.; Petterson, T.M.; De Andrade, M. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J. Thromb. Haemost. 2012, 10, 1521–1531. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3419811/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Tang, W.; Teichert, M.; Chasman, D.I.; Heit, J.A.; Morange, P.E.; Li, G.; Pankratz, N.; Leebeek, F.W.; Paré, G.; de Andrade, M.; et al. A genome-wide association study for venous thromboembolism: The extended cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Genet. Epidemiol. 2013, 37, 512–521. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3990406/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Germain, M.; Saut, N.; Greliche, N.; Dina, C.; Lambert, J.C.; Perret, C.; Cohen, W.; Oudot-Mellakh, T.; Antoni, G.; Alessi, M.C.; et al. Genetics of venous thrombosis: Insights from a new genome wide association study. PLoS ONE 2011, 6, e25581. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3181335/ (accessed on 17 May 2019). [CrossRef]
- Trégouët, D.A.; Heath, S.; Saut, N.; Biron-Andreani, C.; Schved, J.F.; Pernod, G.; Galan, P.; Drouet, L.; Zelenika, D.; Juhan-Vague, I.; et al. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: Results from a GWAS approach. Blood 2009, 113, 5298–5303. Available online: http://www.bloodjournal.org/content/113/21/5298.long?sso-checked=true (accessed on 17 May 2019). [CrossRef]
- Gorski, M.M.; de Haan, H.G.; Mancini, I.; Lotta, L.A.; Bucciarelli, P.; Passamonti, S.M.; Cairo, A.; Pappalardo, E.; van Hylckama Vlieg, A.; Martinelli, I.; et al. Next-generation DNA sequencing to identify novel genetic risk factors for cerebral vein thrombosis. Thromb. Res. 2018, 169, 76–81. Available online: https://www.thrombosisresearch.com/article/S0049-3848(18)30385-2/fulltext (accessed on 17 May 2019). [CrossRef]
- Manco, L.; Silva, C.; Fidalgo, T.; Martinho, P.; Sarmento, A.B.; Ribeiro, M.L. Venous thromboembolism risk associated with ABO, F11 and FGG loci. Blood Coagul. Fibrinolysis 2018, 6, 528–532. Available online: https://insights.ovid.com/pubmed?pmid=29995659 (accessed on 17 May 2019). [CrossRef]
- Reilly, M.P.; Li, M.; He, J.; Ferguson, J.F.; Stylianou, I.M.; Mehta, N.N.; Burnett, M.S.; Devaney, J.M.; Knouff, C.W.; Thompson, J.R.; et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: Two genome-wide association studies. Lancet 2011, 377, 383–392. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3297116/ (accessed on 17 May 2019). [CrossRef]
- Williams, F.M.; Carter, A.M.; Hysi, P.G.; Surdulescu, G.; Hodgkiss, D.; Soranzo, N.; Traylor, M.; Bevan, S.; Dichgans, M.; Rothwell, P.M.; et al. Ischemic stroke is associated with the ABO locus: The EuroCLOT study. Ann. Neurol. 2013, 73, 16–31. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3582024/ (accessed on 22 Jun 2019). [CrossRef] [PubMed]
- Ling, X.; Zheng, Y.; Tao, J.; Zheng, Z.; Chen, L. Association study of polymorphisms in the ABO gene with ischemic stroke in the Chinese population. BMC Neurol. 2016, 16, 146. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4992279/ (accessed on 17 May 2019). [CrossRef] [PubMed][Green Version]
- IBC 50K CAD Consortium. Large-scale gene-centric analysis identifies novel variants for coronary artery disease. PLoS Genet. 2011, 7, e1002260. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178591/ (accessed on 17 May 2019).
- Schunkert, H.; König, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–338. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3119261/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Bruzelius, M.; Strawbridge, R.J.; Trégouët, D.A.; Wiggins, K.L.; Gertow, K.; Sabater-Lleal, M.; Öhrvik, J.; Bergendal, A.; Silveira, A.; Sundström, A.; et al. Influence of coronary artery disease-associated genetic variants on risk of venous thromboembolism. Thromb. Res. 2014, 134, 426–432. Available online: https://www.thrombosisresearch.com/article/S0049-3848(14)00184-4/fulltext (accessed on 17 May 2019). [CrossRef] [PubMed]
- Franchini, M.; Lippi, G. Relative risks of thrombosis and bleeding in different ABO blood groups. Semin. Thromb. Hemost. 2016, 42, 112–117. Available online: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0035-1564832 (accessed on 17 May 2019). [PubMed]
- Zhang, H.; Mooney, C.J.; Reilly, M.P. ABO blood groups and cardiovascular diseases. Review Article. Int. J. Vasc. Med. 2012, 2012, 641917. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3485501/ (accessed on 17 May 2019). [PubMed]
- Barbalic, M.; Dupuis, J.; Dehghan, A.; Bis, J.C.; Hoogeveen, R.C.; Schnabel, R.B.; Nambi, V.; Bretler, M.; Smith, N.L.; Peters, A.; et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum. Mol. Genet. 2010, 19, 1863–1872. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2850624/ (accessed on 17 May 2019). [CrossRef]
- Souto, J.C.; Almasy, L.; Muñiz-Diaz, E.; Soria, J.M.; Borrell, M.; Bayén, L.; Mateo, J.; Madoz, P.; Stone, W.; Blangero, J.; et al. Functional effects of the ABO locus polymorphism on plasma levels of von Willebrand factor, factor VIII, and activated partial thromboplastin time. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2024–2028. Available online: https://www.ahajournals.org/action/doSearch?AllField=Souto+JC&publication=atvb (accessed on 17 May 2019). [CrossRef]
- Chen, Y.; Chen, C.; Ke, X.; Xiong, L.; Shi, Y.; Li, J.; Tan, X.; Ye, S. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ. Cardiovasc. Genet. 2014, 7, 43–48. Available online: https://www.ahajournals.org/doi/full/10.1161/CIRCGENETICS.113.000299?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed (accessed on 17 May 2019). [CrossRef] [PubMed]
- Paterson, A.D.; Lopes-Virella, M.F.; Waggott, D.; Boright, A.P.; Hosseini, S.M.; Carter, R.E.; Shen, E.; Mirea, L.; Bharaj, B.; Sun, L.; et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1958–1967. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3147250/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Zhong, M.; Zhang, H.; Reilly, J.P.; Chrisitie, J.D.; Ishihara, M.; Kumagai, T.; Azadi, P.; Reilly, M.P. ABO blood group as a model for platelet glycan modification in arterial thrombosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1570–1578. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4521621/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Zhong, X.; Kriz, R.; Seehra, J.; Kumar, R. N-linked glycosylation of platelet P2Y12 ADP receptor is essential for signal transduction but not for ligand binding or cell surface expression. FEBS Lett. 2004, 562, 111–117. Available online: https://febs.onlinelibrary.wiley.com/doi/full/10.1016/S0014-5793%2804%2900191-7?sid=nlm%3Apubmed (accessed on 17 May 2019). [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC334765/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Olsson, M.L.; Chester, M.A. A rapid and simple ABO genotype screening method using a novel B/O2 versus A/O2 discriminating nucleotide substitution at the ABO locus. Vox Sang. 1995, 69, 242–247. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1423-0410.1995.tb02602.x?sid=nlm%3Apubmed (accessed on 17 May 2019). [PubMed]
- Delaneau, O.; Zagury, J.-F.; Marchini, J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods 2013, 10, 5–6. Available online: https://www.nature.com/articles/nmeth.2307 (accessed on 17 May 2019). [CrossRef]
- Howie, B.N.; Donnelly, P.; Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009, 5, e1000529. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2689936/ (accessed on 17 May 2019). [CrossRef]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer Associates Inc.: Sunderland, MA, USA, 1998. [Google Scholar]
- Shabalin, A.A. Matrix eQTL: Ultra fast eQTL analysis via large matrix operations. Bioinformatics 2012, 28, 1353–1358. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348564/ (accessed on 17 May 2019). [CrossRef]
- Ziyatdinov, A.; Brunel, H.; Martinez-Perez, A.; Buil, A.; Perera, A.; Soria, J.M. Solarius: An R interface to SOLAR for variance component analysis in pedigrees. Bioinformatics 2016, 32, 1901–1902. Available online: https://academic.oup.com/bioinformatics/article/32/12/1901/1744140 (accessed on 17 May 2019). [CrossRef]
- Almasy, L.; Blangero, J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 1998, 62, 1198–1211. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1377101/ (accessed on 17 May 2019). [CrossRef] [PubMed]
- Self, S.G.; Liang, K.Y. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J. Am. Stat. Assoc. 1987, 82, 605–610. [Google Scholar] [CrossRef]
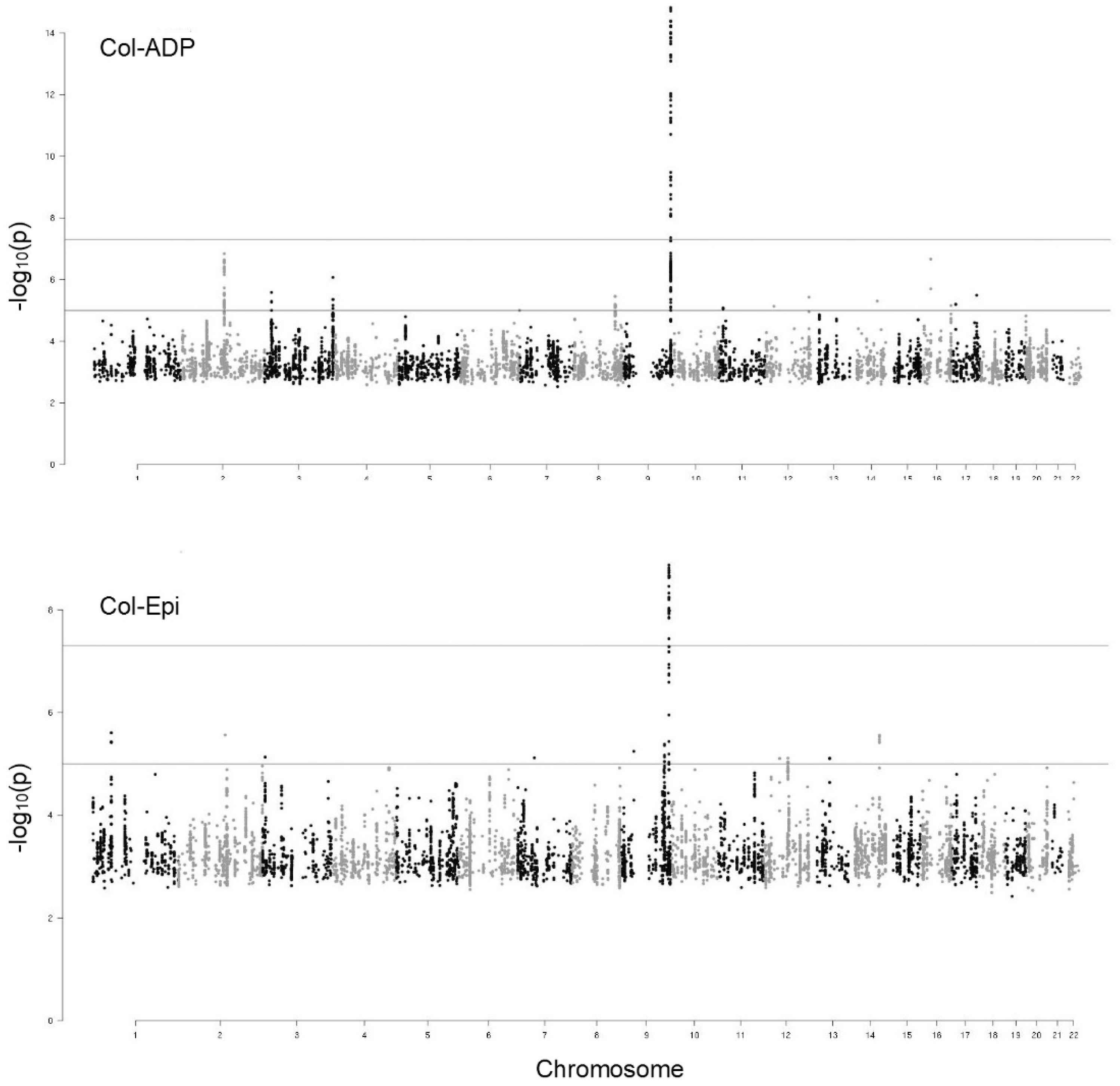
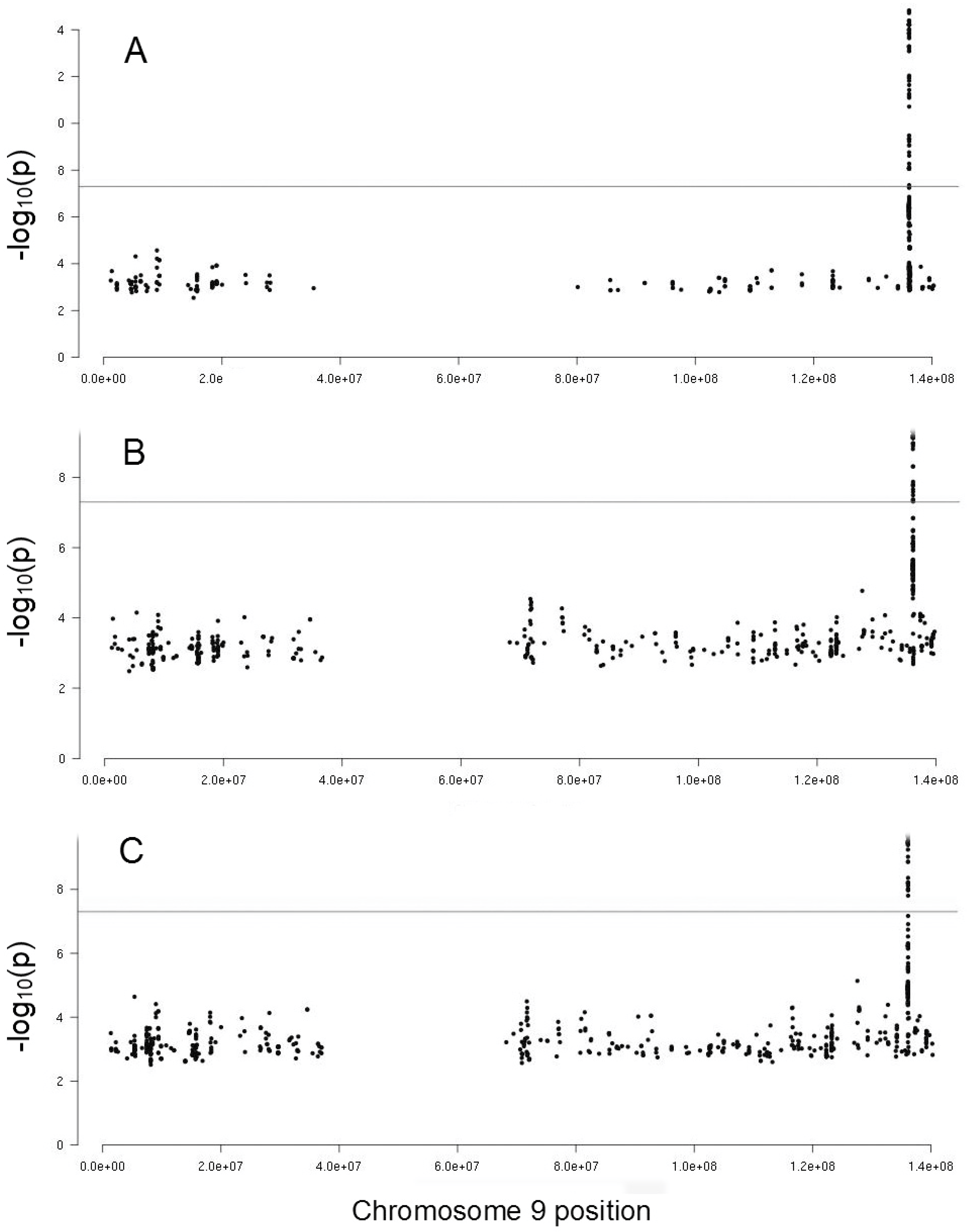
| Phenotype | Col-Epi | VWF | FVIII | ABO |
|---|---|---|---|---|
| Col-ADP | ρ = 0.7917 (5.80 × 10−9) | ρ = −0.7002 (1.02 × 10−10) | ρ = −0.6209 (2.66 × 10−8) | ρ = 0.5895 (7.01 × 10−9) |
| Col-Epi | - | ρ = −0.6342 (7.14 × 10−8) | ρ = −0.5947 (3.97 × 10−7) | ρ = 0.4477 (0.0003) |
| SNP | Position (bp) | Location | MAF | Association with Col-ADP p-Value | Association with Col-Epi p-Value | Association with Thrombosis-Related Conditions [References] | Association with Variations of Biological Factors [References] |
|---|---|---|---|---|---|---|---|
| rs8176719 | 136132908 | coding, 5-UTR, intron | 0.459 | 5.21 × 10−14 | 1.88 × 10−9 | VTE [18,25,26,29] | |
| rs687621 | 136137065 | intron | 0.432 | 1.49 × 10−15 | 3.50 × 10−9 | VTE [18,26], MI [31], LVCES [32] | VWF [17,18,38], ICAM-1 [38] |
| rs687289 | 136137106 | intron | 0.433 | 1.93 × 10−15 | 5.72 × 10−9 | MI [31], LVCES [32] | FVIII [16,17], ICAM-1 [38] |
| rs2519093 | 136141870 | intron | 0.312 | 1.16 × 10−12 | 6.01 × 10−9 | VTE [25], LVCES [32] | - |
| rs514659 | 136142203 | intron | 0.433 | 6.04 × 10−15 | 1.04 × 10−8 | VTE [26], MI [31], LVCES [32], LAA [33] | VWF [38] |
| rs644234 | 136142217 | intron | 0.460 | 1.42 × 10−14 | 2.18 × 10−9 | MI [31], LVCES [32] | E-selectin [38] |
| rs643434 | 136142355 | intron | 0.460 | 1.43 × 10−14 | 2.18 × 10−9 | MI [31], LVCES [32] | - |
| rs545971 | 136143372 | Intron | 0.433 | 6.03 × 10−15 | 1.04 × 10−8 | MI [31], LVCES [32] | - |
| rs612169 | 136143442 | intron | 0.433 | 6.05 × 10−15 | 1.04 × 10−8 | MI [31], LVCES [32] | ICAM-1, E-selectin [38] |
| rs674302 | 136146664 | intron | 0.433 | 6.06 × 10−15 | 1.04 × 10−8 | MI [31], LVCES [32] | - |
| rs500498 | 136148647 | intron | 0.408 | 5.56 × 10−08 | - | VTE [27], LVCES [32] | ICAM-1, E-selectin [38] |
| rs505922 | 136149229 | intron | 0.433 | 9.68 × 10−15 | 1.12 × 10−8 | VTE [27,28], MI [31], LVCES [32] | - |
| rs529565 | 136149500 | intron | 0.434 | 1.86 × 10−14 | 1.01 × 10−8 | VTE [29,30], MI [31], LVCES [32], LAA [33] | - |
| rs630014 | 136149722 | intron | 0.407 | 8.74 × 10−10 | - | VTE [27,28], LVCES [32] | E-selectin [38] |
| rs651007 | 136153875 | intergenic | 0.204 | 8.02 × 10−12 | 2.32 × 10−9 | LVCES [32], LAA [33], CAD [34] | VWF, ICAM-1, E-selectin, cholesterol [38] |
| rs579459 | 136154168 | intergenic | 0.340 | 3.75 × 10−12 | 1.35 × 10−9 | LVCES [32], CAD [35], CAD+VTE [36] | ICAM-1, E- and P-selectin [38] |
| rs649129 | 136154304 | intergenic | 0.338 | 6.88 × 10−12 | 2.19 × 10−9 | LVCES [32] | ICAM-1, LDL-cholesterol [38,39] |
| rs495828 | 136154867 | intergenic | 0.340 | 7.71 × 10−12 | 2.28 × 10−9 | VTE [25,27], LVCES [32] | ACE [38] |
| rs633862 | 136155444 | intergenic | 0.391 | 2.41 × 10−9 | - | LVCES [32] | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pujol-Moix, N.; Martinez-Perez, A.; Sabater-Lleal, M.; Llobet, D.; Vilalta, N.; Hamsten, A.; Souto, J.C.; Soria, J.M. Influence of ABO Locus on PFA-100 Collagen-ADP Closure Time Is Not Totally Dependent on the Von Willebrand Factor. Results of a GWAS on GAIT-2 Project Phenotypes. Int. J. Mol. Sci. 2019, 20, 3221. https://doi.org/10.3390/ijms20133221
Pujol-Moix N, Martinez-Perez A, Sabater-Lleal M, Llobet D, Vilalta N, Hamsten A, Souto JC, Soria JM. Influence of ABO Locus on PFA-100 Collagen-ADP Closure Time Is Not Totally Dependent on the Von Willebrand Factor. Results of a GWAS on GAIT-2 Project Phenotypes. International Journal of Molecular Sciences. 2019; 20(13):3221. https://doi.org/10.3390/ijms20133221
Chicago/Turabian StylePujol-Moix, Núria, Angel Martinez-Perez, Maria Sabater-Lleal, Dolors Llobet, Noèlia Vilalta, Anders Hamsten, Joan Carles Souto, and José Manuel Soria. 2019. "Influence of ABO Locus on PFA-100 Collagen-ADP Closure Time Is Not Totally Dependent on the Von Willebrand Factor. Results of a GWAS on GAIT-2 Project Phenotypes" International Journal of Molecular Sciences 20, no. 13: 3221. https://doi.org/10.3390/ijms20133221
APA StylePujol-Moix, N., Martinez-Perez, A., Sabater-Lleal, M., Llobet, D., Vilalta, N., Hamsten, A., Souto, J. C., & Soria, J. M. (2019). Influence of ABO Locus on PFA-100 Collagen-ADP Closure Time Is Not Totally Dependent on the Von Willebrand Factor. Results of a GWAS on GAIT-2 Project Phenotypes. International Journal of Molecular Sciences, 20(13), 3221. https://doi.org/10.3390/ijms20133221






