Intestinal Preservation Injury: A Comparison Between Rat, Porcine and Human Intestines
Abstract
:1. Introduction
2. Results
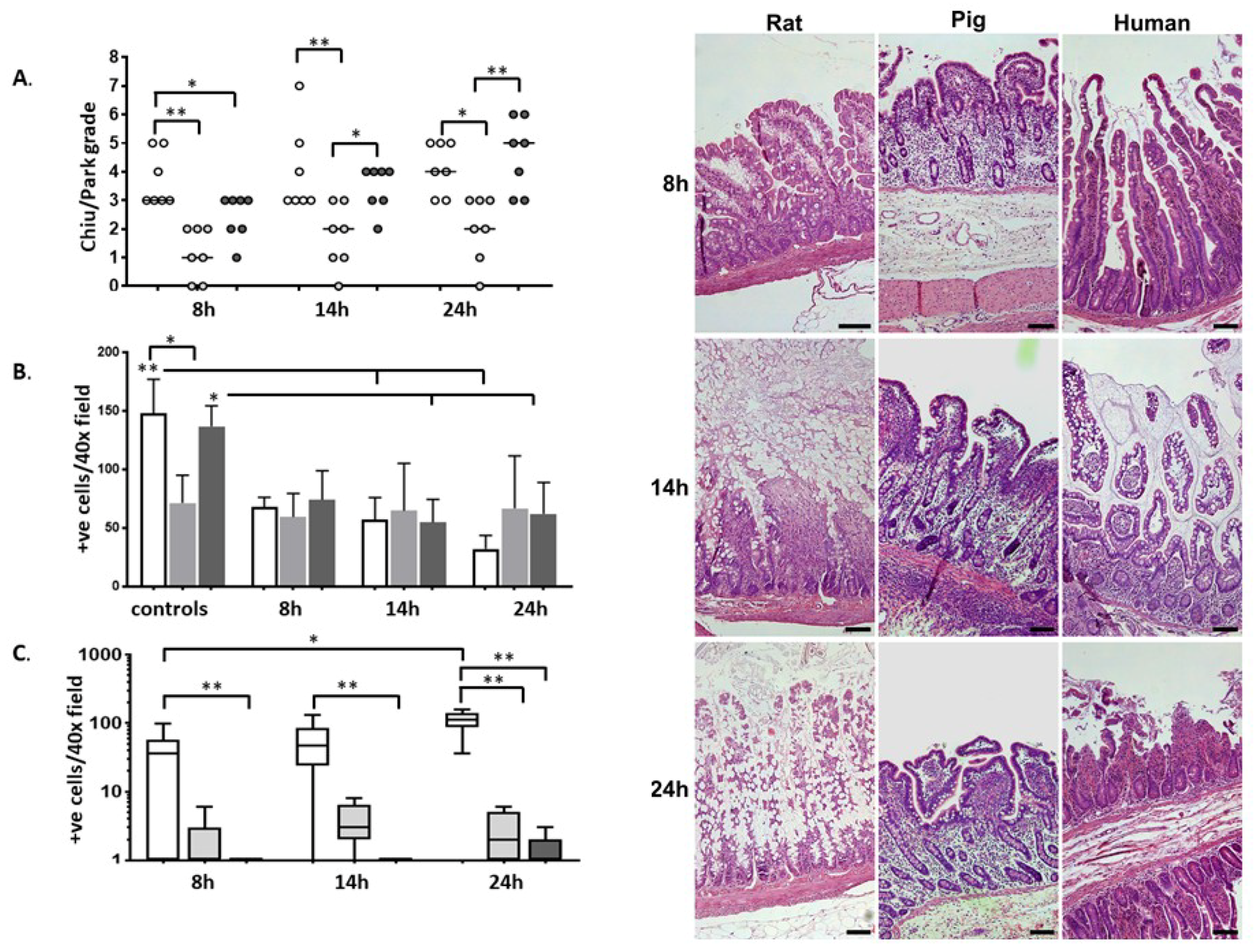
2.1. Histology
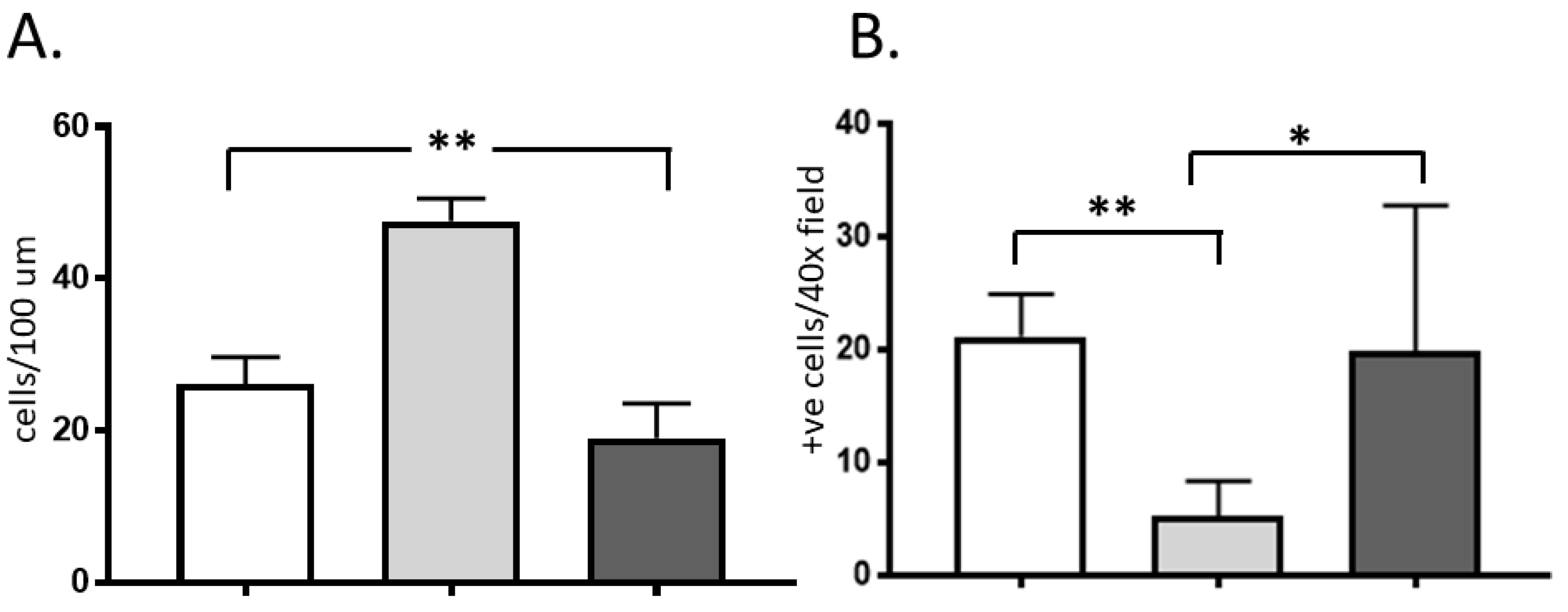
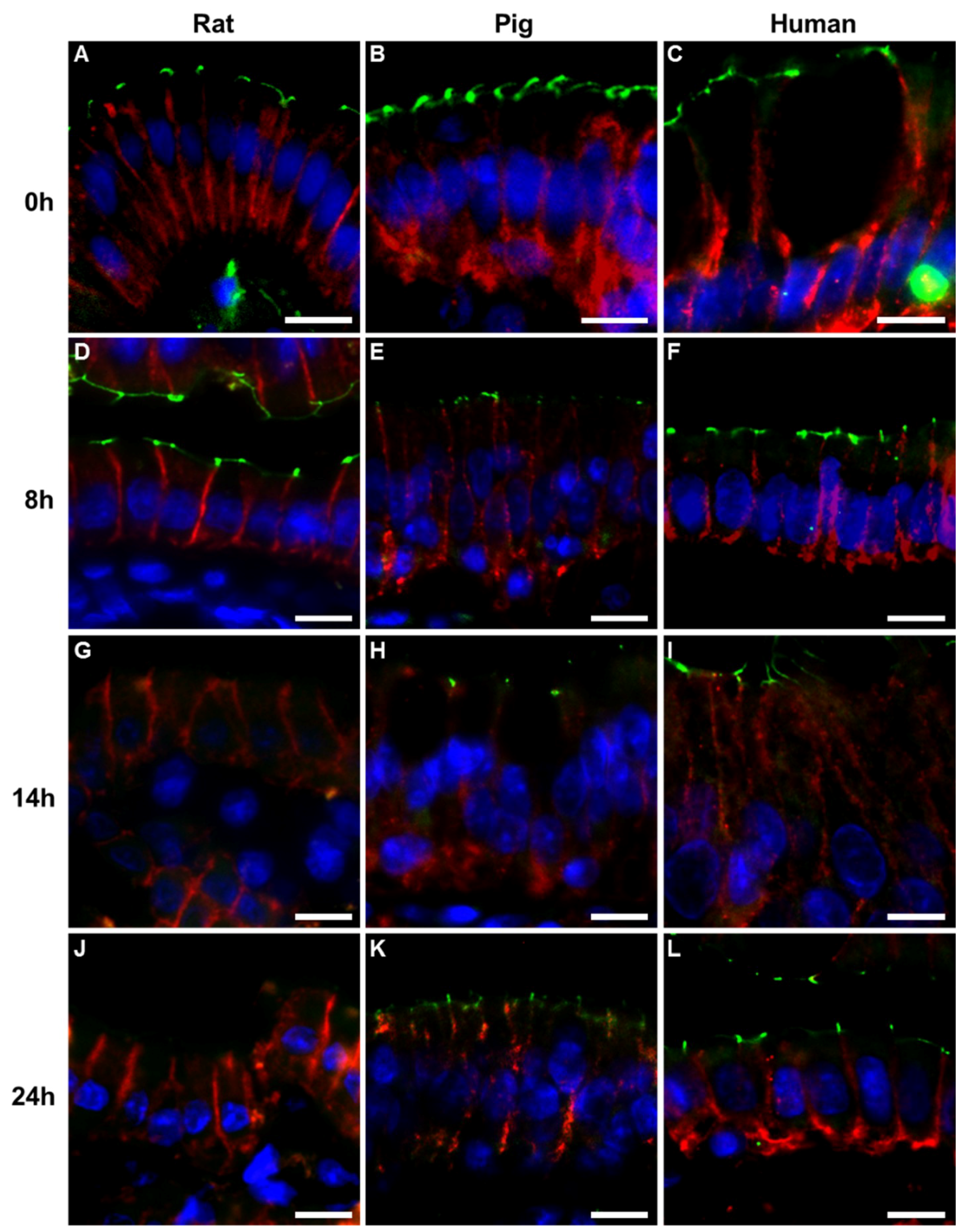
2.2. Immunohistochemistry
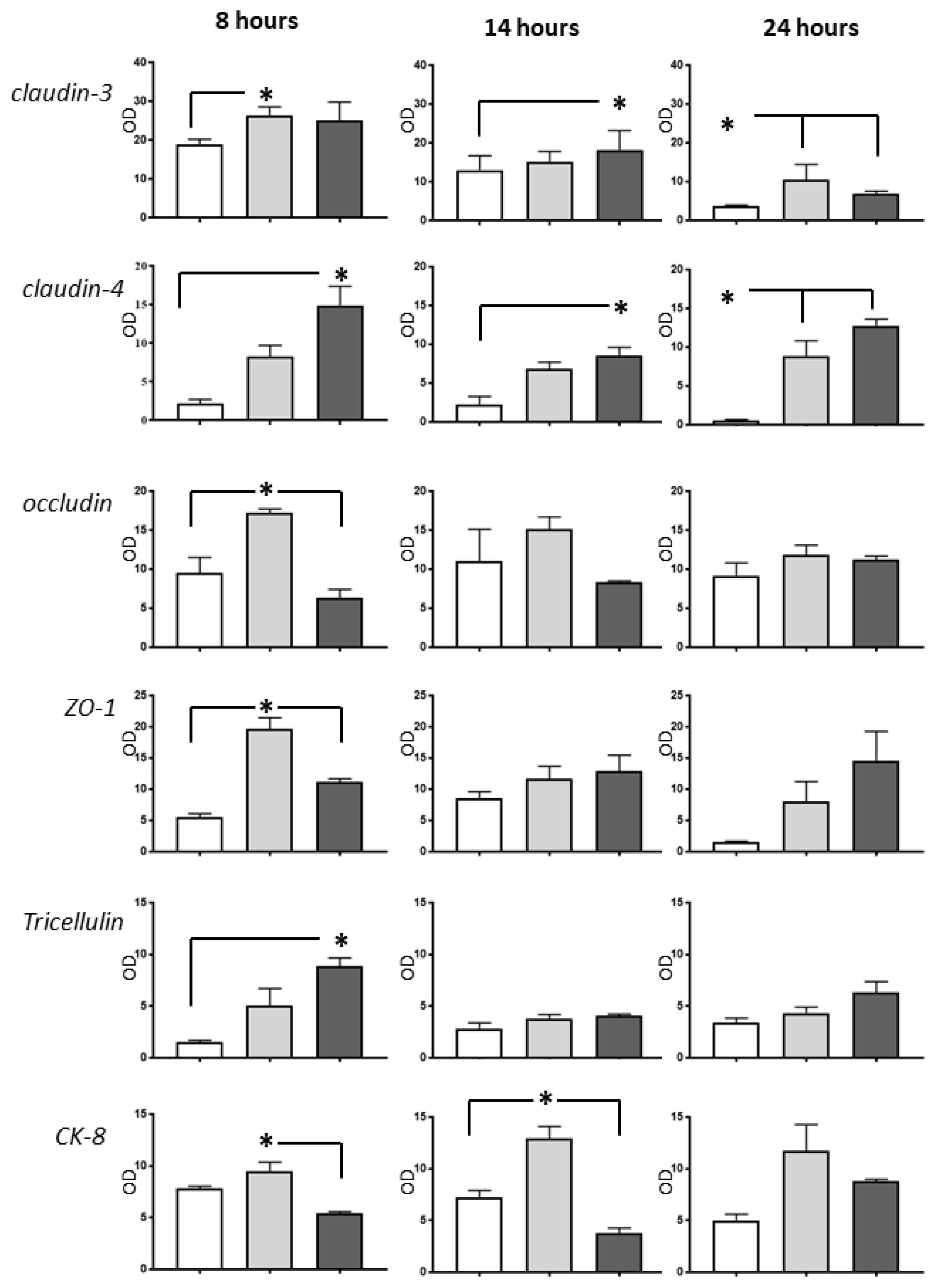
2.3. Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Animals, Surgery, and Sampling
4.2. Human Organ Donors
4.3. Histology
4.3.1. Light Microscopy
4.3.2. Immunofluorescence
4.3.3. Western Blot Analyses of Intestinal Mucosa
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abu-Elmagd, K.M.; Costa, G.; Bond, G.J.; Soltys, K.; Sindhi, R.; Wu, T.; Koritsky, D.A.; Schuster, B.; Martin, L.; Cruz, R.J.; et al. Five hundred intestinal and multivisceral transplantations at a single center: Major advances with new challenges. Ann. Surg. 2009, 250, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Clouse, J.W.; Kubal, C.A.; Fridell, J.A.; Mangus, R.S. Posttransplant complications in adult recipients of intestine grafts without bowel decontamination. J. Surg. Res. 2018, 225, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Huard, G.; Schiano, T.D.; Moon, J.; Iyer, K. Severe acute cellular rejection after intestinal transplantation is associated with poor patient and graft survival. Clin. Transplant. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.; Simrén, M.; Jalanko, H.; Oltean, M.; Saalman, R.; Gudjonsdottir, A.; Gäbel, M.; Borg, H.; Edenholm, M.; Bentdal, O.; et al. Fifteen years’ experience of intestinal and multivisceral transplantation in the Nordic countries. Scand J. Gastroenterol. 2015, 50, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Tesi, R.J.; Jaffe, B.M.; McBride, V.; Haque, S. Histopathologic changes in human small intestine during storage in Viaspan organ preservation solution. Arch. Pathol. Lab. Med. 1997, 121, 714. [Google Scholar] [PubMed]
- deRoover, A.; de Leval, L.; Gilmaire, J.; Detry, O.; Boniver, J.; Honoré, P.; Meurisse, M. A new model for human intestinal preservation: Comparison of University of Wisconsin and Celsior preservation solutions. Transplant. Proc. 2004, 36, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Churchill, T.A. Organ-specific solutions and strategies for the intestinal preservation. Int. Rev. Immunol. 2014, 33, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.; Hurlbut, D.; Zhong, R.; Wang, P.Z.; Chen, H.F.; Garcia, B.; Behme, R.; Stiller, C.; Duff, J. Intestinal permeability and bacterial translocation following small bowel transplantation in the rat. Transplantation 1991, 52, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Zhu, C.; Mera, S.; Pullerits, R.; Mattsby-Baltzer, I.; Mölne, J.; Hallberg, E.; Blomgren, K.; Olausson, M. Reduced liver injury and cytokine release after transplantation of preconditioned intestines. J. Surg. Res. 2009, 154, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.; Duff, J.; Zhong, R.; Garcia, B.; Lipohar, C.; Keown, P.; Stiller, C. Successful intestinal transplantation in pigs treated with cyclosporine. Transplantation 1988, 45, 279–284. [Google Scholar] [CrossRef]
- Pirenne, J.; Benedetti, E.; Gruessner, A.; Moon, C.; Hakim, N.; Fryer, J.P.; Troppmann, C.; Nakhleh, R.E.; Gruessner, R.W. Combined transplantation of small and large bowel. FK506 versus cyclosporine A in a porcine model. Transplantation 1996, 61, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, M.P.; Kuusanmäki, P.; Lauronen, J.; Paavonen, T.; Halttunen, J. Effects of ileum transplantation and chronic rejection on absorption and synthesis of cholesterol in pigs. Pediatr. Surg. Int. 2003, 19, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Weih, S.; Kessler, M.; Fonouni, H.; Golriz, M.; Nickkholgh, A.; Schmidt, J.; Holland-Cunz, S.; Mehrabi, A. Review of various techniques of small bowel transplantation in pigs. J. Surg. Res. 2011, 171, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, N.; Tahara, K.; Schuchtrup, S.; Websky, M.V.; Overhaus, M.; Schmidt, J.; Wirz, S.; Abu-Elmagd, K.M.; Kalff, J.C.; Hirner, A.; et al. Perioperative glycine treatment attenuates ischemia/reperfusion injury and ameliorates smooth muscle dysfunction in intestinal transplantation. Transplantation 2008, 85, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Joshi, M.; Björkman, E.; Oltean, S.; Casselbrant, A.; Herlenius, G.; Olausson, M. Intraluminal polyethylene glycol stabilizes tight junctions and improves intestinal preservation in the rat. Am. J. Transplant 2012, 12, 2044–2051. [Google Scholar] [CrossRef]
- Oltean, M.; Jiga, L.; Hellström, M.; Söfteland, J.; Papurica, M.; Hoinoiu, T.; Ionac, M.; Casselbrant, A. A sequential assessment of the preservation injury in porcine intestines. J. Surg. Res. 2017, 216, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T.; Roberts, M.C.; Rhoads, J.M.; Argenzio, R.A. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery 1997, 121, 526–534. [Google Scholar] [CrossRef]
- Ikeda, H.; Yang, C.L.; Tong, J.; Nishimaki, H.; Masuda, K.; Takeo, T.; Kasai, K.; Itoh, G. Rat small intestinal goblet cell kinetics in the process of restitution of surface epithelium subjected to ischemia-reperfusion injury. Dig. Dis. Sci. 2002, 47, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Hundscheid, I.H.; Lenaerts, K.; Boonen, B.; Renes, I.B.; Verheyen, F.K.; Dejong, C.H.; von Meyenfeldt, M.F.; Beets, G.L.; Buurman, W.A. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut 2013, 62, 250–258. [Google Scholar] [CrossRef]
- Lopez-Pedrosa, J.M.; Torres, M.I.; Fernández, M.I.; Ríos, A.; Gil, A. Severe malnutrition alters lipid composition and fatty acid profile of small intestine in newborn piglets. J Nutr. 1998, 128, 224–233. [Google Scholar] [CrossRef]
- Kubes, P.; Hunter, J.; Granger, D.N. Ischemia/reperfusion-induced feline intestinal dysfunction: Importance of granulocyte recruitment. Gastroenterology 1992, 103, 807–812. [Google Scholar] [CrossRef]
- Dabrowska, D.; Jablonska, E.; Iwaniuk, A.; Garley, M. Many ways–one destination: Different types of neutrophils death. Int. Rev. Immunol. 2019, 38, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Kohli, V.; Selzner, M.; Madden, J.F.; Bentley, R.C.; Clavien, P.A. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 1999, 67, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Jani, A.; Ljubanovic, D.; Faubel, S.; Kim, J.; Mischak, R.; Edelstein, C.L. Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am. J. Transplant 2004, 4, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M.; Hellström, M.; Ciuce, C.; Zhu, C.; Casselbrant, A. Luminal solutions protect mucosal barrier during extended preservation. J. Surg. Res. 2015, 194, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Casselbrant, A.; Söfteland, J.M.; Hellström, M.; Malinauskas, M.; Oltean, M. Luminal polyethylene glycol alleviates intestinal preservation injury irrespective of molecular size. J. Pharmacol. Exp. Ther. 2018, 366, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Hodin, C.M.; de Haan, J.J.; Derikx, J.P.; Rouschop, K.M.; Verheyen, F.K.; van Dam, R.M.; Dejong, C.H.; Buurman, W.A.; Lenaerts, K. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology 2011, 140, 529–539. [Google Scholar] [CrossRef]
- Kwon, O.; Phillips, C.L.; Molitoris, B.A. Ischemia induces alterations in actin filaments in renal vascular smooth muscle cells. Am. J. Physiol. Renal. Physiol. 2002, 282, F1012-9. [Google Scholar] [CrossRef]
- Shi, T.; Moulton, V.R.; Lapchak, P.H.; Deng, G.M.; Dalle Lucca, J.J.; Tsokos, G.C. Ischemia-mediated aggregation of the actin cytoskeleton is one of the major initial events resulting in ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G339–G347. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.R. Molecular basis of epithelial barrier regulation: From basic mechanisms to clinical application. Am. J. Pathol. 2006, 169, 1901–1909. [Google Scholar] [CrossRef]
- Takizawa, Y.; Kishimoto, H.; Kitazato, T.; Tomita, M.; Hayashi, M. Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion. Int. J. Pharm. 2012, 426, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Chiba, H.; Yokozaki, H.; Sakai, N.; Sugimoto, K.; Wada, T.; Kojima, T.; Yamashita, T.; Sawada, N. Differential expression and subcellular localization of claudin-7, -8, -12, -13, and -15 along the mouse intestine. J. Histochem. Cytochem. 2006, 54, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, L.G.; ‘t Hart, N.A.; Ottens, P.J.; van den Berg, A.; Ploeg, R.J.; van Goor, H.; Leuvenink, H.G. Brain death induces inflammation in the donor intestine. Transplantation 2008, 86, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pullerits, R.; Oltean, S.; Flodén, A.; Oltean, M. Circulating resistin levels are early and significantly increased in deceased brain dead organ donors, correlate with inflammatory cytokine response and remain unaffected by steroid treatment. J. Transl. Med. 2015, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.S.; Kaufman, S.S.; Girlanda, R.; Little, C.M.; Rekhtman, Y.; Raofi, V.; Laurin, J.M.; Shetty, K.; Fennelly, E.M.; Johnson, L.B.; et al. Utilization of donors who have suffered cardiopulmonary arrest and resuscitation in intestinal transplantation. Transplantation 2008, 86, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Fröhlich Königsrainer, A.; Schaffer, R.; Schaub, F.; Pratschke, J.; Pascher, A.; Steurer, W.; Nadalin, S. Organ donation: When should we consider intestinal donation. Transpl. Int. 2012, 25, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Park, P.O.; Haglund, U.; Bulkley, G.B.; Falt, K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 1990, 107, 574. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Søfteland, J.M.; Casselbrant, A.; Biglarnia, A.-R.; Linders, J.; Hellström, M.; Pesce, A.; Padma, A.M.; Jiga, L.P.; Hoinoiu, B.; Ionac, M.; et al. Intestinal Preservation Injury: A Comparison Between Rat, Porcine and Human Intestines. Int. J. Mol. Sci. 2019, 20, 3135. https://doi.org/10.3390/ijms20133135
Søfteland JM, Casselbrant A, Biglarnia A-R, Linders J, Hellström M, Pesce A, Padma AM, Jiga LP, Hoinoiu B, Ionac M, et al. Intestinal Preservation Injury: A Comparison Between Rat, Porcine and Human Intestines. International Journal of Molecular Sciences. 2019; 20(13):3135. https://doi.org/10.3390/ijms20133135
Chicago/Turabian StyleSøfteland, John Mackay, Anna Casselbrant, Ali-Reza Biglarnia, Johan Linders, Mats Hellström, Antonio Pesce, Arvind Manikantan Padma, Lucian Petru Jiga, Bogdan Hoinoiu, Mihai Ionac, and et al. 2019. "Intestinal Preservation Injury: A Comparison Between Rat, Porcine and Human Intestines" International Journal of Molecular Sciences 20, no. 13: 3135. https://doi.org/10.3390/ijms20133135
APA StyleSøfteland, J. M., Casselbrant, A., Biglarnia, A.-R., Linders, J., Hellström, M., Pesce, A., Padma, A. M., Jiga, L. P., Hoinoiu, B., Ionac, M., & Oltean, M. (2019). Intestinal Preservation Injury: A Comparison Between Rat, Porcine and Human Intestines. International Journal of Molecular Sciences, 20(13), 3135. https://doi.org/10.3390/ijms20133135





