Post-Mortem Immunohistochemical Evidence of β2-Adrenergic Receptor Expression in the Adrenal Gland
Abstract
1. Introduction
2. Results
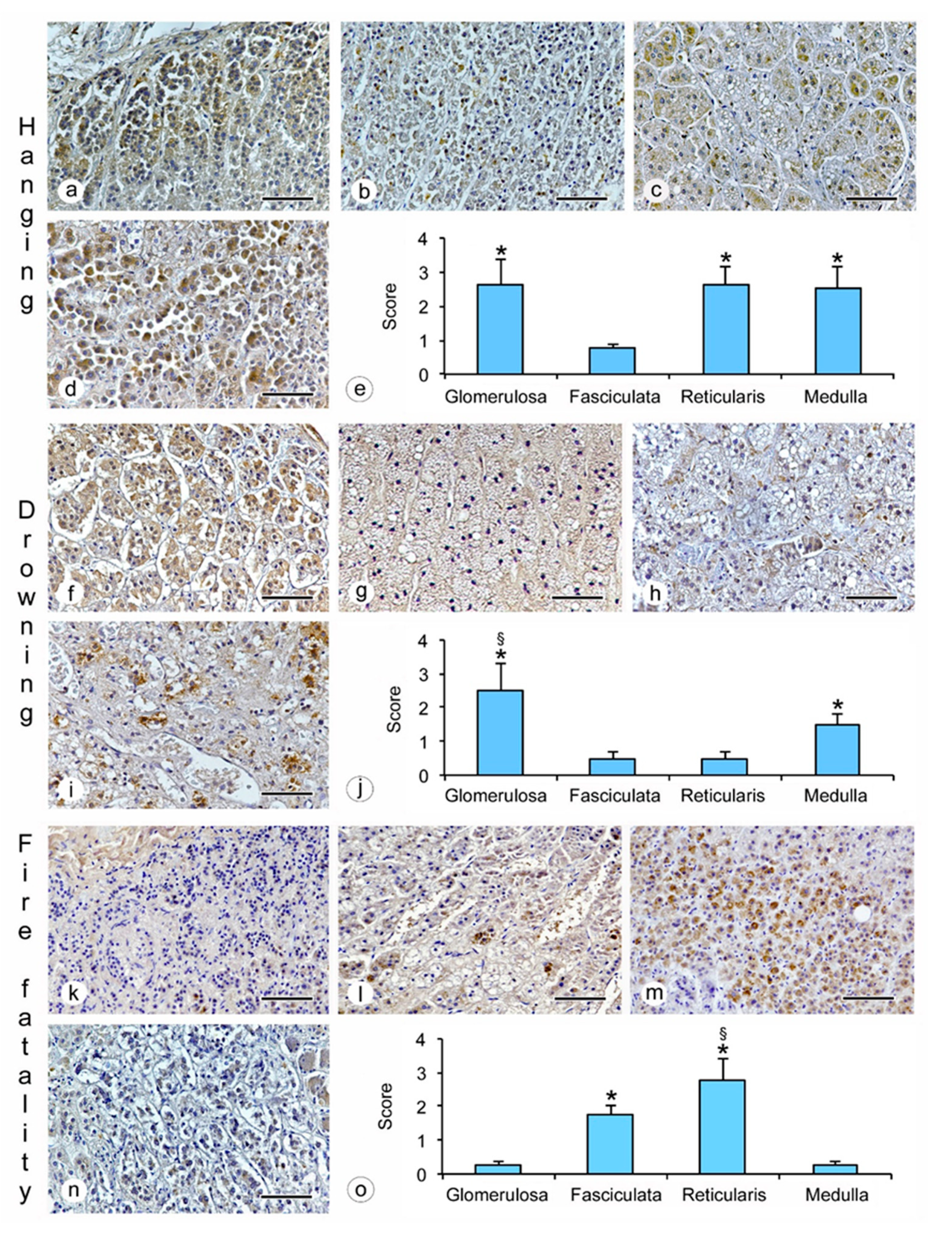
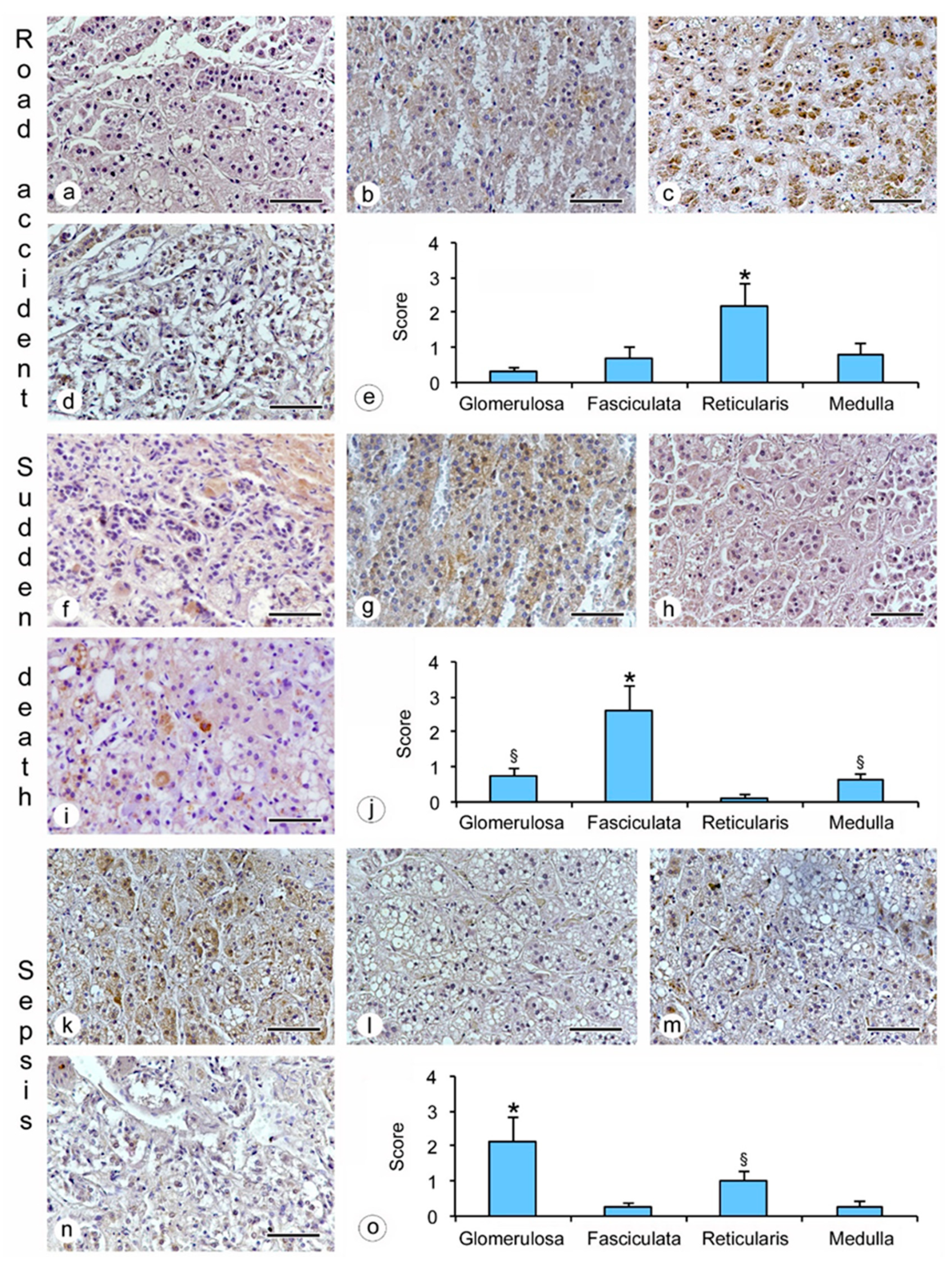
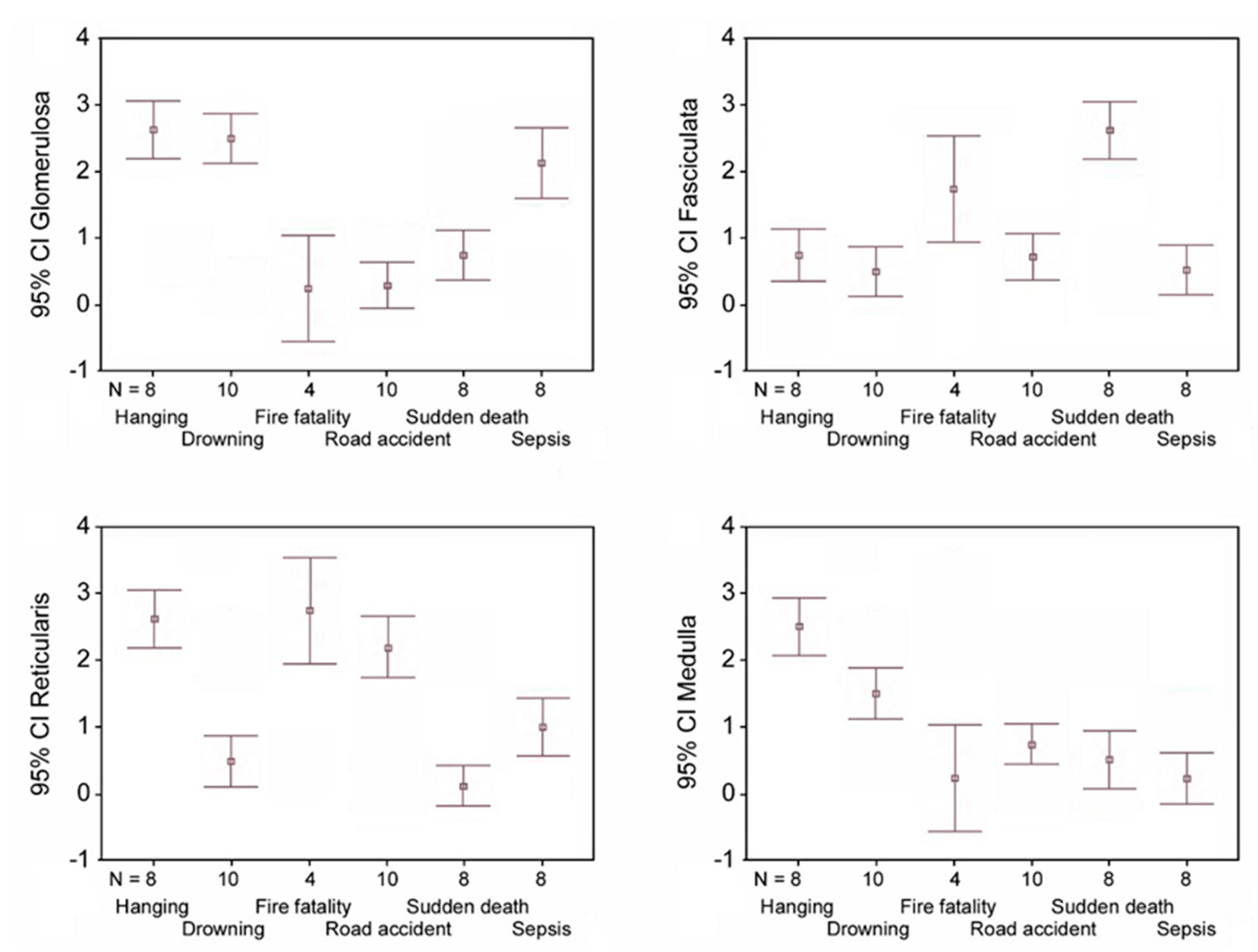
- hanging: the immunostaining of β2-AR was higher in the glomerulosa, reticularis, and medulla (Figure 1a,c,d), while a lower expression was observed in the fasciculata (Figure 1b). The statistical analysis confirmed the significant differences among the zones (p < 0.05 versus the fasciculata zone) (Figure 1e);
- drowning: the β2-AR immunostaining was higher in the glomerulosa and the medulla (Figure 1f,i), and significantly lower in the fasciculata and in the reticularis (Figure 1g,h). The statistical analysis also showed significant differences (p < 0.05) between the glomerulosa and the medulla (Figure 1j).
3. Discussion
- (i)
- to histologically demonstrate β2-AR expression in the human cortex;
- (ii)
- to provide suggestions on the possible involvement of β2-AR in human cortex hormonal stimulation, and
- (iii)
- to study β2-AR expression in the adrenal gland in different causes of death.
4. Materials and Methods
4.1. Immunohistochemistry Analysis
4.2. Morphometric Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goldstein, D.S. Adrenal responses to stress. Cell. Mol. Neurobiol. 2010, 30, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, Q.Y.; Hu, H.T.; Zhang, M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol. Ther. 2010, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Differential responses of components of the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 13–22. [Google Scholar] [PubMed]
- Motzer, S.A.; Hertig, V. Stress, stress response, and health. Nurs. Clin. N. Am 2004, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.L.; Ishikawa, T.; Michiue, T.; Li, D.R.; Zhao, D.; Quan, L.; Oritani, S.; Bessho, Y.; Maeda, H. Postmortem serum catecholamine levels in relation to the cause of death. Forensic Sci. Int. 2007, 173, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bańka, K.; Teresiński, G.; Buszewicz, G.; Mądro, R. Glucocorticosteroids as markers of death from hypothermia. Forensic Sci. Int. 2013, 229, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Inamori-Kawamoto, O.; Quan, L.; Michiue, T.; Chen, H.; Wang, Q.; Zhu, B.L.; Maeda, H. Postmortem urinary catecholamine levels with regard to the cause of death. Leg. Med. (Tokyo) 2014, 16, 344–349. [Google Scholar] [CrossRef]
- Ishikawa, T.; Quan, L.; Michiue, T.; Kawamoto, O.; Wang, Q.; Chen, J.H.; Zhu, B.L.; Maeda, H. Postmortem catecholamine levels in pericardial and cerebrospinal fluids with regard to the cause of death in medicolegal autopsy. Forensic Sci. Int. 2013, 228, 52–60. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yoshida, C.; Michiue, T.; Perdekamp, M.G.; Pollak, S.; Maeda, H. Immunohistochemistry of catecholamines in the hypothalamic-pituitary-adrenal system with special regard to fatal hypothermia and hyperthermia. Leg. Med. (Tokyo) 2010, 12, 121–127. [Google Scholar] [CrossRef]
- Aragona, F. L’immagine istologica delle surrenali quale test psicologico postmortale. Riv. Ital. Med. Leg. 1990, 12, 125–138. [Google Scholar]
- Belda, X.; Fuentes, S.; Daviu, N.; Nadal, R.; Armario, A. Stress-induced sensitization: The hypothalamic-pituitary-adrenal axis and beyond. Stress 2015, 18, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Spadari, R.C.; Cavadas, C.; de Carvalho, A.E.T.S.; Ortolani, D.; de Moura, A.L.; Vassalo, P.F. Role of beta-adrenergic receptors and sirtuin signaling in the heart during aging, heart failure, and adaptation to stress. Cell. Mol. Neurobiol. 2018, 38, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Ma, J.; Ma, Q.; Guo, K.; Guo, J.; Li, X.; Li, W.; Liu, J.; Huang, C.; Wang, F.; et al. β2-AR-HIF-1α: A novel regulatory axis for stress-induced pancreatic tumor growth and angiogenesis. Curr. Mol. Med. 2013, 13, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, H.; Kanaizumi, E.; Himi, T. Immunohistochemical localization of alpha and beta adrenergic receptors in the human nasal turbinate. Auris Nasus Larynx 2016, 43, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Antunes, G.; Simoes de Souza, F.M. Olfactory receptor signaling. Methods Cell Biol. 2016, 132, 127–145. [Google Scholar] [PubMed]
- Dixon, R.A.; Kobilka, B.K.; Strader, D.J.; Benovic, J.L.; Dohlman, H.G.; Frielle, T.; Bolanowski, M.A.; Bennett, C.D.; Rands, E.; Diehl, R.E.; et al. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature 1986, 321, 75–79. [Google Scholar] [CrossRef]
- Emorine, L.J.; Marullo, S.; Briend-Sutren, M.M.; Patey, G.; Tate, K.; Delavier-Klutchko, C.; Strosberg, A.D. Molecular characterization of the human beta 3-adrenergic receptor. Science 1989, 245, 1118–1121. [Google Scholar] [CrossRef]
- Bylund, D.B.; Eikenberg, D.C.; Hieble, J.P.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Molinoff, P.B.; Ruffolo, R.R., Jr.; Trendelenburg, U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar]
- Daly, C.J.; McGrath, C.J. Previously unsuspected widespread cellular and tissue distribution of β-adrenoceptors and its relevance to drug action. Trends Pharmacol. Sci. 2011, 32, 219–226. [Google Scholar] [CrossRef]
- Cesetti, T.; Hernández-Guijo, J.M.; Baldelli, P.; Carabelli, V.; Carbone, E. Opposite action of beta1- and beta2-adrenergic receptors on Ca(V)1 L-channel current in rat adrenal chromaffin cells. J. Neurosci. 2003, 23, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Cortez, V.; Santana, M.; Marques, A.P.; Mota, A.; Rosmaninho-Salgado, J.; Cavadas, C. Regulation of catecholamine release in human adrenal chromaffin cells by β-adrenoceptors. Neurochem. Int. 2012, 60, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lightly, E.R.; Walker, S.W.; Bird, I.M.; Williams, B.C. Subclassification of beta-adrenoceptors responsible for steroidogenesis in primary cultures of bovine adrenocortical zona fasciculata/reticularis cells. Br. J. Pharmacol. 1990, 99, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart-Bornstein, M.; Bornstein, S.R. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann. N. Y. Acad. Sci. 2008, 1148, 112–117. [Google Scholar] [CrossRef] [PubMed]
- McRae, A.L.; Saladin, M.E.; Brady, K.T.; Upadhyaya, H.; Back, S.E.; Timmerman, M.E. Stress reactivity: Biological and subjective responses to the cold pressor and Trier Social stressors. Hum. Psychopharmacol. 2006, 21, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, M.A.; Cunningham, L.A.; Kleitman, N. The role of adrenal nerves in the regulation of adrenocortical functions. Ann. N. Y. Acad. Sci. 1987, 512, 449–464. [Google Scholar] [CrossRef]
- Charlton, B.G.; McGadey, J.; Russell, D.; Neal, D.E. Noradrenergic innervation of the human adrenal cortex as revealed by dopamine-beta-hydroxylase immunohistochemistry. J. Anat. 1992, 180, 501–506. [Google Scholar]
- Lowrance, S.A.; Ionadi, A.; McKay, E.; Douglas, X.; Johnson, J.D. Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology 2016, 68, 163–170. [Google Scholar] [CrossRef]
- Heym, C. Immunocytochemical correlates of an extrapituitary adrenocortical regulation in man. Histol. Histopathol. 1997, 12, 567–581. [Google Scholar]
- Ehrhart-Bornstein, M.; Hinson, J.P.; Bornstein, S.R.; Scherbaum, W.A.; Vinson, G.P. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr. Rev. 1998, 19, 101–143. [Google Scholar] [CrossRef]
- Scheuer, D.A.; Bechtold, A.G. Glucocorticoids modulate baroreflex control of heart rate in conscious normotensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R475–R483. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, A.G.; Scheuer, D.A. Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1003–R1011. [Google Scholar] [CrossRef] [PubMed]
- Chandran, D.S.; Ali, N.; Jaryal, A.K.; Jyotsna, V.P.; Deepak, K.K. Decreased autonomic modulation of heart rate and altered cardiac sympathovagal balance in patients with Cushing’s syndrome: Role of endogenous hypercortisolism. Neuroendocrinology 2013, 97, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Ueng, J.P.; Chai, C.Y. Effects of asphyxia on arterial blood pressure, formation of nitric oxide in medulla and blood parameters in the cat. Chin. J. Physiol. 2005, 48, 51–56. [Google Scholar] [PubMed]
- Borovsky, V.; Herman, M.; Dunphy, G.; Caplea, A.; Ely, D. CO2 asphyxia increases plasma norepinephrine in rats via sympathetic nerves. Am. J. Physiol. 1998, 274, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Kodikara, S. Attempted suicidal hanging: An uncomplicated recovery. Am. J. Forensic Med. Pathol. 2012, 33, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Rybnikova, E.; Glushchenko, T.; Churilova, A.; Pivina, S.; Samoilov, M. Expression of glucocorticoid and mineralocorticoid receptors in hippocampus of rats exposed to various modes of hypobaric hypoxia: Putative role in hypoxic preconditioning. Brain Res. 2011, 1381, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Chapman, D.F.; Nunez, S.Y.; Zivin, J.A. Dehydroepiandrosterone sulfate is neuroprotective in a reversible spinal cord ischemia model: Possible involvement of GABA(A) receptors. Stroke 2000, 31, 1953–1956. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Araujo, D.M. Preclinical development of neurosteroids as neuroprotective agents for the treatment of neurodegenerative diseases. Int. Rev. Neurobiol. 2001, 46, 379–397. [Google Scholar]
- Juhász-Vedres, G.; Rózsa, E.; Rákos, G.; Dobszay, M.B.; Kis, Z.; Wölfling, J.; Toldi, J.; Párducz, A.; Farkas, T. Dehydroepiandrosterone sulfate is neuroprotective when administered either before or after injury in a focal cortical cold lesion model. Endocrinology 2006, 147, 683–686. [Google Scholar] [CrossRef]
- Lür, G.; Rákos, G.; Juhász-Vedres, G.; Farkas, T.; Kis, Z.; Toldi, J. Effects of dehydroepiandrosterone sulfate on the evoked cortical activity of controls and of brain-injured rats. Cell. Mol. Neurobiol. 2006, 26, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Gąsińska, E.; Bujalska-Zadrożny, M.; Sar, M.; Makulska-Nowak, H. Influence of acute and subchronic oral administration of dehydroepiandrosterone (DHEA) on nociceptive threshold in rats. Pharmacol. Rep. 2012, 64, 965–969. [Google Scholar] [CrossRef]
- Patte-Mensah, C.; Meyer, L.; Kibaly, C.; Mensah-Nyagan, A.G. Regulatory effect of dehydroepiandrosterone on spinal cord nociceptive function. Front. Biosci. 2010, 2, 1528–1537. [Google Scholar] [CrossRef][Green Version]
- Salgado, D.R.; Rocco, J.R.; Silva, E.; Vincent, J.L. Modulation of the renin-angiotensin-aldosterone system in sepsis: A new therapeutic approach? Expert Opin. Ther. Targets 2010, 14, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.B.; Friedman, G.; Viana, M.V.; Tonietto, T.; Saltz, H.; Czepielewski, M.A. Aldosterone secretion in patients with septic shock: A prospective study. Arq. Bras. Endocrinol. Metabol. 2013, 57, 636–641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cumming, A.D.; Driedger, A.A.; McDonald, J.W.; Lindsay, R.M.; Solez, K.; Linton, A.L. Vasoactive hormones in the renal response to systemic sepsis. Am. J. Kidney Dis. 1988, 11, 23–32. [Google Scholar] [CrossRef]
- Hilgenfeldt, U.; Kienapfel, G.; Kellermann, W.; Schott, R.; Schmidt, M. Renin–angiotensin system in sepsis. Clin. Exp. Hypertens. A 1987, 9, 1493–1504. [Google Scholar] [CrossRef]
- Bierens, J.J.; Lunetta, P.; Tipton, M.; Warner, D.S. Physiology of drowning: A review. Physiology (Bethesda) 2016, 31, 147–166. [Google Scholar] [CrossRef]
- Conn, A.W.; Miyasaka, K.; Katayama, M.; Fujita, M.; Orima, H.; Barker, G.; Bohn, D. A canine study of cold water drowning in fresh versus salt water. Crit. Care Med. 1995, 23, 2029–2037. [Google Scholar] [CrossRef]
- Ventura Spagnolo, E.; Stassi, C.; Mondello, C.; Zerbo, S.; Milone, L.; Argo, A. Forensic microbiology applications: A systematic review. Leg. Med. (Tokyo) 2018, 36, 73–80. [Google Scholar] [CrossRef]
- Tsokos, M. Postmortem diagnosis of sepsis. Forensic Sci. Int. 2007, 165, 155–164. [Google Scholar] [CrossRef]
- Ventura Spagnolo, E.; Mondello, C.; Di Mauro, D.; Vermiglio, G.; Asmundo, A.; Filippini, E.; Alibrandi, A.; Rizzo, G. Analysis on sarcoglycans expression as markers of septic cardiomyopathy in sepsis-related death. Int. J. Legal Med. 2018, 132, 1685–1692. [Google Scholar] [CrossRef]
- Yajima, D.; Inokuchi, G.; Makino, Y.; Motomura, A.; Chiba, F.; Torimitsu, S.; Yamaguchi, R.; Hoshioka, Y.; Malakienė, D.; Raudys, R.; et al. Diagnosis of drowning by summation of sodium, potassium, and chloride ion levels in sphenoidal sinus fluid: Differentiating between freshwater and seawater drowning and its application to brackish water and bathtub deaths. Forensic Sci. Int. 2018, 284, 219–225. [Google Scholar] [CrossRef]
- Mondello, C.; Cardia, L.; Ventura-Spagnolo, E. Immunohistochemical detection of early myocardial infarction: A systematic review. Int. J. Leg. Med. 2017, 131, 411–421. [Google Scholar] [CrossRef]
- Mondello, C.; Cardia, L.; Bartoloni, G.; Asmundo, A.; Ventura Spagnolo, E. Immunohistochemical study on dystrophin expression in CAD-related sudden cardiac death: A marker of early myocardial ischaemia. Int. J. Leg. Med. 2018, 132, 1333–1339. [Google Scholar] [CrossRef]
- Schulze, K.; Ebert, L.C.; Ruder, T.D.; Fliss, B.; Poschmann, S.A.; Gascho, D.; Thali, M.J.; Flach, P.M. The gas bubble sign-a reliable indicator of laryngeal fractures in hanging on post-mortem CT. Br. J. Radiol. 2018, 91, 20170479. [Google Scholar] [CrossRef]
| Cortex | Medulla | ||||||
|---|---|---|---|---|---|---|---|
| Case | Age | Sex | Cause of Death | Glomerulosa | Fasciculata | Reticularis | |
| 1 | 53 | F | Hanging | 3 | 1 | 3 | 3 |
| 2 | 44 | F | 3 | 1 | 3 | 3 | |
| 3 | 57 | F | 2 | 1 | 2 | 2 | |
| 4 | 38 | M | 3 | 0 | 2 | 3 | |
| 5 | 66 | M | 3 | 1 | 3 | 2 | |
| 6 | 70 | M | 2 | 0 | 2 | 2 | |
| 7 | 64 | M | 3 | 1 | 3 | 3 | |
| 8 | 19 | M | 2 | 1 | 3 | 2 | |
| 9 | 79 | M | Road accident | 0 | 1 | 2 | 1 |
| 10 | 73 | M | 0 | 1 | 2 | 1 | |
| 11 | 23 | M | 1 | 0 | 3 | 1 | |
| 12 | 30 | F | 0 | 0 | 2 | 0 | |
| 13 | 66 | F | 0 | 1 | 1 | 1 | |
| 14 | 58 | F | 1 | 1 | 2 | 0 | |
| 15 | 19 | M | 1 | 1 | 3 | 1 | |
| 16 | 80 | F | 0 | 0 | 2 | 1 | |
| 17 | 75 | F | 0 | 1 | 3 | 1 | |
| 18 | 60 | F | 0 | 1 | 2 | 1 | |
| 19 | 19 | M | Fire fatality | 0 | 2 | 3 | 0 |
| 20 | 52 | M | 0 | 2 | 3 | 0 | |
| 21 | 74 | M | 1 | 2 | 2 | 1 | |
| 22 | 40 | F | 0 | 1 | 3 | 0 | |
| 23 | 33 | F | Sudden death | 1 | 3 | 0 | 1 |
| 24 | 27 | M | 1 | 3 | 0 | 1 | |
| 25 | 61 | M | 0 | 2 | 0 | 0 | |
| 26 | 63 | F | 1 | 2 | 1 | 1 | |
| 27 | 68 | M | 0 | 3 | 0 | 0 | |
| 28 | 56 | M | 1 | 2 | 0 | 1 | |
| 29 | 41 | M | 1 | 3 | 0 | 0 | |
| 30 | 73 | M | 1 | 3 | 0 | 1 | |
| 31 | 67 | F | Sepsis | 2 | 0 | 1 | 0 |
| 32 | 82 | F | 2 | 0 | 1 | 0 | |
| 33 | 62 | M | 1 | 0 | 1 | 0 | |
| 34 | 72 | F | 3 | 0 | 1 | 1 | |
| 35 | 74 | M | 2 | 0 | 0 | 0 | |
| 36 | 54 | F | 3 | 1 | 2 | 1 | |
| 37 | 76 | F | 2 | 0 | 1 | 0 | |
| 38 | 52 | F | 2 | 1 | 1 | 0 | |
| 39 | 18 | M | Drowning | 3 | 1 | 1 | 2 |
| 40 | 21 | M | 2 | 0 | 0 | 1 | |
| 41 | 18 | M | 3 | 0 | 1 | 2 | |
| 42 | 23 | F | 2 | 1 | 1 | 1 | |
| 43 | 58 | M | 3 | 0 | 1 | 2 | |
| 44 | 53 | F | 2 | 1 | 0 | 2 | |
| 45 | 18 | F | 3 | 0 | 1 | 1 | |
| 46 | 22 | M | 2 | 1 | 0 | 2 | |
| 47 | 18 | M | 2 | 0 | 0 | 1 | |
| 48 | 36 | M | 3 | 1 | 0 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura Spagnolo, E.; Mondello, C.; Cardia, L.; Minutoli, L.; Puzzolo, D.; Asmundo, A.; Macaione, V.; Alibrandi, A.; Malta, C.; Baldino, G.; et al. Post-Mortem Immunohistochemical Evidence of β2-Adrenergic Receptor Expression in the Adrenal Gland. Int. J. Mol. Sci. 2019, 20, 3065. https://doi.org/10.3390/ijms20123065
Ventura Spagnolo E, Mondello C, Cardia L, Minutoli L, Puzzolo D, Asmundo A, Macaione V, Alibrandi A, Malta C, Baldino G, et al. Post-Mortem Immunohistochemical Evidence of β2-Adrenergic Receptor Expression in the Adrenal Gland. International Journal of Molecular Sciences. 2019; 20(12):3065. https://doi.org/10.3390/ijms20123065
Chicago/Turabian StyleVentura Spagnolo, Elvira, Cristina Mondello, Luigi Cardia, Letteria Minutoli, Domenico Puzzolo, Alessio Asmundo, Vincenzo Macaione, Angela Alibrandi, Consuelo Malta, Gennaro Baldino, and et al. 2019. "Post-Mortem Immunohistochemical Evidence of β2-Adrenergic Receptor Expression in the Adrenal Gland" International Journal of Molecular Sciences 20, no. 12: 3065. https://doi.org/10.3390/ijms20123065
APA StyleVentura Spagnolo, E., Mondello, C., Cardia, L., Minutoli, L., Puzzolo, D., Asmundo, A., Macaione, V., Alibrandi, A., Malta, C., Baldino, G., & Micali, A. (2019). Post-Mortem Immunohistochemical Evidence of β2-Adrenergic Receptor Expression in the Adrenal Gland. International Journal of Molecular Sciences, 20(12), 3065. https://doi.org/10.3390/ijms20123065





