Disruption of the Metal Ion Environment by EDTA for Silk Formation Affects the Mechanical Properties of Silkworm Silk
Abstract
1. Introduction
2. Results and Discussion
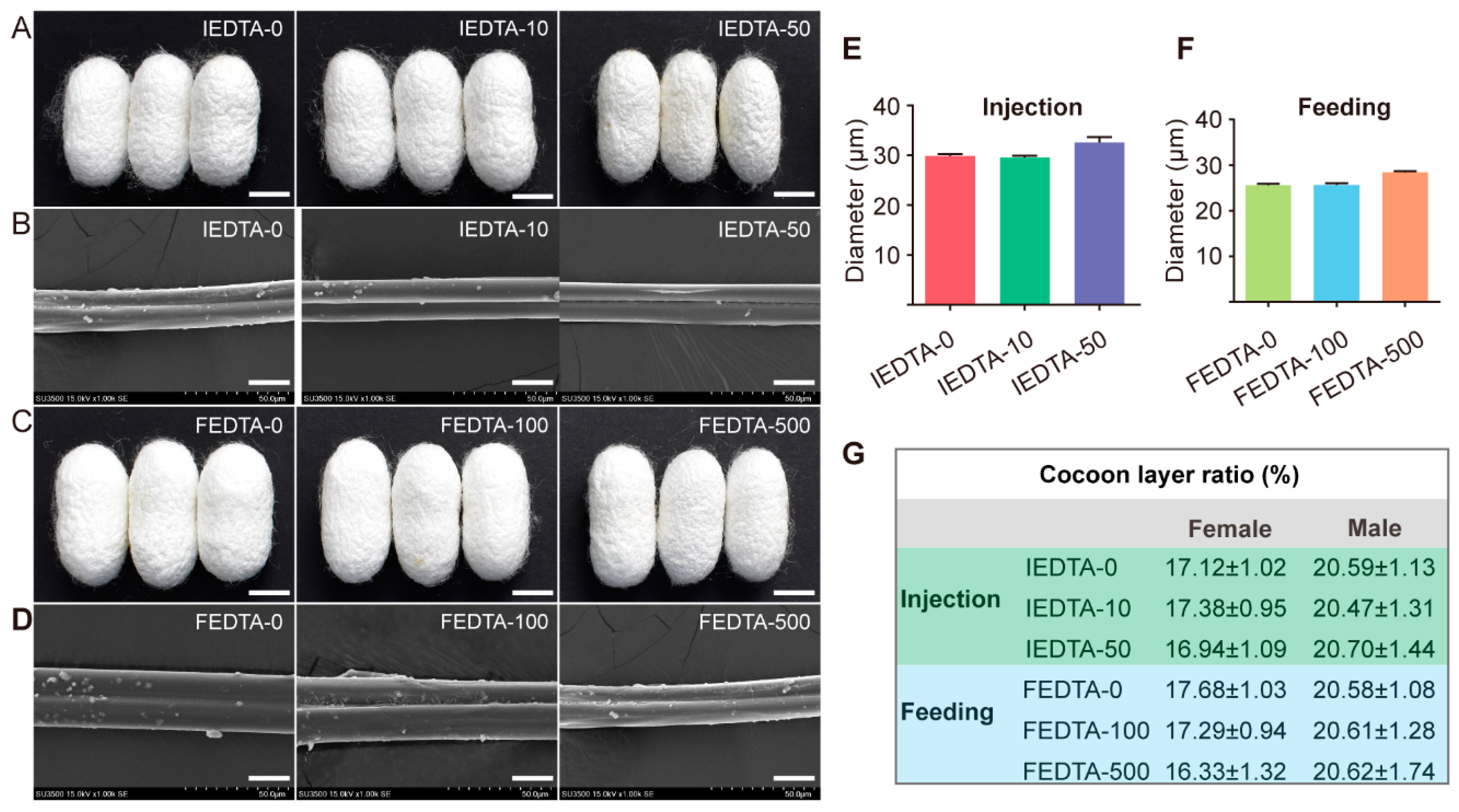
2.1. Observation of Morphological Characteristics
2.2. EDTA Disturbed the Ionic Environment for Silk Fiber Formation
2.3. Secondary Structure Analysis
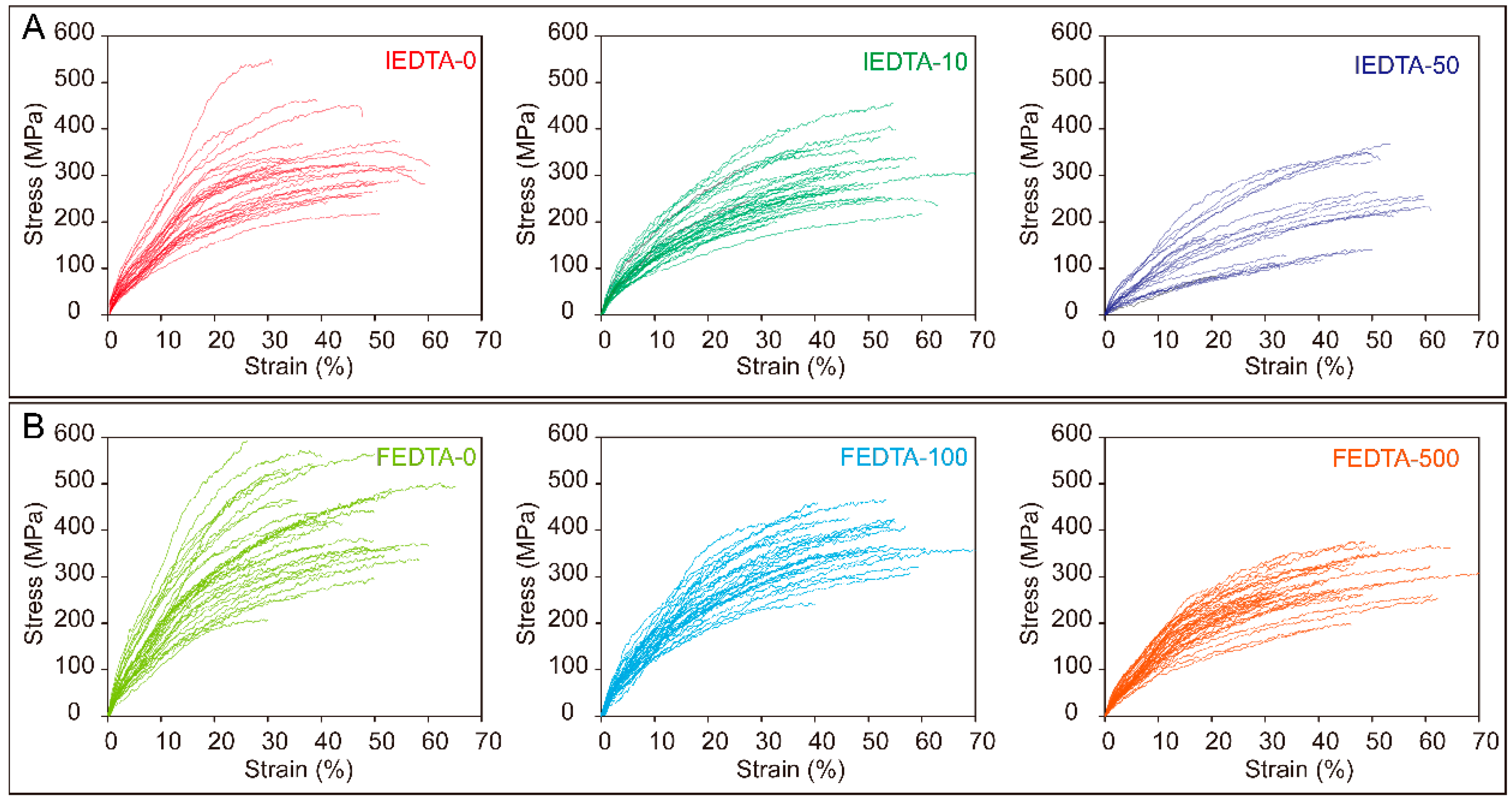
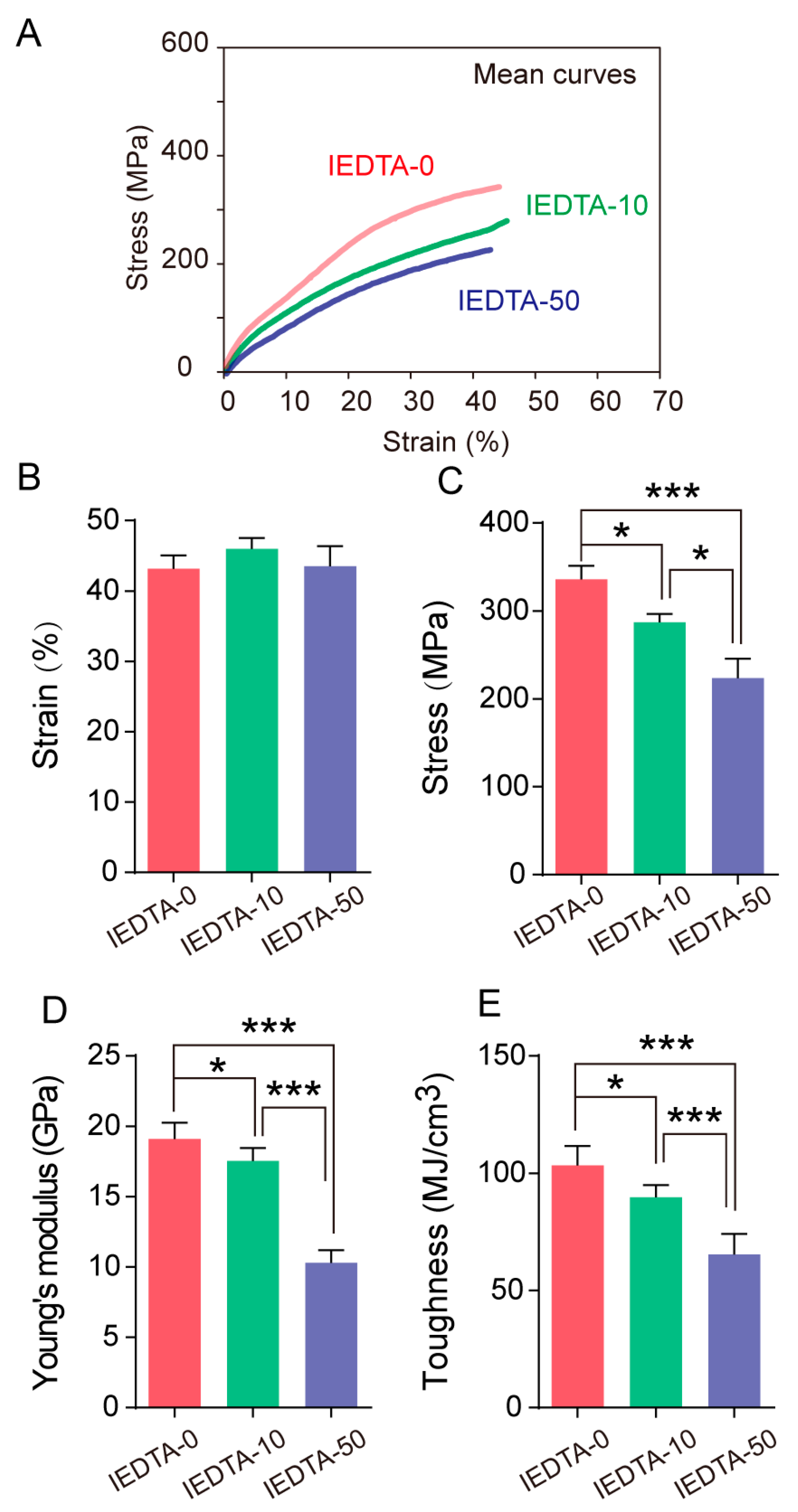
2.4. Mechanical Performance of Silk Fiber
2.5. Current Advances in the Effect of Metal Ions on the Structure and Performance of Silk In Vitro and In Vivo
3. Materials and Methods
3.1. Silkworms
3.2. Experimental Design
3.2.1. Injecting EDTA to Silkworms (IEDTA)
3.2.2. Feeding EDTA to Silkworms (FEDTA)
3.3. Elemental Analysis
3.4. FTIR Spectroscopy of a Single Silk Fiber
3.5. Mechanical Testing of Silk Fiber
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EDTA | Ethylenediaminetetraacetic acid |
| EDTA·2Na·2H2O | Ethylenediaminetetraacetic acid disodium salt |
| FTIR | Fourier Transform Infrared Spectroscopy |
References
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins Struct. Funct. Genet. 2001, 44, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Omenetto, F.G.; Kaplan, D.L. New opportunities for an ancient material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Kikuchi, Y.; Kojima, K.; Tamura, T.; Kuwabara, N.; Nakamura, T.; Asakura, T. Mechanical properties of regenerated Bombyx mori silk fibers and recombinant silk fibers produced by transgenic silkworms. J. Biomater. Sci. Polym. Ed. 2010, 21, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Teule, F.; Miao, Y.G.; Sohn, B.H.; Kim, Y.S.; Hull, J.J.; Fraser, M.J., Jr.; Lewis, R.V.; Jarvis, D.L. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Natl. Acad. Sci. USA 2012, 109, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Lan, X.; Zhang, Y.; Zhao, T.; Wang, Y.; Kajiura, Z.; Nakagaki, M. Transgenic silkworms (Bombyx mori) produce recombinant spider dragline silk in cocoons. Mol. Biol. Rep. 2010, 37, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, Y.; Sezutsu, H.; Nakajima, K.; Tamada, Y.; Kojima, K. High-toughness silk produced by a transgenic silkworm expressing spider (Araneus ventricosus) dragline silk protein. PLoS ONE 2014, 9, e105325. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Li, L.L.; Zhang, M.Y.; Liu, S.L.; Jiang, L.H.; Shen, Q. Directly obtaining high strength silk fiber from silkworm by feeding carbon nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Koh, L.D.; Li, D.; Ji, B.; Zhang, Y.; Yeo, J.; Guan, G.; Han, M.Y.; Zhang, Y.W. Peptide–Graphene Interactions Enhance the Mechanical Properties of Silk Fibroin. ACS Appl. Mater. Interfaces 2015, 7, 21787–21796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.; Zhang, M.; Jian, M.; Zhang, Y. Feeding Single-Walled Carbon Nanotubes or Graphene to Silkworms for Reinforced Silk Fibers. Nano Lett. 2016, 16, 6695–6700. [Google Scholar] [CrossRef]
- Wu, G.H.; Song, P.; Zhang, D.Y.; Liu, Z.Y.; Li, L.; Huang, H.M.; Zhao, H.P.; Wang, N.N.; Zhu, Y.Q. Robust composite silk fibers pulled out of silkworms directly fed with nanoparticles. Int. J. Biol. Macromol. 2017, 104, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.N.; Stewart, R.J. Aquatic caddisworm silk is solidified by environmental metal ions during the natural fiber-spinning process. FASEB J. 2019, 33, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Rajkhowa, R.; Naik, R.; Wang, L.J.; Smith, S.V.; Wang, X.G. An Investigation Into Transition Metal Ion Binding Properties of Silk Fibers and Particles Using Radioisotopes. J. Appl. Polym. Sci. 2011, 119, 3630–3639. [Google Scholar] [CrossRef]
- Zong, X.H.; Zhou, P.; Shao, Z.Z.; Chen, S.M.; Chen, X.; Hu, B.W.; Deng, F.; Yao, W.H. Effect of pH and copper(II) on the conformation transitions of silk fibroin based on EPR, NMR, and Raman spectroscopy. Biochemistry 2004, 43, 11932–11941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Shao, Z.; Zhou, P.; Knight, D.P.; Vollrath, F. Copper in the silk formation process of Bombyx mori silkworm. FEBS Lett. 2003, 554, 337–341. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Shao, Z.; Huang, Y.; Knight, D.P. Effect of metallic ions on silk formation in the Mulberry silkworm, Bombyx mori. J. Phys. Chem. B 2005, 109, 16937–16945. [Google Scholar] [CrossRef]
- Liu, Q.S.; Wang, X.; Tan, X.Y.; Xie, X.Q.; Li, Y.; Zhao, P.; Xia, Q.Y. A strategy for improving the mechanical properties of silk fiber by directly injection of ferric ions into silkworm. Mater. Des. 2018, 146, 134–141. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, Q.; Chen, Q.; Xia, Q.; Zhao, P. In vivo effects of metal ions on conformation and mechanical performance of silkworm silks. Biochim. Biophys. Acta 2017, 1861, 567–576. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Xie, K.; Yi, Q.; Chen, Q.; Wang, X.; Shen, H.; Xia, Q.; Zhao, P. Ca2+ and endoplasmic reticulum Ca2+-ATPase regulate the formation of silk fibers with favorable mechanical properties. J. Insect Physiol. 2015, 73, 53–59. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, P.; Li, Y.; Yi, Q.; Ma, S.; Xie, K.; Chen, H.; Xia, Q. Modifying the Mechanical Properties of Silk Fiber by Genetically Disrupting the Ionic Environment for Silk Formation. Biomacromolecules 2015, 16, 3119–3125. [Google Scholar] [CrossRef]
- Salama, P.; Berk, D. Photocatalytic oxidation of Ni-EDTA in a well-mixed reactor. Ind. Eng. Chem. Res. 2005, 44, 7071–7077. [Google Scholar] [CrossRef]
- Pitter, P.; Sykora, V. Biodegradability of ethylenediamine-based complexing agents and related compounds. Chemosphere 2001, 44, 823–826. [Google Scholar] [CrossRef]
- Lin, Q.; Pan, H.; Yao, K.; Pan, Y.; Long, W. Competitive removal of Cu-EDTA and Ni-EDTA via microwave-enhanced Fenton oxidation with hydroxide precipitation. Water Sci. Technol. 2015, 72, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Wang, S.W. Electrolytic recovery of binary metals and EDTA from strong complexed solutions. Water Res. 2000, 34, 3179–3185. [Google Scholar] [CrossRef]
- Miller, L.M.; Dumas, P. Chemical imaging of biological tissue with synchrotron infrared light. BBA Biomembr. 2006, 1758, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Q. Synchrotron IR microspectroscopy for protein structure analysis: Potential and questions. Spectrosc. Int. J. 2006, 20, 229–251. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, J.; Jordan, J.S.; Wang, X.; Henning, R.W.; Yarger, J.L. Structural Comparison of Various Silkworm Silks: An Insight into the Structure-Property Relationship. Biomacromolecules 2018, 19, 906–917. [Google Scholar] [CrossRef]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Li, S.; Feng, Q.L. Advances in Silkworm Studies Accelerated by the Genome Sequencing of Bombyx mori. Annu Rev. Entomol 2014, 59, 513–536. [Google Scholar] [CrossRef]
- Karlin, S.; Zhu, Z.Y. Classification of mononuclear zinc metal sites in protein structures. Proc. Natl. Acad. Sci. USA 1997, 94, 14231–14236. [Google Scholar] [CrossRef]
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The Mechanical Role of Metal Ions in Biogenic Protein-Based Materials. Angew. Chem. Int. Ed. 2014, 53, 12026–12044. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Cavallaro, G. Minimal Functional Sites Allow a Classification of Zinc Sites in Proteins. PLoS ONE 2011, 6, e26325. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.M.; Nowicki, M.W.; Walkinshaw, M.D. Metals in protein structures: A review of their principal features. Crystallogr. Rev. 2010, 16, 247–302. [Google Scholar] [CrossRef]
- Politi, Y.; Priewasser, M.; Pippel, E.; Zaslansky, P.; Hartmann, J.; Siegel, S.; Li, C.; Barth, F.G.; Fratzl, P. A Spider’s Fang: How to Design an Injection Needle Using Chitin-Based Composite Material. Adv. Funct. Mater. 2012, 22, 2519–2528. [Google Scholar] [CrossRef]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and functional aspects of metal sites in biology. Chem. Rev. 1996, 96, 2239–2314. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Deng, Y.-B.; Zhou, P. Folding process of silk fibroin induced by ferric and ferrous ions. J. Mol. Struct. 2009, 938, 305–310. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, X.; Knight, D.P.; Zong, X.H.; Deng, F.; Yao, W.H. Effects of pH and calcium ions on the conformational transitions in silk fibroin using 2D Raman correlation spectroscopy and 13C solid-state NMR. Biochemistry 2004, 43, 11302–11311. [Google Scholar] [CrossRef]
- Ruan, Q.X.; Zhou, P.; Hu, B.W.; Ji, D. An investigation into the effect of potassium ions on the folding of silk fibroin studied by generalized two-dimensional NMR-NMR correlation and Raman spectroscopy. FEBS J. 2008, 275, 219–232. [Google Scholar] [CrossRef]
- Xu, L.H.; Tu, S.D.; Chen, C.H.; Zhao, J.; Zhang, Y.; Zhou, P. Effect of EGCG On Fe(III)-Induced Conformational Transition of Silk Fibroin, a Model of Protein Related to Neurodegenerative Diseases. Biopolymers 2016, 105, 100–107. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jiang, T.; Zheng, Y.W.; Zhou, P. Interference of EGCG on the Zn(II)-induced conformational transition of silk fibroin as a model protein related to neurodegenerative diseases. Soft Matt. 2012, 8, 5543–5549. [Google Scholar] [CrossRef]
- Deng, Y.B.; Ji, D.; Sun, P.C.; Knight, D.; Cai, J.H.; Zhou, P. The Role of Mn(II) in Silk Fibroin Based on EPR and NMR Spectroscopy. Spectrosc. Lett. 2011, 44, 176–185. [Google Scholar] [CrossRef]
- Chang, H.; Cheng, T.; Wu, Y.; Hu, W.; Long, R.; Liu, C.; Zhao, P.; Xia, Q. Transcriptomic Analysis of the Anterior Silk Gland in the Domestic Silkworm (Bombyx mori)—Insight into the Mechanism of Silk Formation and Spinning. PLoS ONE 2015, 10, e0139424. [Google Scholar] [CrossRef] [PubMed]
- Nichol, H.; Law, J.H.; Winzerling, J.J. Iron metabolism in insects. Ann. Rev. Entomol. 2002, 47, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.K. Calcium—How and why? J. Biosci. 2001, 26, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.M. The geometry of metal-ligand interactions relevant to proteins. II. Angles at the metal atom, additional weak metal-donor interactions. Acta Crystallogr. D 2000, 56, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Tainer, J.A.; Roberts, V.A.; Getzoff, E.D. Metal-Binding Sites in Proteins. Curr. Opin. Biotechnol. 1991, 2, 582–591. [Google Scholar] [CrossRef]


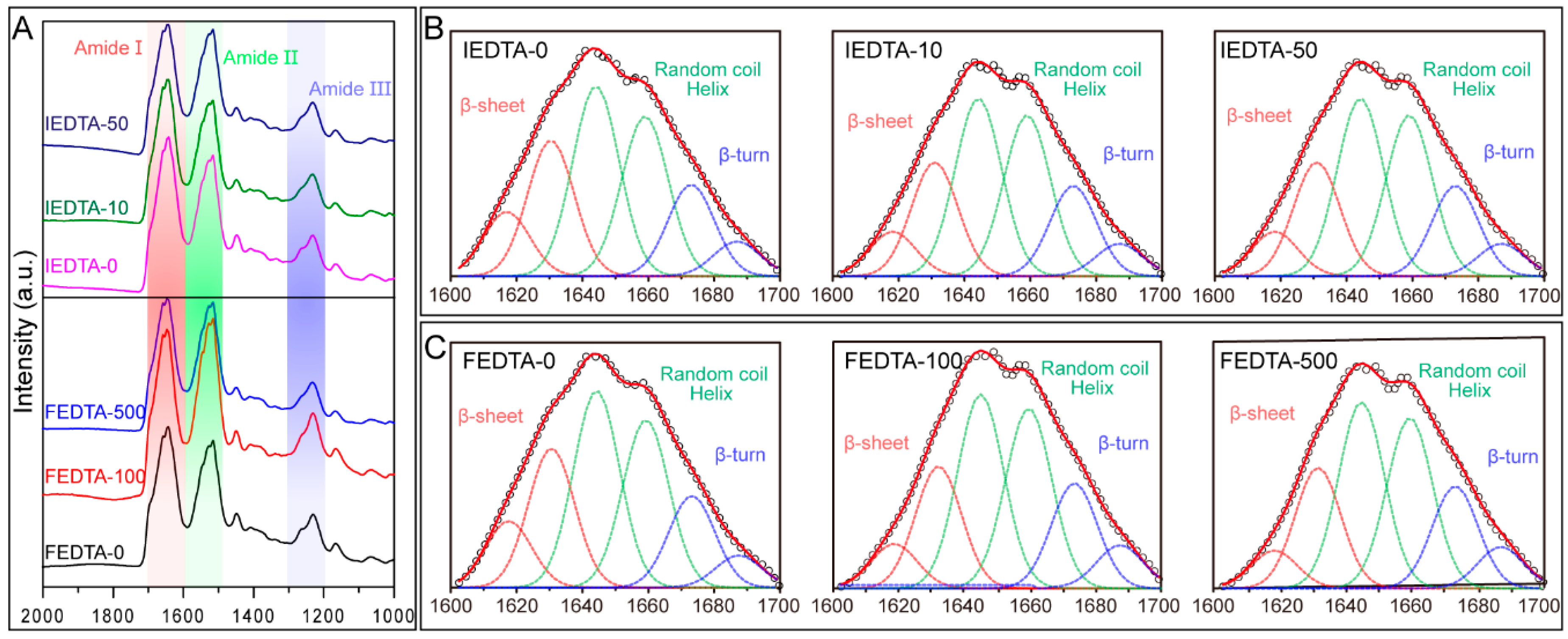

| Secondary Structures (%) | Injecting EDTA | Feeding EDTA | ||||
|---|---|---|---|---|---|---|
| 0 mM | 10 mM | 50 mM | 0 mM | 100 mM | 500 mM | |
| β-sheet | 32.29 ± 1.41 | 26.85 ± 1.60 | 28.27 ± 1.7 | 28.22 ± 3.0 | 26.4 ± 1.5 | 26.37 ± 2.8 |
| Random coil and Helix | 53.1 ± 2.58 | 53.25 ± 1.0 | 53.25 ± 1.2 | 56.8 ± 5.0 | 53 ± 0.6 | 54 ± 1.1 |
| β-turn | 13.56 ± 0.80 | 20.06 ± 2.2 | 18.6 ± 1.0 | 14.93 ± 3.0 | 20.4 ± 1.0 | 19.4 ± 2.0 |
| Metal Ions | In Vitro | In Vivo |
|---|---|---|
| Secondary Structure | Mechanical Performance | |
| Ca2+ | α-helical intermediate conformation [37] | Injection of CaCl2: strain increased 30.9% [18] |
| Forming a stable protein network [15,18] | Transgenic silkworm: strength/strain increased 21.1%/73.1% [19] | |
| Na+ | Break down the protein network [15] | Transgenic silkworm: the average tenacity, Young’s modulus, and toughness of the transgenic silks decreased significantly [19] |
| K+ | Decrease β-sheet like conformation and break down the protein network [15,38] | Injection of KCl: strength increased 71.4% [17] |
| Cu2+ | β-sheet increased [13,15,17] | Injection of CuCl2: strength increased 51.9% [17] |
| Fe2+ | Less influence [36] | Not reported |
| Fe3+ | β-sheet increased [16,36,39] | Injection of FeCl3: strength/toughness increased 13%/29% [16] |
| Mg2+ | β-sheet increased [15] | Not reported |
| Zn2+ | β-sheet increased [15,40] | Not reported |
| Mn2+ | Stabilizing the Silk I state [41] | Not reported |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wang, X.; Tan, X.; Xie, X.; Dong, H.; Li, X.; Li, Y.; Zhao, P.; Xia, Q. Disruption of the Metal Ion Environment by EDTA for Silk Formation Affects the Mechanical Properties of Silkworm Silk. Int. J. Mol. Sci. 2019, 20, 3026. https://doi.org/10.3390/ijms20123026
Liu Q, Wang X, Tan X, Xie X, Dong H, Li X, Li Y, Zhao P, Xia Q. Disruption of the Metal Ion Environment by EDTA for Silk Formation Affects the Mechanical Properties of Silkworm Silk. International Journal of Molecular Sciences. 2019; 20(12):3026. https://doi.org/10.3390/ijms20123026
Chicago/Turabian StyleLiu, Qingsong, Xin Wang, Xiaoyin Tan, Xiaoqian Xie, Haonan Dong, Xinning Li, Yi Li, Ping Zhao, and Qingyou Xia. 2019. "Disruption of the Metal Ion Environment by EDTA for Silk Formation Affects the Mechanical Properties of Silkworm Silk" International Journal of Molecular Sciences 20, no. 12: 3026. https://doi.org/10.3390/ijms20123026
APA StyleLiu, Q., Wang, X., Tan, X., Xie, X., Dong, H., Li, X., Li, Y., Zhao, P., & Xia, Q. (2019). Disruption of the Metal Ion Environment by EDTA for Silk Formation Affects the Mechanical Properties of Silkworm Silk. International Journal of Molecular Sciences, 20(12), 3026. https://doi.org/10.3390/ijms20123026





