Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice, Ethics, and Sample Collection
2.2. DSS and SERM Treatments
2.3. Quantification of Histological Changes in Colon Tissue
2.4. Immunohistochemistry to Determine the Number of Mrc-1+ Monocytes in Colon
2.5. Gene Expression Analysis
2.6. Circulating Cytokine Analysis
2.7. Nuclear Receptor Affinity Assays
2.8. Statistical Analysis
3. Results
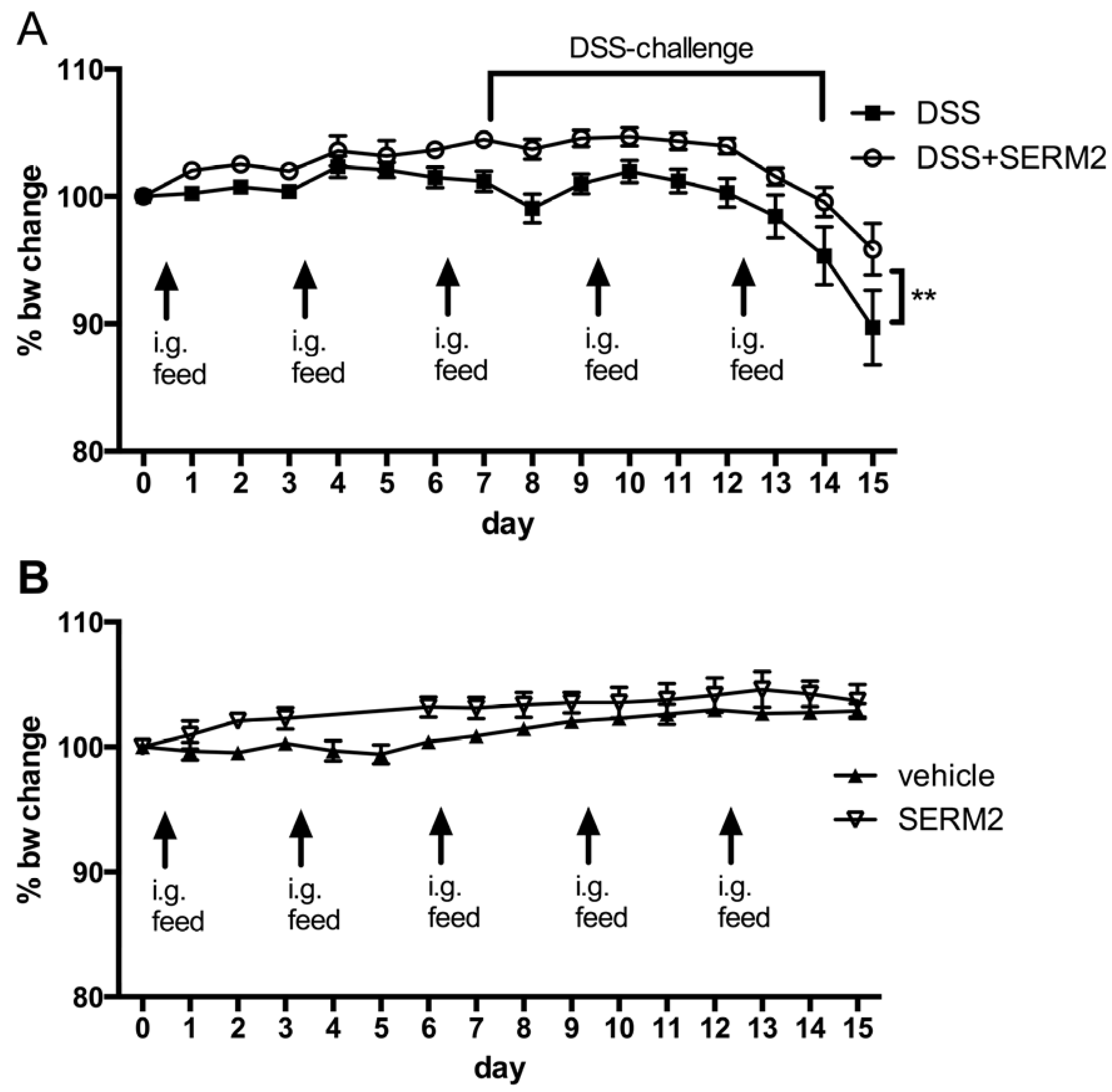
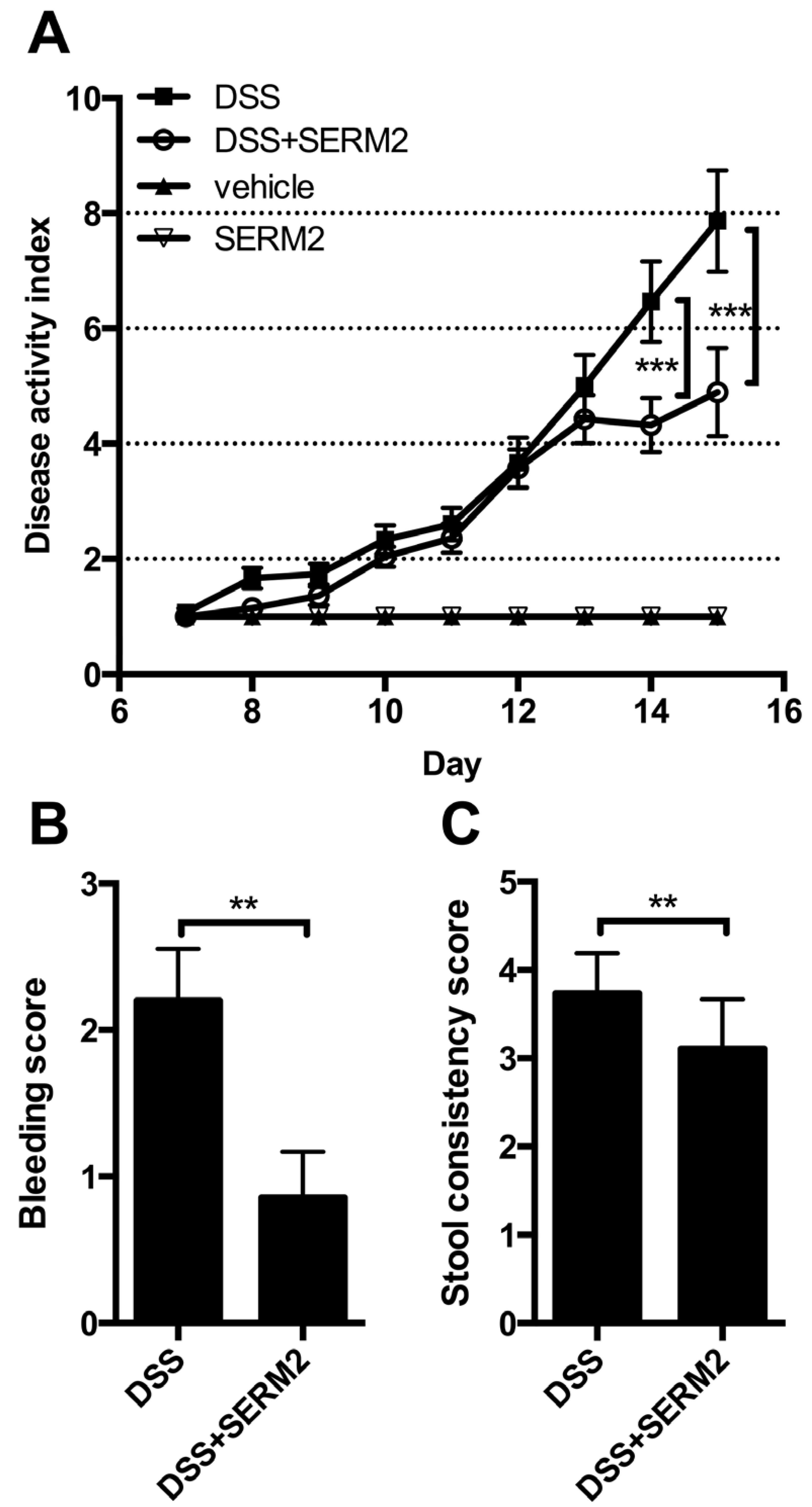
3.1. Pharmacological Doses of SERM2 Decrease the Severity of DSS-Induced Colitis and Sustain the Recovery
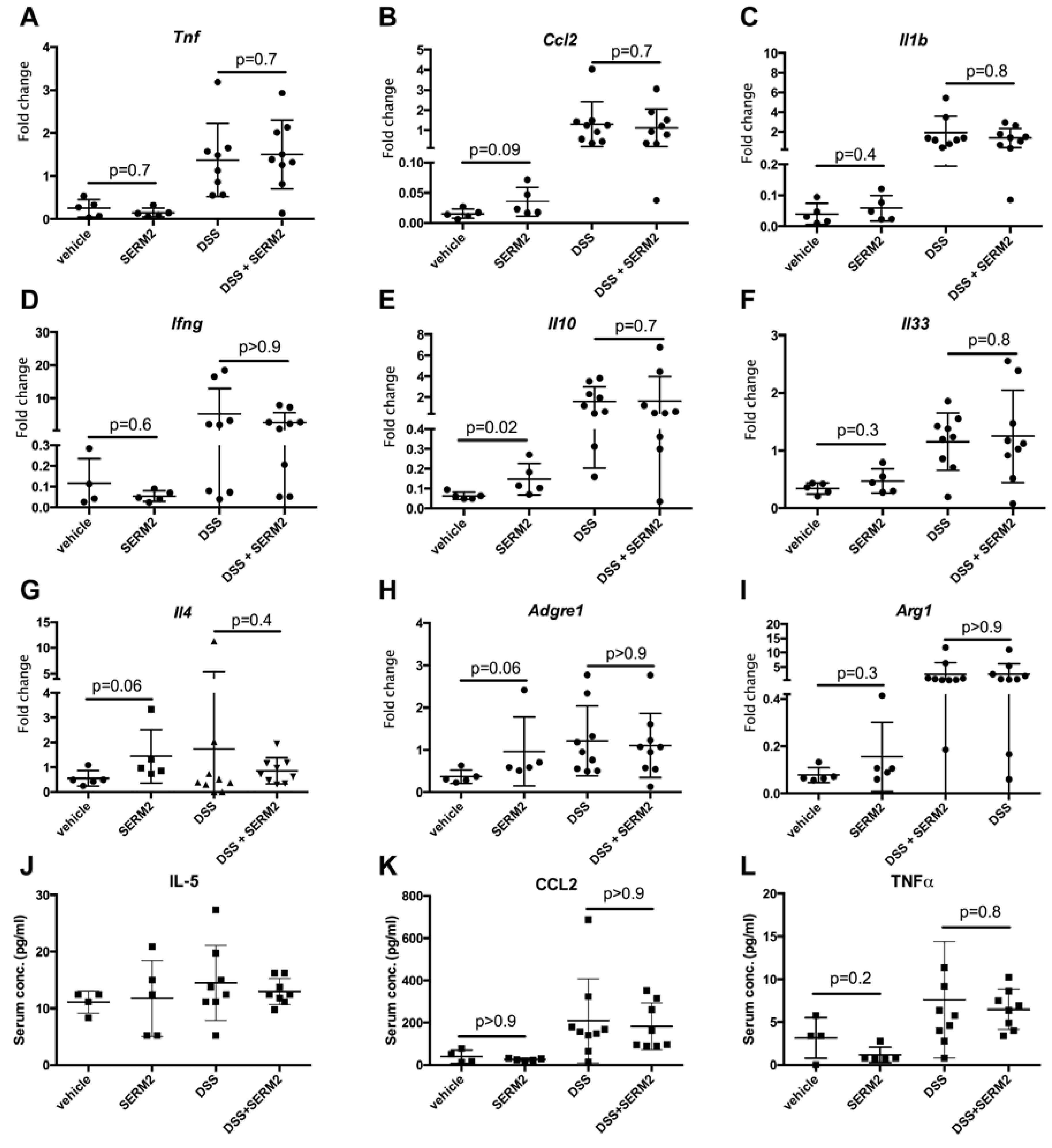
3.2. SERM2 Stimulates the Expression of Anti-Inflammatory Cytokines in the Colon, but Does Not Affect Th1-Type Mediators.
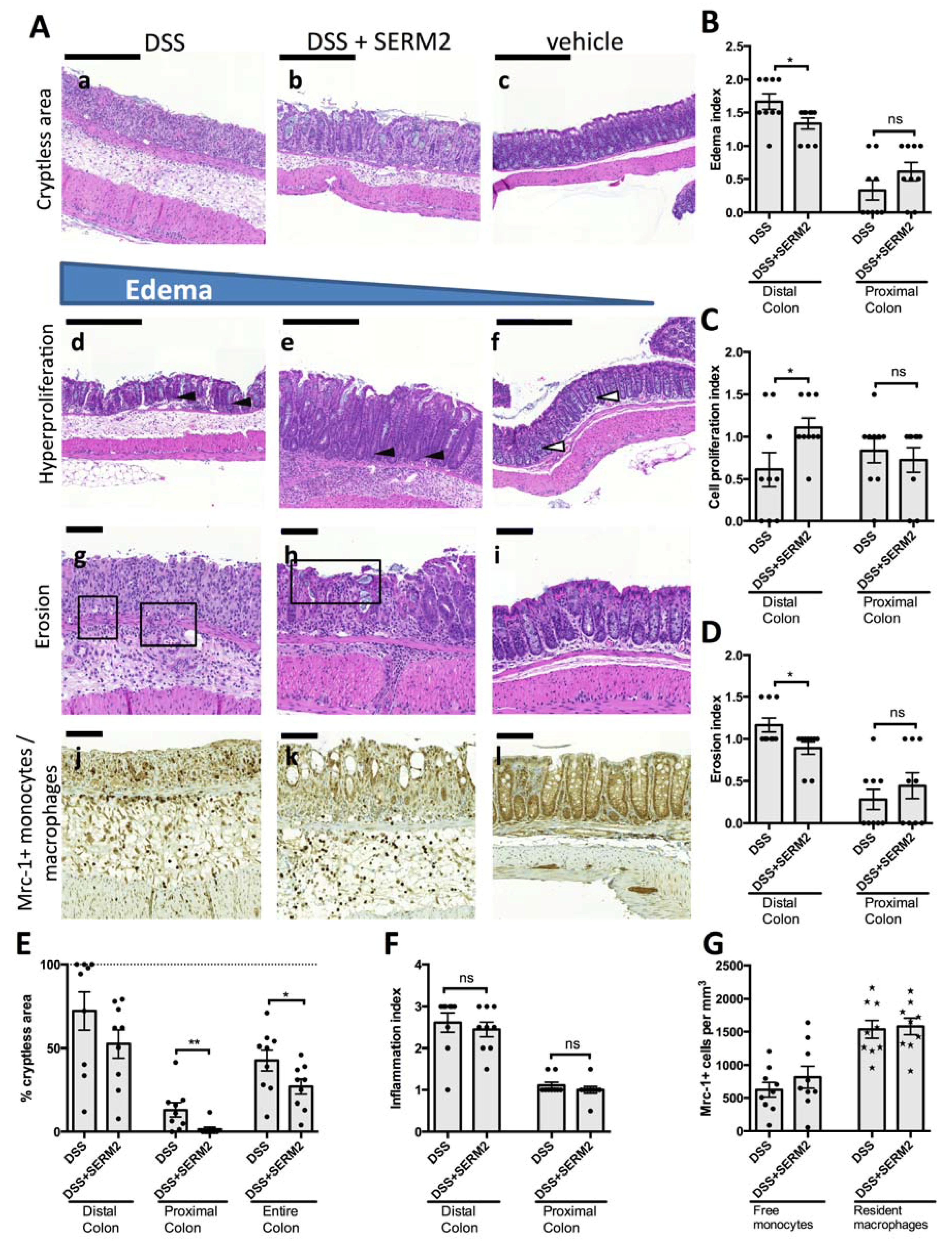
3.3. SERM2 Ameliorates Colitis by Preventing Tissue Damages and Inducing Mrc-1+ Monocyte Migration
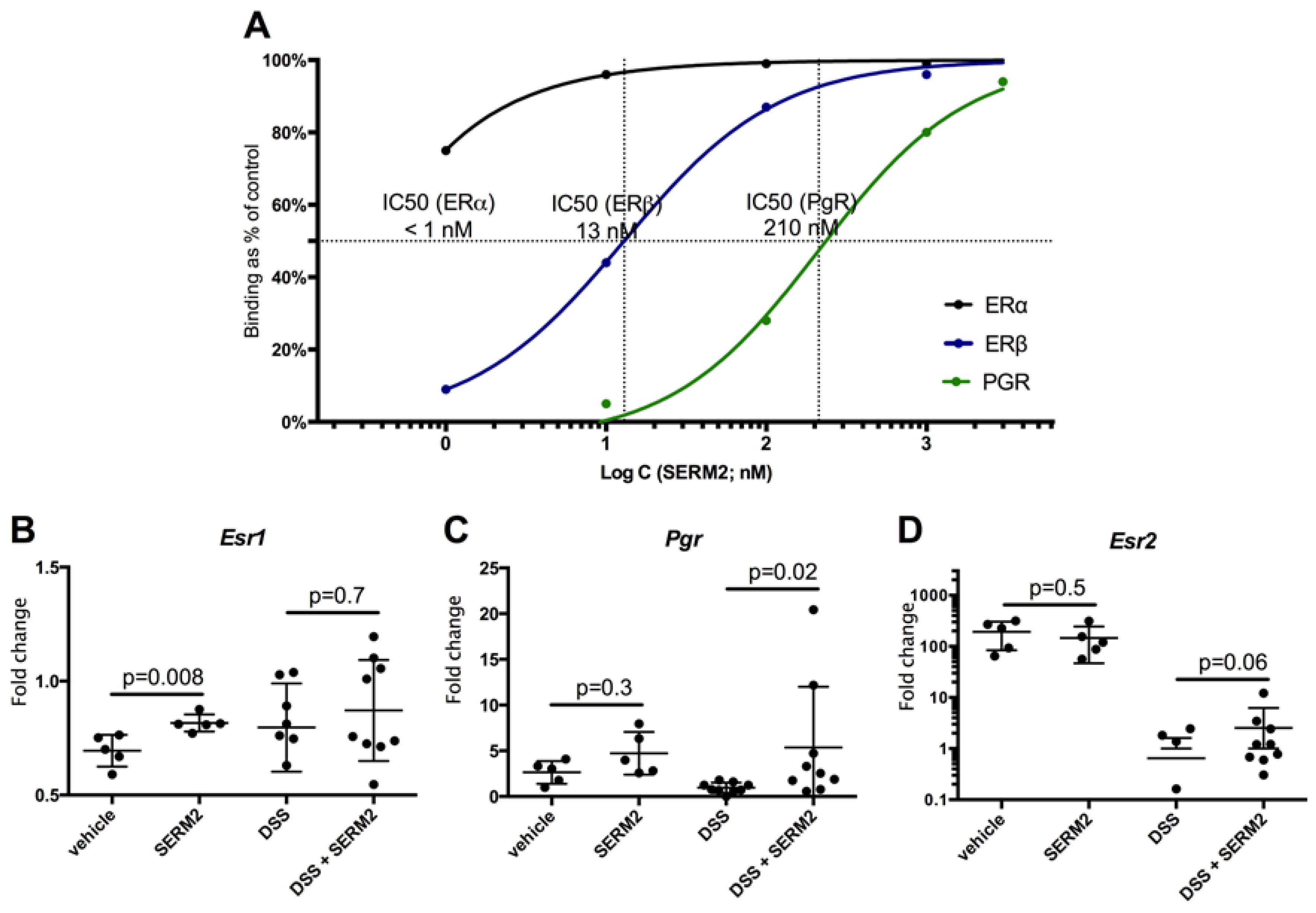
3.4. SERM2 Binds with ERα, ERβ, and PGR In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Androgen receptor |
| CCL | C-C chemokine ligand |
| CD | Crohn’s disease |
| DAI | disease activity index |
| DSS | dextran sodium sulfate |
| ER | estrogen receptor |
| GPER | G protein coupled estrogen receptor |
| GR | glucocorticoid receptor |
| IBD | inflammatory bowel disease |
| Mrc-1 | mannose receptor-1 |
| PGR | progesterone receptor |
| SERM | selective estrogen receptor modulator |
| UC | ulcerative colitis |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Liu, T.-C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Kühl, A.A.; Erben, U.; Kredel, L.I.; Siegmund, B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front. Immunol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nagao-kitamoto, H.; Kitamoto, S.; Kuffa, P.; Kamada, N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest. Res. 2016, 14, 127–138. [Google Scholar] [CrossRef]
- Bain, C.C.; Mowat, A.M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 2014, 260, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.; Shao, B.Z.; Xu, Z.Q.; Chen, X.W.; Liu, C. Intestinal autophagy and its pharmacological control in inflammatory bowel disease. Front. Immunol. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Salame, T.-M.; Jung, S. Guardians of the gut—Murine intestinal macrophages and dendritic cells. Front. Immunol. 2015, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Ip, E.E.K.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; Balschun, T.; Karlsen, T.H.; Sventoraityte, J.; Nikolaus, S.; Mayr, G.; Domingues, F.S.; Albrecht, M.; Nothnagel, M.; Ellinghaus, D.; et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat. Genet. 2008, 40, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, E.; Bernshtein, B.; Friedlander, G.; Walker, C.R.; Yona, S.; Kim, K.W.; Brenner, O.; Krauthgamer, R.; Varol, C.; Müller, W.; et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 2014, 40, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T.L. IL-10 modulates DSS-induced colitis through a macrophage–ROS–NO axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Iwakiri, R.; Amemori, S.; Yamaguchi, K.; Fujise, T.; Otani, H.; Shimoda, R.; Tsunada, S.; Sakata, H.; Ikeda, Y.; et al. Comparison of the efficacy of granulocyte and monocyte/macrophage adsorptive apheresis and leukocytapheresis in active ulcerative colitis patients: A prospective randomized study. Eur. J. Gastroenterol. Hepatol. 2008, 20, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Iwakiri, R.; Ikeda, Y.; Kishi, T.; Tsuruoka, N.; Shimoda, R. Factors affecting short- and long-term effects of leukocyte removal therapy in active ulcerative colitis. J. Gastroenterol. Hepatol. 2013, 28, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P.; Hanauer, S.B. Ulcerative colitis. BMJ 2013, 346, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Härkönen, P.L.; Väänänen, H.K. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann. N. Y. Acad. Sci. 2006, 1089, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Waliszewski, P.; Blaszczyk, M.; Wolinska-Witort, E.; Drews, M.; Snochowski, M.; Hurst, R.E. Molecular study of sex steroid receptor gene expression in human colon and in colorectal carcinomas. J. Surg. Oncol. 1997, 64, 3–11. [Google Scholar] [CrossRef]
- Phiel, K.L.; Henderson, R.A.; Adelman, S.J.; Elloso, M.M. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol. Lett. 2005, 97, 107–113. [Google Scholar] [CrossRef]
- Pierdominici, M.; Maselli, A.; Varano, B.; Barbati, C.; Spada, C.; Zullo, A.; Lorenzetti, R.; Rosati, M.; Rainaldi, G.; Limiti, M.R.; et al. Linking estrogen receptor β expression with inflammatory bowel disease activity. Oncotarget 2015, 6, 40443–40451. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Straub, R.H. Estrogen metabolism and autoimmunity. Autoimmun. Rev. 2012, 11, A460–A464. [Google Scholar] [CrossRef]
- Pozzi, S.; Benedusi, V.; Maggi, A.; Vegeto, E. Estrogen action in neuroprotection and brain inflammation. Ann. N. Y. Acad. Sci. 2006, 1089, 302–323. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Wang, H.; Wu, T.C.J.; Sicotte, N.L.; Nakamura, K.; Kurth, F.; Itoh, N.; Bardens, J.; Bernard, J.T.; Corboy, J.R.; et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2015, 15, 35–46. [Google Scholar] [CrossRef]
- Bábíčková, J.; Tóthová, Ľ.; Lengyelová, E.; Bartoňová, A.; Hodosy, J.; Gardlík, R.; Celec, P. Sex Differences in Experimentally Induced Colitis in Mice: A Role for Estrogens. Inflammation 2015, 38, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.C.; Hillhouse, A.E.; Myles, M.H.; Lubahn, D.B.; Bryda, E.C.; Davis, J.W.; Franklin, C.L. The Role of Estrogen Signaling in a Mouse Model of Inflammatory Bowel Disease: A Helicobacter Hepaticus Model. PLoS ONE 2014, 9, e94209. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.F.; Deng, Y.; Bercik, P.; Collins, S.M. Modulatory effects of estrogen in two murine models of experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G27–G36. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xu, W.; Chen, Y.; Qiu, W.; Shu, Y.; Wu, A.; Dai, Y.; Bao, J.; Lu, Z.; Hu, X. Raloxifene suppresses experimental autoimmune encephalomyelitis and NF-kB-dependent CCL20 expression in reactive astrocytes. PLoS ONE 2014, 9, e94320. [Google Scholar]
- Perez, B.; Henriquez, C.; Sarmiento, J.; Morales, N.; Folch, H.; Galesio, J.S.; Uberti, B.; Morán, G. Tamoxifen as a new therapeutic tool for neutrophilic lung inflammation. Respirology 2016, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Taha, S.; Jasim, A.; Elghany, S.A.; Sultan, A.; AlKhateeb, A.; Othman, M.; Jun, F.; Taurin, S.; Bakhiet, M. Styrene maleic acid encapsulated raloxifene micelles for management of inflammatory bowel disease. Clin. Transl. Med. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Polari, L.; Wiklund, A.; Sousa, S.; Kangas, L.; Linnanen, T.; Härkönen, P.; Määttä, J. SERMs Promote Anti-Inflammatory Signaling and Phenotype of CD14+ Cells. Inflammation 2018, 41, 1157–1171. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Rana, A.; Ahlawat, A.; Reddy, K.; Puneet, V.B. Animal models of inflammatory bowel disease: A review. Inflammopharmacology 2014, 22, 219–233. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, M.; Yamada, S.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Asghar, M.N.; Silvander, J.S.G.; Helenius, T.O.; Lähdeniemi, I.A.K.; Alam, C.; Fortelius, L.E.; Holmsten, R.O.; Toivola, D.M. The Amount of Keratins Matters for Stress Protection of the Colonic Epithelium. PLoS ONE 2015, 10, e0127436. [Google Scholar] [CrossRef]
- Ten Hove, T.; Drillenburg, P.; Wijnholds, J.; Te Velde, A.A.; Van Deventer, S.J.H. Differential susceptibility of multidrug resistance protein-1Deficient Mice to DSS and TNBS-Induced Colitis. Dig. Dis. Sci. 2002, 47, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Langen, M.L.; Hotte, N.; Dieleman, L.A.; Albert, E.; Mulder, C.; Madsen, K.L.; Kl, M. Estrogen receptor-B signaling modulates epithelial barrier function. Am. J. Physiol. 2011, 300, G621–G626. [Google Scholar]
- Linares, P.M.; Algaba, A.; Urzainqui, A.; Guijarro-rojas, M.; Gonzalez-Tajuelo, R.; Garrido, J.; Chaparro, M.; Gispert, J.P.; Bermejo, F.; Guerra, I.; et al. Ratio of Circulating Estrogen Receptors Beta and Alpha (ERb/ERa) Indicates Endoscopic Activity in Patients with Crohn’s Disease. Dig. Dis. Sci. 2017, 62, 2744–2754. [Google Scholar] [CrossRef]
- Lügering, N.L.; Kucharzik, T.; Stein, H.; Günther, W.; Lügering, A.; Hasilik, A.; Domschke, W.; Reinhard, S. IL-10 Synergizes with IL-4 and IL-13 in Inhibiting Lysosomal Enzyme Secretion by Human Monocytes and Lamina Propria Mononuclear Cells from Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 1998, 43, 706–714. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F. IL-10 and Macrophages Orchestrate Gut Homeostasis. Immunity 2014, 40, 637–639. [Google Scholar] [CrossRef]
- Suuronen, T.; Nuutinen, T.; Huuskonen, J.; Ojala, J.; Thornell, A.; Salminen, A. Anti-inflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflamm. Res. 2005, 54, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.E.; Santos-Galindo, M.; Garcia-Segura, L.M. Selective estrogen receptor modulators regulate reactive microglia after penetrating brain injury. Front. Aging Neurosci. 2014, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ma, X. Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-kB pathway after spinal cord injury in rats. Neurol. Sci. 2014, 35, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Aggelakopoulou, M.; Kourepini, E.; Paschalidis, N.; Panoutsakopoulou, V. ERβ in CD4 + T Cells Is Crucial for Ligand-Mediated Suppression of Central Nervous System Autoimmunity. J. Immunol. 2016, 196, 4947–4956. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.A.; Havran, H.L.; Quereshy, H.A.; Kuang, S.; De Salvo, C.; Pizarro, T.T. Estrogen Receptor α Loss-of-Function Protects Female Mice From DSS-Induced Experimental Colitis. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 630–633.e1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Ngo, D.; Jones, M.; Simpson, E.; Fritzemeier, K.H.; Morand, E.F. Endogenous estrogen regulation of inflammatory arthritis and cytokine expression in male mice, predominantly via estrogen receptor α. Arthritis Rheum. 2010, 62, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Sobolewska-włodarczyk, A.; Cygankiewicz, A.I.; Jacenik, D. G protein-coupled receptor 30 (GPR30) expression pattern in inflammatory bowel disease patients suggests its key role in the inflammatory process. A preliminary study. J. Gastrointest. Liver Dis. 2017, 26, 29–35. [Google Scholar]
- Jacenik, D.; Cygankiewicz, A.I.; Fichna, J.; Mokrowiecka, A.; Małecka-Panas, E.; Krajewska, W.M. Estrogen signaling deregulation related with local immune response modulation in irritable bowel syndrome. Mol. Cell. Endocrinol. 2018, 471, 89–96. [Google Scholar] [CrossRef]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone receptor modulates ERa action in breast cancer. Nature 2015, 523, 313–317. [Google Scholar] [CrossRef]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef]
- Ellmann, S.; Sticht, H.; Thiel, F.; Beckmann, M.W.; Strick, R.; Strissel, P.L. Estrogen and progesterone receptors: From molecular structures to clinical targets. Cell. Mol. Life Sci. 2009, 66, 2405–2426. [Google Scholar] [CrossRef] [PubMed]
- Wiegratz, I.; Kuhl, H. Progestogen therapies: Differences in clinical effects? Trends Endocrinol. Metab. 2004, 15, 277–285. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polari, L.; Anttila, S.; Helenius, T.; Wiklund, A.; Linnanen, T.; Toivola, D.M.; Määttä, J. Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis. Int. J. Mol. Sci. 2019, 20, 3007. https://doi.org/10.3390/ijms20123007
Polari L, Anttila S, Helenius T, Wiklund A, Linnanen T, Toivola DM, Määttä J. Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis. International Journal of Molecular Sciences. 2019; 20(12):3007. https://doi.org/10.3390/ijms20123007
Chicago/Turabian StylePolari, Lauri, Santeri Anttila, Terhi Helenius, Anu Wiklund, Tero Linnanen, Diana M. Toivola, and Jorma Määttä. 2019. "Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis" International Journal of Molecular Sciences 20, no. 12: 3007. https://doi.org/10.3390/ijms20123007
APA StylePolari, L., Anttila, S., Helenius, T., Wiklund, A., Linnanen, T., Toivola, D. M., & Määttä, J. (2019). Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis. International Journal of Molecular Sciences, 20(12), 3007. https://doi.org/10.3390/ijms20123007





