Differentially Expressed Mitochondrial Proteins in Human MCF7 Breast Cancer Cells Resistant to Paclitaxel
Abstract
1. Introduction
2. Results
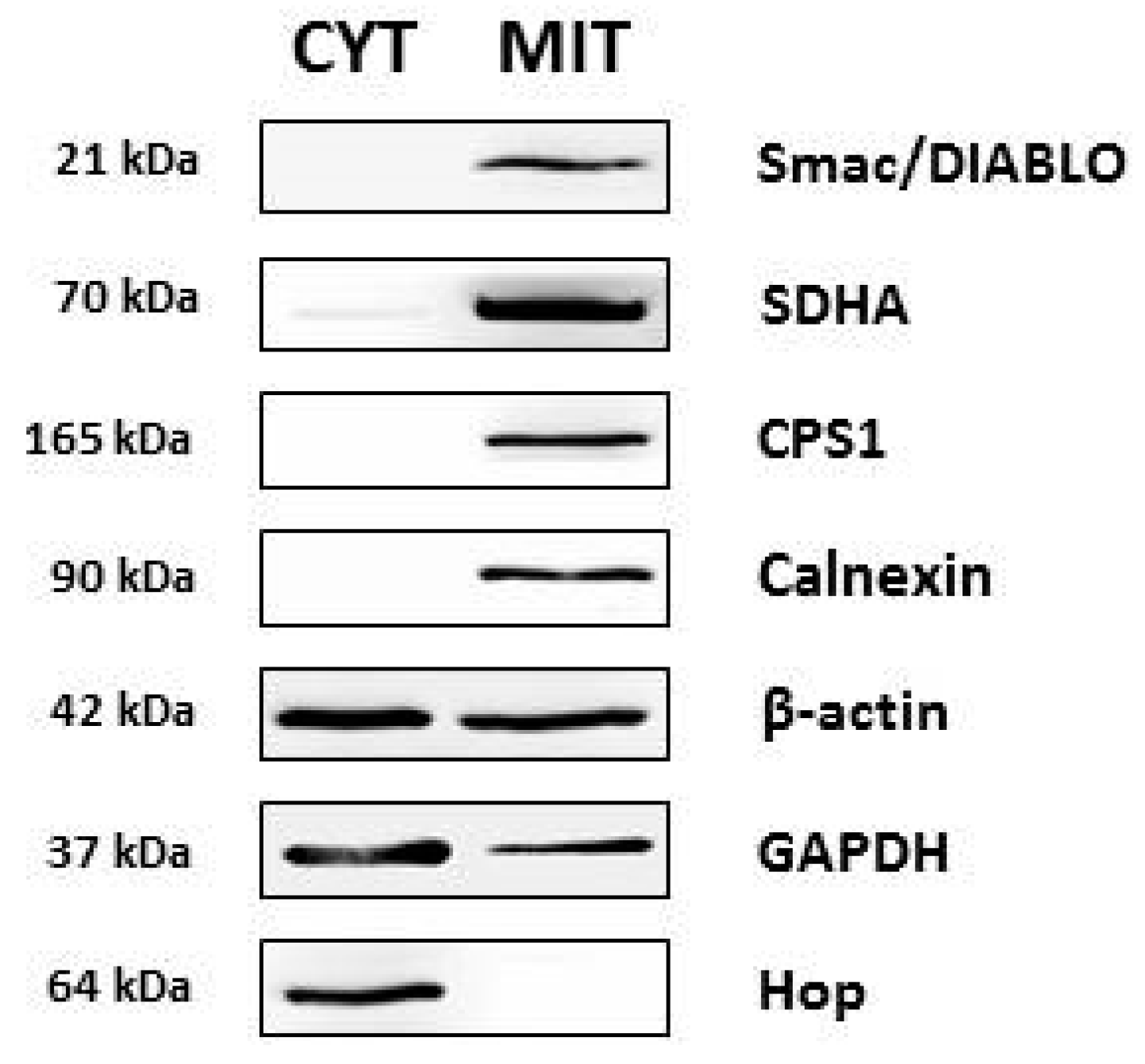
2.1. Isolation of Mitochondrial Fraction
2.2. Two-Dimensional Electrophoresis of Mitochondrial Fraction
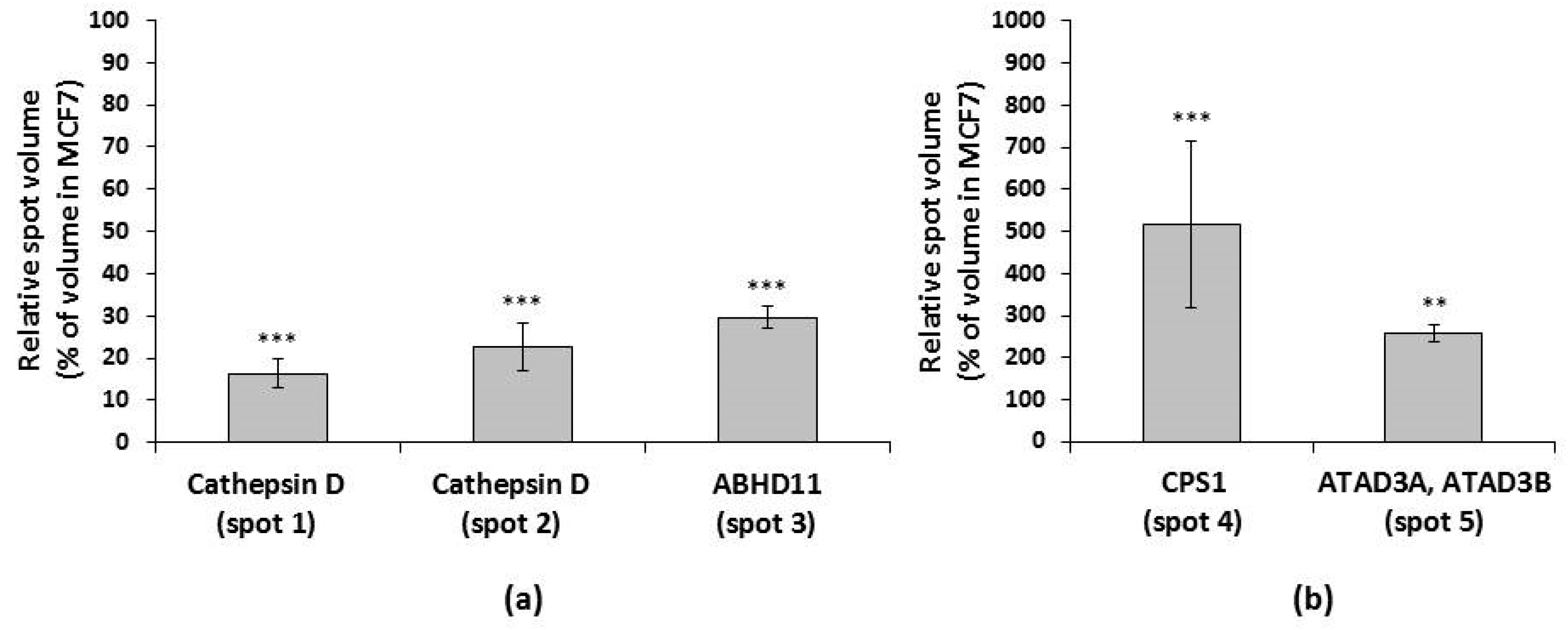
2.3. Spot Analysis and Protein Identification
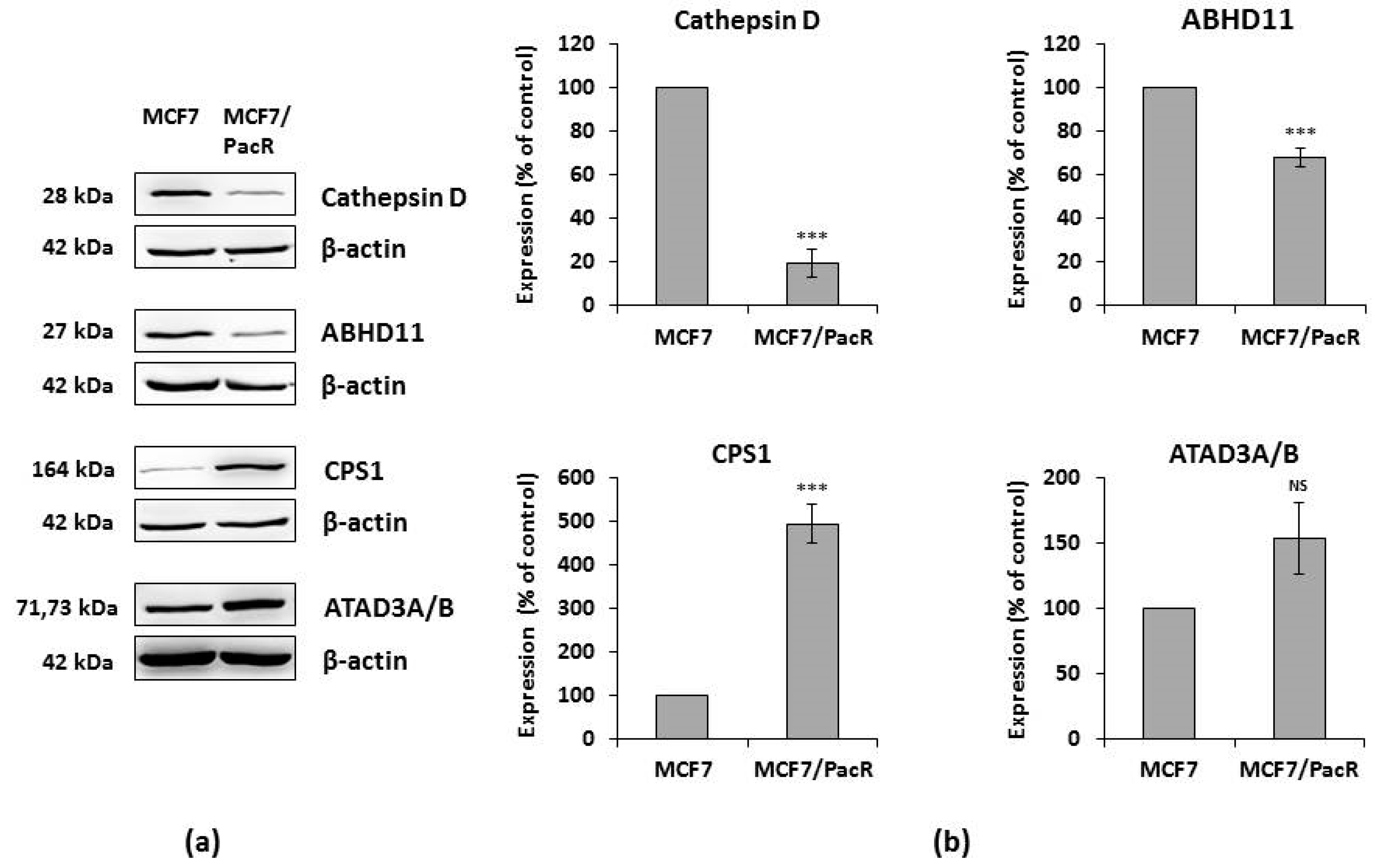
2.4. Western Blot of Identified Proteins
2.5. Distribution of CPS1 within Cells
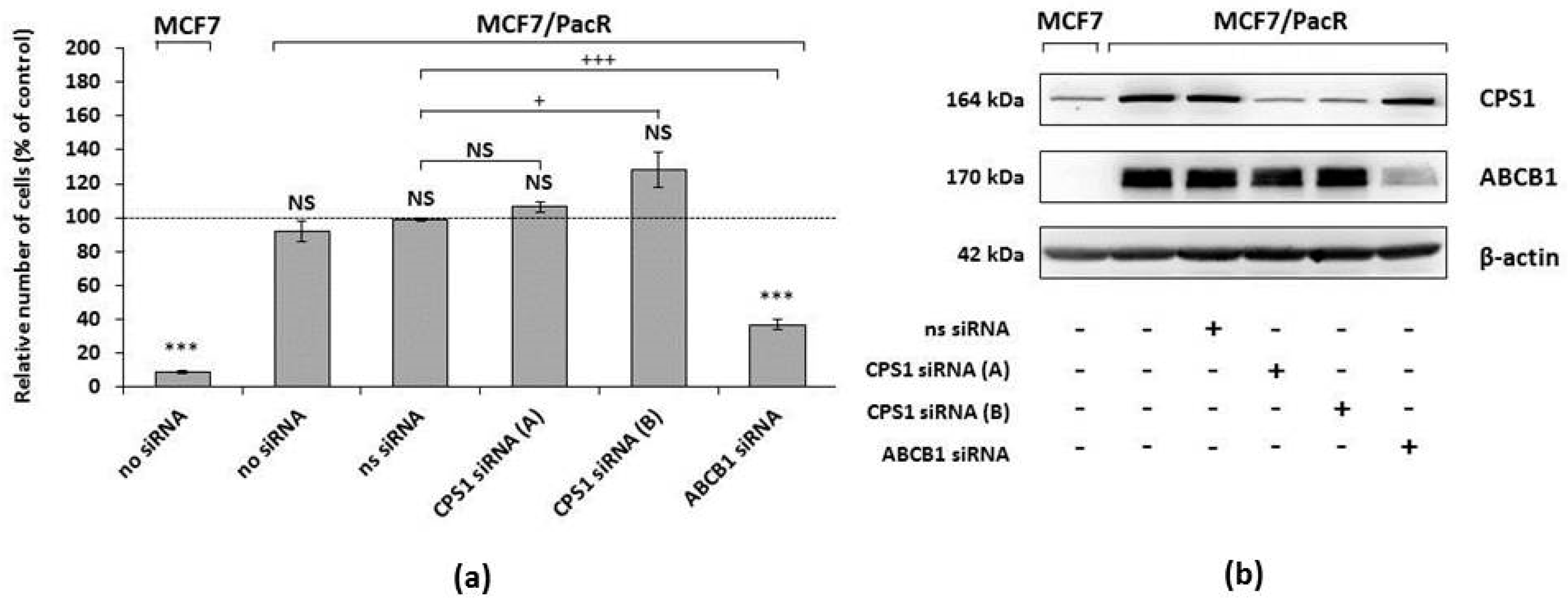
2.6. Effect of CPS1 Silencing on Resistance to Paclitaxel
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Preparation of Mitochondria-Enriched Fraction
4.4. Two-Dimensional Electrophoresis
4.5. Analysis of Two-Dimensional Electrophoresis Gels
4.6. Mass Spectrometry
4.7. Whole-Cell Lysate Preparation
4.8. Western Blot Analysis
4.9. Confocal Microscopy
4.10. FACS Analysis
4.11. siRNA Silencing and Its Effect on Resistance to Paclitaxel
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-PAGE | Two-dimensional polyacrylamide gel electrophoresis |
| ABCB1 | ATP-binding cassette transporter member B1 |
| ABCB4 | ATP-binding cassette transporter member B4 |
| ABCC2 | ATP-binding cassette transporter member C2 |
| ABCC3 | ATP-binding cassette transporter member C3 |
| ABHD11 | Alpha/beta hydrolase domain-containing protein 11 |
| ATAD3A | ATPase family AAA domain-containing protein 3A |
| ATAD3B | ATPase family AAA domain-containing protein 3B |
| CPS1 | Carbamoyl phosphate synthetase 1 |
| COX IV | Cytochrome c oxidase subunit IV |
| Diablo | Direct inhibitor of apoptosis-binding protein with low pI |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HER2 | Human epidermal growth factor receptor 2 |
| Hop | Hsp70-Hsp90 organizing protein |
| HSP27 | Heat-shock protein 27 |
| MALDI-TOF | Matrix-assisted laser desorption/ionization-time-of-flight |
| MAP2 | Microtubule-associated protein 2 |
| MCF7 | Michigan Cancer Foundation 7 |
| SDHA | Succinate dehydrogenase complex flavoprotein, subunit alpha |
| STK11 | Serine/threonine kinase 11 |
| Smac | Second mitochondria-derived activator of caspase |
| WBSCR21 | Williams–Beuren syndrome chromosomal region 21 protein |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Theriault, R.L.; Carlson, R.W.; Allred, C.; Anderson, B.O.; Burstein, H.J.; Edge, S.B.; Farrar, W.B.; Forero, A.; Giordano, S.H.; Goldstein, L.J.; et al. National comprehensive cancer network. Breast cancer, version 3.2013: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2013, 11, 753–760. [Google Scholar] [CrossRef]
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Panel members. Tailoring therapies—Improving the management of early breast cancer: St Gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Mustacchi, G.; De Laurentiis, M. The role of taxanes in triple-negative breast cancer: Literature review. Drug Des. Dev. Ther. 2015, 9, 4303–4318. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Manfredi, J.J.; Parness, J.; Horwitz, S.B. Taxol binds to cellular microtubules. J. Cell Biol. 1982, 94, 688–696. [Google Scholar] [CrossRef]
- Snyder, J.P.; Nettles, J.H.; Cornett, B.; Downing, K.H.; Nogales, E. The binding conformation of Taxol in beta-tubulin: A model based on electron crystallographic density. Proc. Natl. Acad. Sci. USA 2001, 98, 5312–5316. [Google Scholar] [CrossRef]
- Xiao, H.; Verdier-Pinard, P.; Fernandez-Fuentes, N.; Burd, B.; Angeletti, R.; Fiser, A.; Horwitz, S.B.; Orr, G.A. Insights into mechanism of microtubule stabilization by Taxol. Proc. Natl. Acad. Sci. USA 2006, 103, 10166–10173. [Google Scholar] [CrossRef]
- Woods, C.M.; Zhu, J.; McQueney, P.A.; Bollag, D.; Lazarides, E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol. Med. 1995, 1, 506–526. [Google Scholar] [CrossRef]
- Gascoigne, K.E.; Taylor, S.S. How do anti-mitotic drugs kill cancer cells? J. Cell Sci. 2009, 122, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Ajabnoor, G.M.; Crook, T.; Coley, H.M. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death Dis. 2012, 3, e260. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Hu, S.S.; Dong, Q.; Cai, J.X.; Zhang, W.P.; Sun, J.Y.; Wang, T.T.; Xie, J.J.; He, H.R.; Xing, J.F.; et al. Establishment of paclitaxel-resistant breast cancer cell line and nude mice models, and underlying multidrug resistance mechanisms in vitro and in vivo. Asian Pac. J. Cancer Prev. 2013, 14, 6135–6140. [Google Scholar] [CrossRef] [PubMed]
- Němcová-Fürstová, V.; Kopperová, D.; Balušíková, K.; Ehrlichová, M.; Brynychová, V.; Václavíková, R.; Daniel, P.; Souček, P.; Kovář, J. Characterization of acquired paclitaxel resistance of breast cancer cells and involvement of ABC transporters. Toxicol. Appl. Pharmacol. 2016, 310, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Boichuk, S.; Galembikova, A.; Sitenkov, A.; Khusnutdinov, R.; Dunaev, P.; Valeeva, E.; Usolova, N. Establishment and characterization of a triple negative basal-like breast cancer cell line with multi-drug resistance. Oncol. Lett. 2017, 14, 5039–5045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, J.; Zhang, W.; Liu, G.; Yin, D.; Li, J.; Zhang, S.; Li, H. Establishment of paclitaxel-resistant cell line and the underlying mechanism on drug resistance. Int. J. Gynecol. Cancer 2012, 22, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Duran, G.E.; Wang, Y.C.; Moisan, F.; Francisco, E.B.; Sikic, B.I. Decreased levels of baseline and drug-induced tubulin polymerisation are hallmarks of resistance to taxanes in ovarian cancer cells and are associated with epithelial-to-mesenchymal transition. Br. J. Cancer 2017, 116, 1318–1328. [Google Scholar] [CrossRef]
- Gonçalves, A.; Braguer, D.; Kamath, K.; Martello, L.; Briand, C.; Horwitz, S.; Wilson, L.; Jordan, M.A. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc. Natl. Acad. Sci. USA 2001, 98, 11737–11742. [Google Scholar] [CrossRef]
- Sobue, S.; Mizutani, N.; Aoyama, Y.; Kawamoto, Y.; Suzuki, M.; Nozawa, Y.; Ichihara, M.; Murate, T. Mechanism of paclitaxel resistance in a human prostate cancer cell line, PC3-PR, and its sensitization by cabazitaxel. Biochem. Biophys. Res. Commun. 2016, 479, 808–813. [Google Scholar] [CrossRef]
- Takeda, M.; Mizokami, A.; Mamiya, K.; Li, Y.Q.; Zhang, J.; Keller, E.T.; Namiki, M. The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines. Prostate 2007, 67, 955–967. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, S.C.; Luo, D.; Xie, Y. Study of multi-drug resistant mechanisms in taxol-resistant hepatocellular carcinoma QGY-TR 50 cell line. Biochem. Biophys. Res. Commun. 2001, 280, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Orr, G.A.; Verdier-Pinard, P.; McDaid, H.; Horwitz, S.B. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003, 22, 7280–7295. [Google Scholar] [CrossRef] [PubMed]
- Martínéz, C.; García-Martín, E.; Pizarro, R.M.; García-Gamito, F.J.; Agúndez, J.A.G. Expression of paclitaxel-inactivating CYP3A activity in human colorectal cancer: Implications for drug therapy. Br. J. Cancer 2002, 87, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Smoter, M.; Bodnar, L.; Duchnowska, R.; Stec, R.; Grala, B.; Szczylik, C. The role of Tau protein in resistance to paclitaxel. Cancer Chemother. Pharmacol. 2011, 68, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; Briasoulis, E.; Linardou, H.; Bafaloukos, D.; Papadimitriou, C. Taxane resistance in breast cancer: Mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 2012, 38, 890–903. [Google Scholar] [CrossRef]
- Gao, B.; Russell, A.; Beesley, J.; Chen, X.Q.; Healey, S.; Henderson, M.; Wong, M.; Emmanuel, C.; Galletta, L.; Johnatty, S.E.; et al. Paclitaxel sensitivity in relation to ABCB1 expression, efflux and single nucleotide polymorphism in ovarian cancer. Sci. Rep. 2014, 4, 4669. [Google Scholar] [CrossRef] [PubMed]
- Jelínek, M.; Balušíková, K.; Daniel, P.; Němcová-Fürstová, V.; Kirubakaran, P.; Jaček, M.; Wei, L.; Wang, X.; Vondrášek, J.; Ojima, I.; et al. Substituents at the C3′ and C3′N positions are critical for taxanes to overcome acquired resistance of cancer cells to paclitaxel. Toxicol. Appl. Pharmacol. 2018, 347, 79–91. [Google Scholar] [CrossRef]
- Alam, A.; Kowal, J.; Broude, E.; Roninson, I.; Locher, K.P. Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 2019, 363, 753–756. [Google Scholar] [CrossRef]
- Pavlíková, N.; Bartoňová, I.; Balušíková, K.; Kopperová, D.; Halada, P.; Kovář, J. Differentially expressed proteins in MCF-7 breast cancer cells sensitive and resistant to paclitaxel. Exp. Cell Res. 2015, 333, 1–10. [Google Scholar] [CrossRef]
- Palmfeldt, J.; Bross, P. Proteomics of human mitochondria. Mitochondrion 2017, 33, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant. Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell signalling. J. Cell Sci. 2012, 125, 807–815. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Joshi, A.D.; Mustafa, M.G.; Lichti, C.F.; Elferink, C.J. Homocitrullination is a novel histone H1 epigenetic mark dependent on aryl hydrocarbon receptor recruitment of carbamoyl-phosphate synthase 1. J. Biol. Chem. 2015, 290, 27767–27778. [Google Scholar] [CrossRef]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–625. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Benes, P.; Vetvicka, V.; Fusek, M. Cathepsin d-many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 2008, 68, 12–28. [Google Scholar] [CrossRef]
- Achour, O.; Ashraf, Y.; Bridiau, N.; Kacem, M.; Poupard, N.; Bordenave-Juchereau, S.; Sannier, F.; Lamerant-Fayel, N.; Kieda, C.; Liaudet-Coopman, E.; et al. Alteration of cathepsin D trafficking induced by hypoxia and extracellular acidification in MCF-7 breast cancer cells. Biochimie 2016, 121, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wang, K.; Li, Q.; Zou, Y.; Chen, B.; Gong, Q.; Ho, H.I.; Yin, T.; Zhang, F.; Lu, Y.; et al. The novel autophagy inhibitor alpha-hederin promoted paclitaxel cytotoxicity by increasing reactive oxygen species accumulation in non-small cell lung cancer cells. Int. J. Mol. Sci. 2018, 19, 3221. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Archer, T.K. Upstream stimulatory factors mediate estrogen receptor activation of the cathepsin D promoter. Mol. Endocrinol. 1998, 12, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Dabrosin, C.; Johansson, A.C.; Ollinger, K. Decreased secretion of cathepsin D in breast cancer in vivo by tamoxifen: Mediated by the mannose-6-phosphate/IGF-II receptor? Breast Cancer Res. Treat. 2004, 85, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.C.; Steen, H.; Ollinger, K.; Roberg, K. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 2003, 10, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Emert-Sedlak, L.; Shangary, S.; Rabinovitz, A.; Miranda, M.B.; Delach, S.M.; Johnson, D.E. Involvement of cathepsin D in chemotherapy-induced cytochrome c release, caspase activation, and cell death. Mol. Cancer Ther. 2005, 4, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte-Luis, V.; Montero, J.A.; Kawakami, Y.; Izpisua-Belmonte, J.C.; Hurle, J.M. Lysosomal cathepsins in embryonic programmed cell death. Dev. Biol. 2007, 301, 205–217. [Google Scholar] [CrossRef]
- Castino, R.; Peracchio, C.; Salini, A.; Nicotra, G.; Trincheri, N.F.; Démoz, M.; Valente, G.; Isidoro, C. Chemotherapy drug response in ovarian cancer cells strictly depends on a cathepsin D-Bax activation loop. J. Cell. Mol. Med. 2009, 13, 1096–1109. [Google Scholar] [CrossRef]
- Jancekova, B.; Ondrouskova, E.; Knopfova, L.; Smarda, J.; Benes, P. Enzymatically active cathepsin D sensitizes breast carcinoma cells to TRAIL. Tumour Biol. 2016, 37, 10685–10696. [Google Scholar] [CrossRef]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim. Biophys. Acta 2013, 1831, 792–802. [Google Scholar] [CrossRef]
- Pober, S. Williams-Beuren syndrome. N. Engl. J. Med. 2010, 362, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Arya, M.; Srinivasan, M.; Rajasekharan, R. Human alpha beta hydrolase domain containing protein 11 and its yeast homolog are lipid hydrolases. Biochem. Biophys. Res. Commun. 2017, 487, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, Y.; Tsuji, J.; Fu, S.C.; Tomii, K.; Horton, P.; Imai, K. MitoFates: Improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol. Cell. Proteomics 2015, 14, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Wiedl, T.; Arni, S.; Roschitzki, B.; Grossmann, J.; Collaud, S.; Soltermann, A.; Hillinger, S.; Aebersold, R.; Weder, W. Activity-based proteomics: Identification of ABHD11 and ESD activities as potential biomarkers for human lung adenocarcinoma. J. Proteom. 2011, 74, 1884–1894. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shao, Y.; Zhu, M.; Li, Q.; Yang, F.; Lu, X.; Xu, C.; Xiao, B.; Sun, Y.; Guo, J. Using gastric juice lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of gastric cancer. Tumor Biol. 2016, 37, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Li, L.; Duan, X. Long non-coding RNA ABHD11-AS1 promotes colorectal cancer development through regulation of miR-133a/SOX4 axis. Biosci. Rep. 2018, BSR20181386. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Lv, S.X.; Qiao, Y.; Li, Q.P.; Ye, B.; Wang, C.C.; Miao, L. Long noncoding RNA ABHD11-AS1 predicts the prognosis of pancreatic cancer patients and serves as a promoter by activation the PI3K-AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8630–8639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.L.; Chen, S.; Zong, Z.H.; Guan, X.; Zhao, Y. LncRNA ABHD11-AS1 promotes the development of endometrial carcinoma by targeting cyclin D1. J. Cell. Mol. Med. 2018, 22, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- De Cima, S.; Polo, L.M.; Díez-Fernández, C.; Martínez, A.I.; Cervera, J.; Fita, I.; Rubio, V. Structure of human carbamoyl phosphate synthetase: Deciphering the on/off switch of human ureagenesis. Sci. Rep. 2015, 5, 16950. [Google Scholar] [CrossRef]
- Çeliktas, M.; Tanaka, I.; Tripathi, S.C.; Fahrmann, J.F.; Aguilar-Bonavides, C.; Villalobos, P.; Delgado, O.; Dhillon, D.; Dennison, J.B.; Ostrin, E.J.; et al. Role of CPS1 in cell growth, metabolism and prognosis in LKB1-Inactivated lung adenocarcinoma. J. Natl. Cancer Inst. 2017, 109, 1–9. [Google Scholar] [CrossRef]
- Kim, J.; Hu, Z.; Cai, L.; Li, K.; Choi, E.; Faubert, B.; Bezwada, D.; Rodriguez-Canales, J.; Villalobos, P.; Lin, Y.F.; et al. CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells. Nature 2017, 546, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; McInnes, K.J.; Takagi, K.; Ono, K.; Hunger, N.I.; Wang, L.; Sasano, H.; Simpson, E.R. LKB1 expression is inhibited by estradiol-17β in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2011, 127, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Li, C.F.; Lin, C.Y.; Lee, S.W.; Sheu, M.J.; Lin, L.C.; Chen, T.J.; Wu, T.F.; Hsing, C.H. Overexpression of CPS1 is an independent negative prognosticator in rectal cancers receiving concurrent chemoradiotherapy. Tumour Biol. 2014, 35, 11097–11105. [Google Scholar] [CrossRef] [PubMed]
- Cardona, D.M.; Zhang, X.; Liu, C. Loss of carbamoyl phosphate synthetase I in small-intestinal adenocarcinoma. Am. J. Clin. Pathol. 2009, 132, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Dye, R.B.; Fink, S.P.; Williams, R.C., Jr. Taxol-induced flexibility of microtubules and its reversal by MAP-2 and Tau. J. Biol. Chem. 1993, 268, 6847–6850. [Google Scholar] [PubMed]
- Bauer, J.A.; Chakravarthy, A.B.; Rosenbluth, J.M.; Mi, D.; Seeley, E.H.; De Matos Granja-Ingram, N.; Olivares, M.G.; Kelley, M.C.; Mayer, I.A.; Meszoely, I.M.; et al. Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation. Clin. Cancer Res. 2010, 16, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, S.; Xenarios, I.; Langridge, J.; Vilbois, F.; Parone, P.A.; Martinou, J.C. Proteomic analysis of the mouse liver mitochondrial inner membrane. J. Biol. Chem. 2003, 278, 41566–41571. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Rousseau, D. ATAD3, a vital membrane bound mitochondrial ATPase involved in tumor progression. J. Bioenerg. Biomembr. 2012, 44, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Gilquin, B.; Taillebourg, E.; Cherradi, N.; Hubstenberger, A.; Gay, O.; Merle, N.; Assard, N.; Fauvarque, M.O.; Tomohiro, S.; Kuge, O.; et al. The AAA+ ATPase ATAD3A controls mitochondrial dynamics at the interface of the inner and outer membranes. Mol. Cell. Biol. 2010, 30, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Frickey, T.; Lupas, A.N. Phylogenetic analysis of AAA proteins. J. Struct. Biol. 2004, 146, 2–10. [Google Scholar] [CrossRef]
- Wang, Y.; Bogenhagen, D.F. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J. Biol. Chem. 2006, 281, 25791–25802. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mao, C.C.; Reyes, A.; Sembongi, H.; Di Re, M.; Granycome, C.; Clippingdale, A.B.; Fearnley, I.M.; Harbour, M.; Robinson, A.J.; et al. The AAA+ protein ATAD3 has displacement loop binding properties and is involved in mitochondrial nucleoid organization. J. Cell. Biol. 2007, 176, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, X.; Li, H.; Shull, A.; Kim, J.; Cowell, J.K. Mitochondrial ATAD3A combines with GRP78 to regulate WASF3 metastasis-promoting protein. Oncogene 2016, 35, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.; Goffart, S.; Williams, S.L.; Diaz, F.; Garcia, S.; Nissanka, N.; Area-Gomez, E.; Pohjoismäki, J.; Moraes, C.T. ATAD3 controls mitochondrial cristae structure, influencing mtDNA replication and cholesterol levels in muscle. J. Cell. Sci. 2018, 131, jcs217075. [Google Scholar] [CrossRef] [PubMed]
- Hubstenberger, A.; Labourdette, G.; Baudier, J.; Rousseau, D. ATAD 3A and ATAD 3B are distal 1p-located genes differentially expressed in human glioma cell lines and present in vitro anti-oncogenic and chemoresistant properties. Exp. Cell Res. 2008, 314, 2870–2883. [Google Scholar] [CrossRef] [PubMed]
- You, W.C.; Chiou, S.H.; Huang, C.Y.; Chiang, S.F.; Yang, C.L.; Sudhakar, J.N.; Lin, T.Y.; Chiang, I.P.; Shen, C.C.; Cheng, W.Y.; et al. Mitochondrial protein ATPase family AAA domain containing 3A correlates with radioresistance in glioblastoma. Neuro. Oncol. 2013, 15, 1342–1352. [Google Scholar] [CrossRef]
- Huang, K.H.; Chow, K.C.; Chang, H.W.; Lin, T.Y.; Lee, M.C. ATPase family AAA domain containing 3A is an anti-apoptotic factor and secretion regulator of PSA in prostate cancer. Int. J. Mol. Med. 2011, 28, 9–15. [Google Scholar] [CrossRef]
- Merle, N.; Féraud, O.; Gilquin, B.; Hubstenberger, A.; Kieffer-Jacquinot, S.; Assard, N.; Bennaceur-Griscelli, A.; Honnorat, J.; Baudier, J. ATAD3B is a human embryonic stem cell specific mitochondrial protein, re-expressed in cancer cells, that functions as dominant negative for the ubiquitous ATAD3A. Mitochondrion 2012, 12, 441–448. [Google Scholar] [CrossRef]
- Calcagno, A.M.; Ambudkar, S.V. Molecular mechanisms of drug resistance in single-step and multi-step drug-selected cancer cells. Methods Mol. Biol. 2010, 596, 77–93. [Google Scholar] [CrossRef]
- Jelínek, M.; Balušíková, K.; Kopperová, D.; Němcová-Fürstová, V.; Šrámek, J.; Fidlerová, J.; Zanardi, I.; Ojima, I.; Kovář, J. Caspase-2 is involved in cell death induction by taxanes in breast cancer cells. Cancer Cell Int. 2013, 13, 42. [Google Scholar] [CrossRef]
- Jelínek, M.; Balušíková, K.; Schmiedlová, M.; Němcová-Fürstová, V.; Šrámek, J.; Stančíková, J.; Zanardi, I.; Ojima, I.; Kovář, J. The role of individual caspases in cell death induction by taxanes in breast cancer cells. Cancer Cell Int. 2015, 15, 8. [Google Scholar] [CrossRef] [PubMed]



| Spot No. | Protein Name | DTB No. | No. of Peptides | SC [%] | MS/MS Confirmation | MW [kDa] Th./Exp. | pI Th./Exp. |
|---|---|---|---|---|---|---|---|
| 1 | Cathepsin D | P07339 | 12 | 33 | FDGILGMAYPR | 45/28 | 6.1/4.7 |
| YYTVFDRDNNR | |||||||
| LVDQNIFSFYLSR | |||||||
| 2 | Cathepsin D | P07339 | 15 | 38 | FDGILGMAYPR | 45/28 | 6.1/5.0 |
| YYTVFDRDNNR | |||||||
| LVDQNIFSFYLSR | |||||||
| ISVNNVLPVFDNLMQQK | |||||||
| 3 | Abhydrolase domain-containing protein 11, ABHD11 | Q8NFV4 | 21 | 68 | AINIADELPR | 35/27 | 9.5/7.2 |
| GGAEPRPLPLSYR | |||||||
| TAMLLALQRPELVER | |||||||
| VNLDALTQHLDKILAFPQR | |||||||
| 4 | Carbamoyl-phosphate synthase 1 [ammonia], mitochondrial, CPS1 | P31327 | 14 | 10 | FVHDNYVIR | 164/164 | 6.3/6.0–6.2 |
| GILIGIQQSFRPR | |||||||
| SAYALGGLGSGICPNR | |||||||
| 5 | ATPase family AAA domain-containing protein 3A, ATAD3A | Q9NVI7 | 29 | 38 | TAGTLFGEGFR | 71/71 | 9.1/10.4–10.7 |
| LDSVIEFSIPDSLLIR | |||||||
| LQAYHTQTTPLIEYYR | |||||||
| QRYEDQLKQQQLLNEENLR | |||||||
| ATPase family AAA domain-containing protein 3B, ATAD3B | Q5T9A4 | 17 | 29 | LKEYEAAVEQLKSEQIR | 73/73 | 9.3/10.4–10.7 |
| Protein Name/Abbreviation | Protein Localization | Function | Gene Localizaton | Expression in MCF7/PacR Cells |
|---|---|---|---|---|
| ATP binding cassette subfamily B member 1, ABCB1 | Plasma membrane | Drug efflux [14] | 7q21.12 | Overexpressed, concrete level not determined [14] |
| ATP binding cassette subfamily B member 4, ABCB4 | Plasma membrane | Drug efflux [14] | 7q21.12 | Overexpressed, concrete level not determined [14] |
| ATP binding cassette subfamily C member 2, ABCC2 | Plasma membrane | Drug efflux [14] | 10q24.2 | Overexpressed, concrete level not determined [14] |
| ATP binding cassette subfamily C member 3, ABCC3 | Plasma membrane | Drug efflux [14] | 17q21.33 | Overexpressed, concrete level not determined [14] |
| Abhydrolase domain-containing protein 11, ABHD11 | Mitochondria | Putative lipid hydrolase [52] | 7q11.23 | 68% [this paper] |
| ATPase family AAA domain-containing protein 3A and 3B, ATAD3A, ATAD3B | Mitochondria | Multiple (mitochondrial network, cristae structure, nucleoid binding) [69,71,72,73,74,75] | 1p36.33 | 154% [this paper] |
| Carbamoyl-phosphate synthetase 1, CPS1 | Mitochondria | Enzyme (urea cycle) [59,61] | 2q34 | 494% [this paper] |
| Cathepsin D, CTSD | Lysosomes | Protein degradation, cell death [40,48,49] | 11p15.5 | 28% [30], 19% [this paper] |
| Heat shock protein family B (small) member 1, HSP27 | Cytosol | Signaling [30] | 7q11.23 | 47% [30] |
| Thyroid hormone receptor interactor 6, TRIP6 | Cytosol | Antiapoptotic signaling [30] | 7q22.1 | 650% [30] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, P.; Halada, P.; Jelínek, M.; Balušíková, K.; Kovář, J. Differentially Expressed Mitochondrial Proteins in Human MCF7 Breast Cancer Cells Resistant to Paclitaxel. Int. J. Mol. Sci. 2019, 20, 2986. https://doi.org/10.3390/ijms20122986
Daniel P, Halada P, Jelínek M, Balušíková K, Kovář J. Differentially Expressed Mitochondrial Proteins in Human MCF7 Breast Cancer Cells Resistant to Paclitaxel. International Journal of Molecular Sciences. 2019; 20(12):2986. https://doi.org/10.3390/ijms20122986
Chicago/Turabian StyleDaniel, Petr, Petr Halada, Michael Jelínek, Kamila Balušíková, and Jan Kovář. 2019. "Differentially Expressed Mitochondrial Proteins in Human MCF7 Breast Cancer Cells Resistant to Paclitaxel" International Journal of Molecular Sciences 20, no. 12: 2986. https://doi.org/10.3390/ijms20122986
APA StyleDaniel, P., Halada, P., Jelínek, M., Balušíková, K., & Kovář, J. (2019). Differentially Expressed Mitochondrial Proteins in Human MCF7 Breast Cancer Cells Resistant to Paclitaxel. International Journal of Molecular Sciences, 20(12), 2986. https://doi.org/10.3390/ijms20122986





