Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications
Abstract
1. Introduction
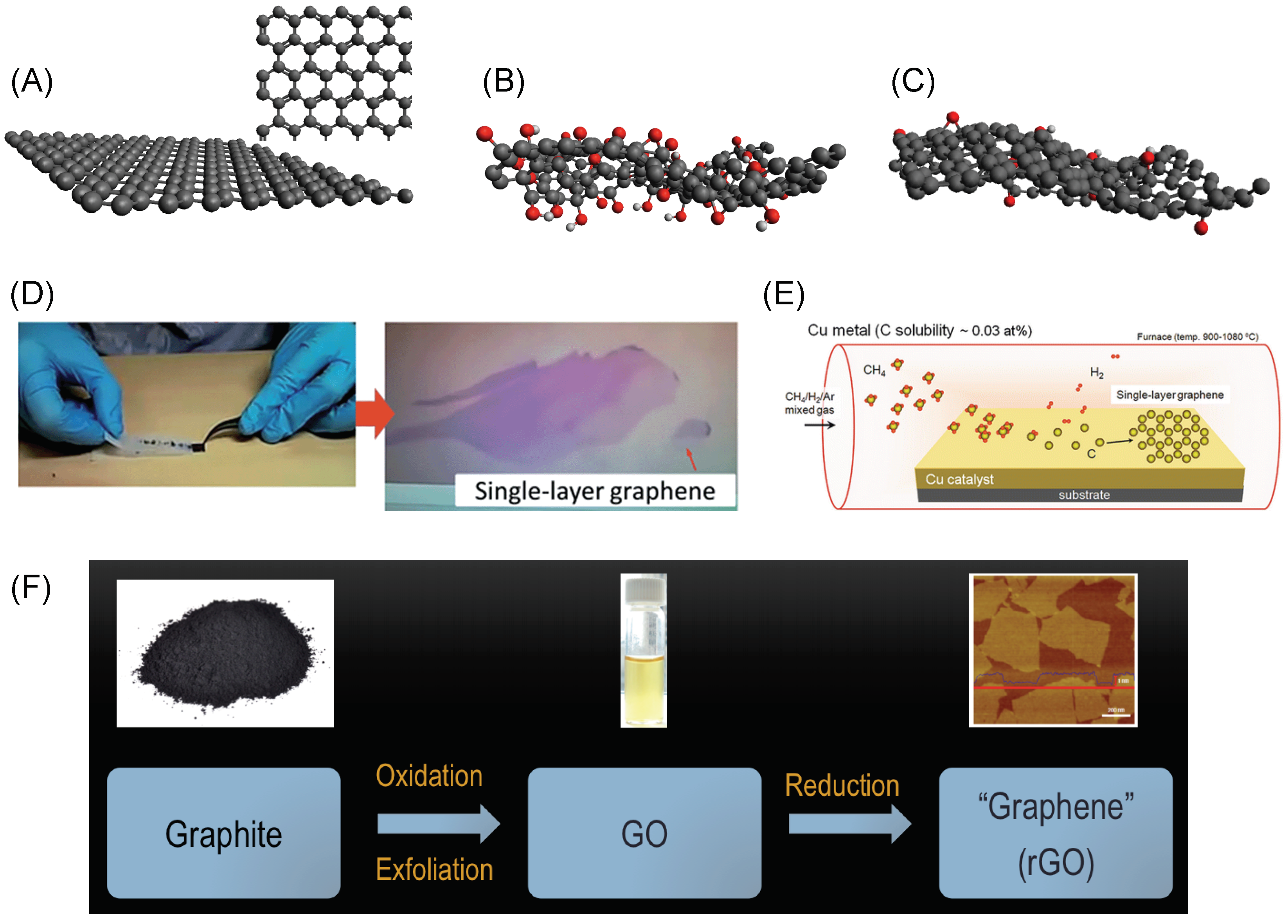
1.1. Graphene and Its Derivatives: Synthesis and Properties
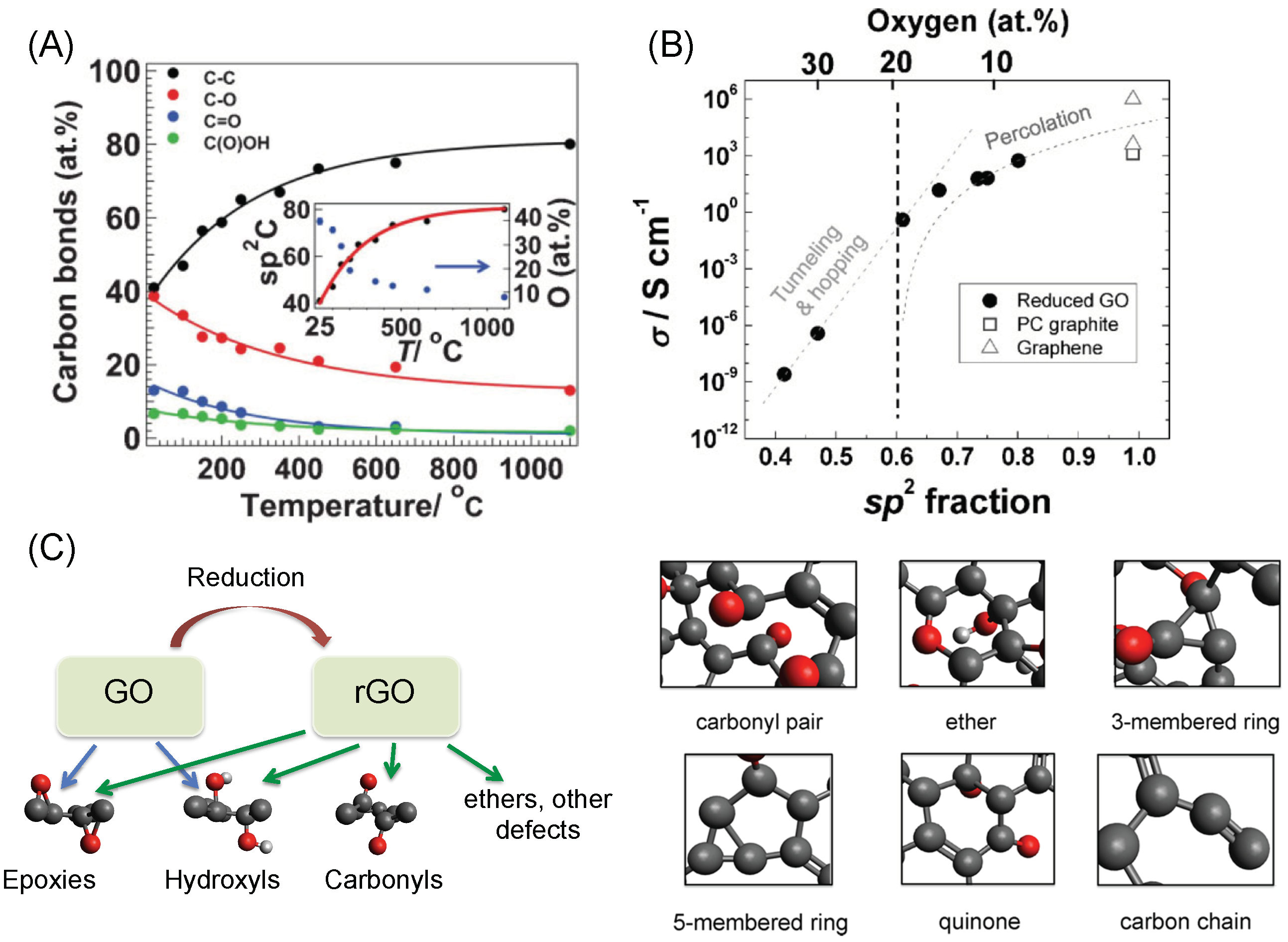
1.2. GO and rGO: Structure–Property Relationships
2. Graphene/Graphene Oxide Nanomaterials Based Electrochemical Biosensors
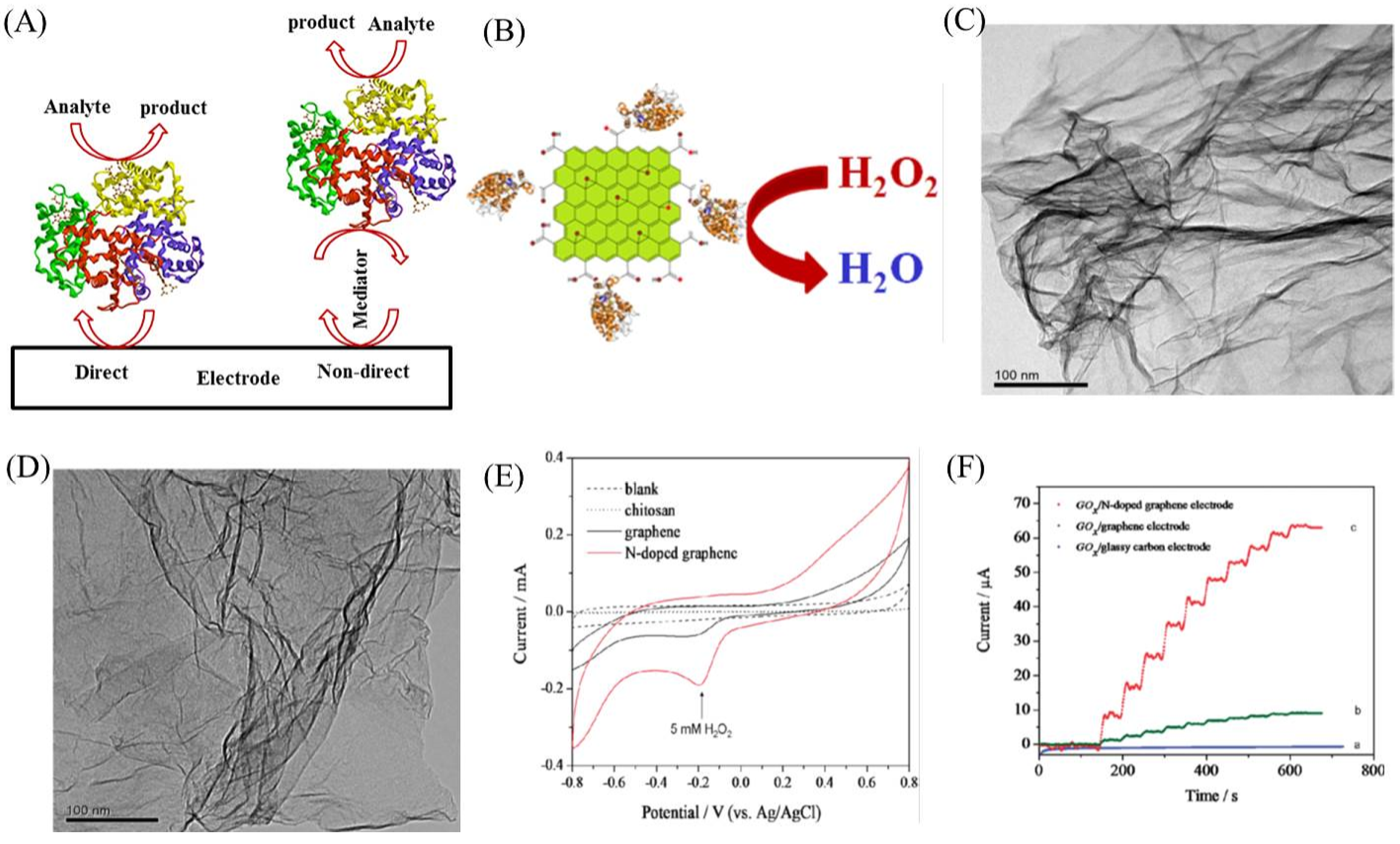
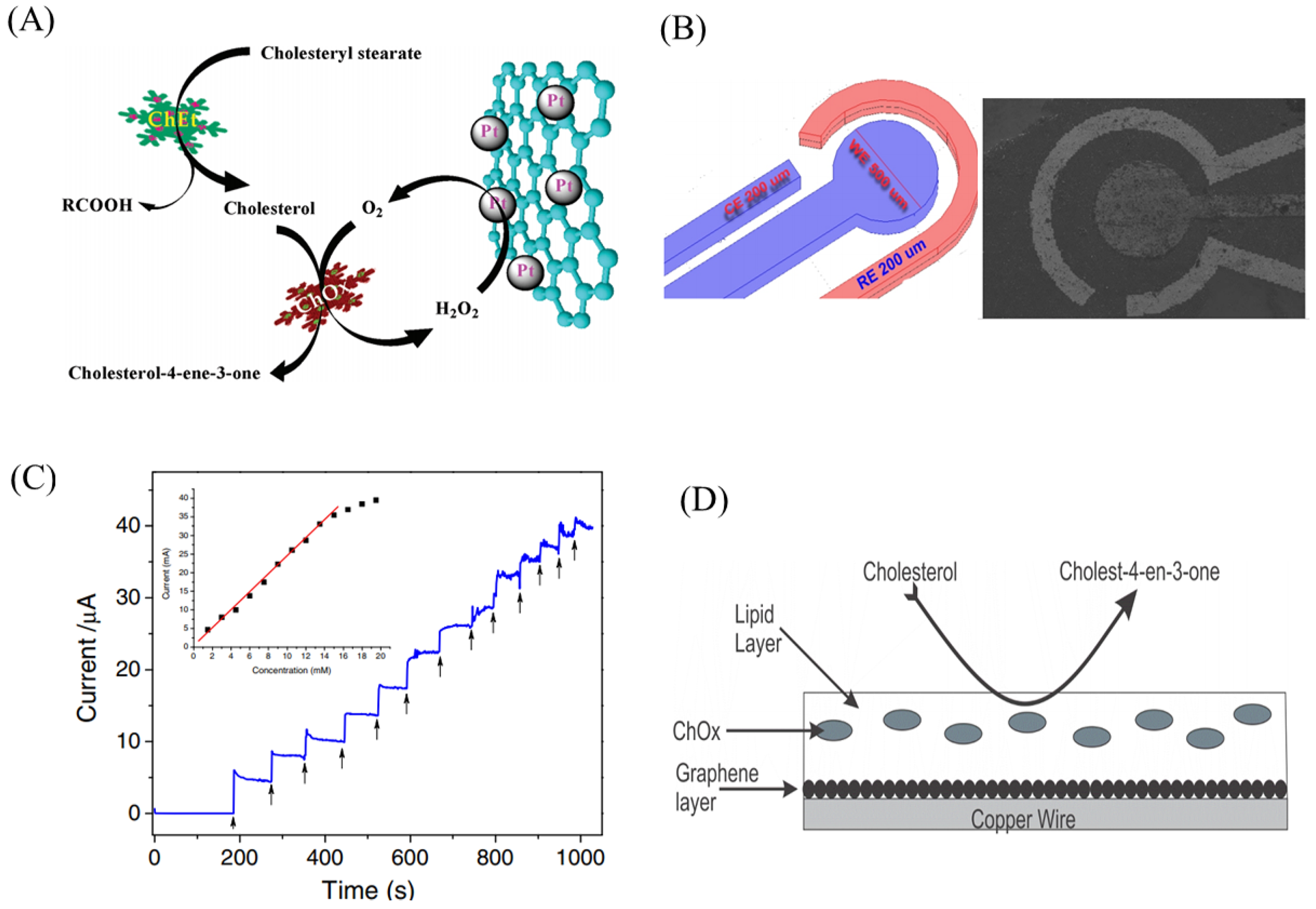
2.1. Enzymatic Biosensor
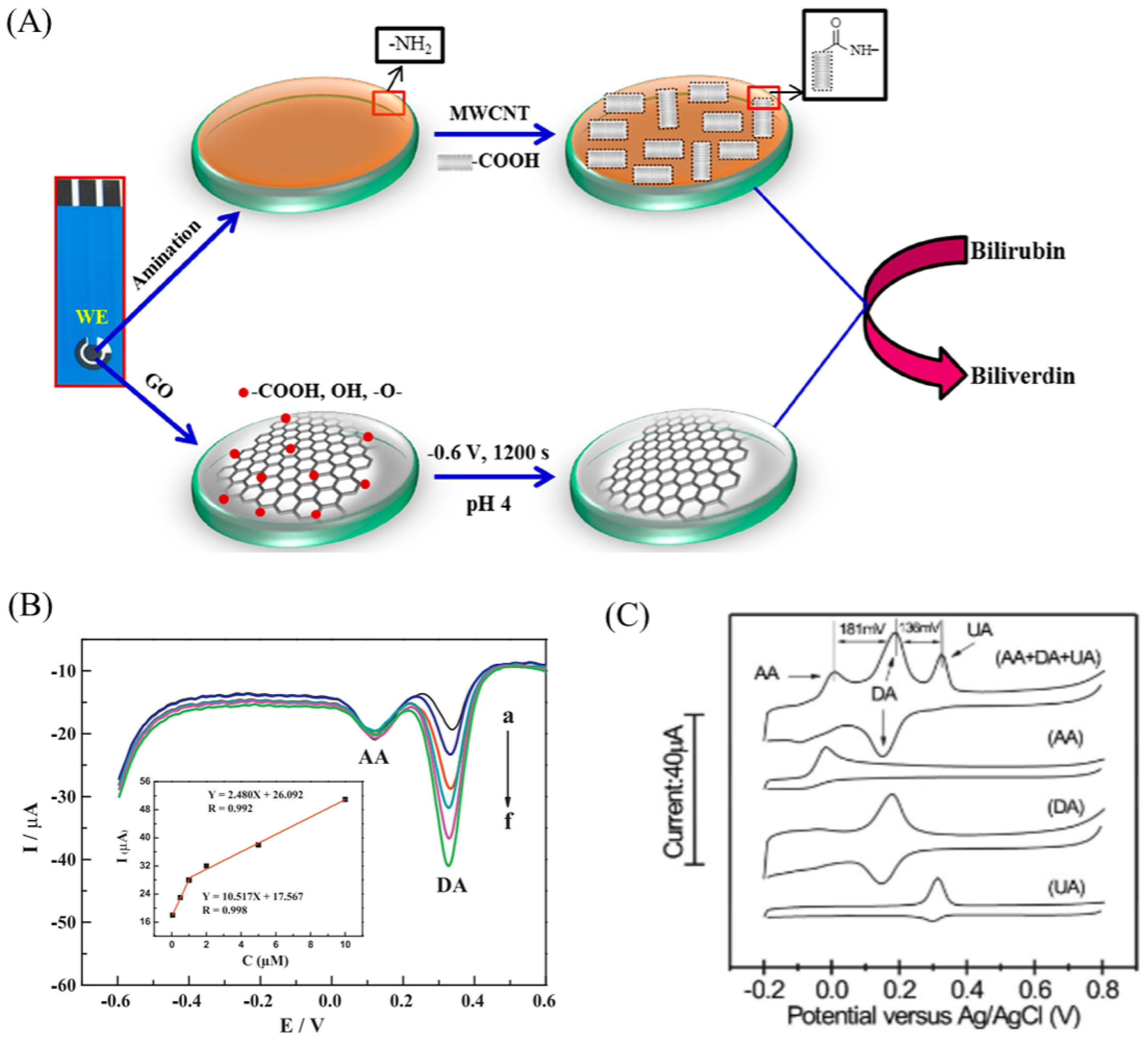
2.2. Non-Enzymatic Biosensor
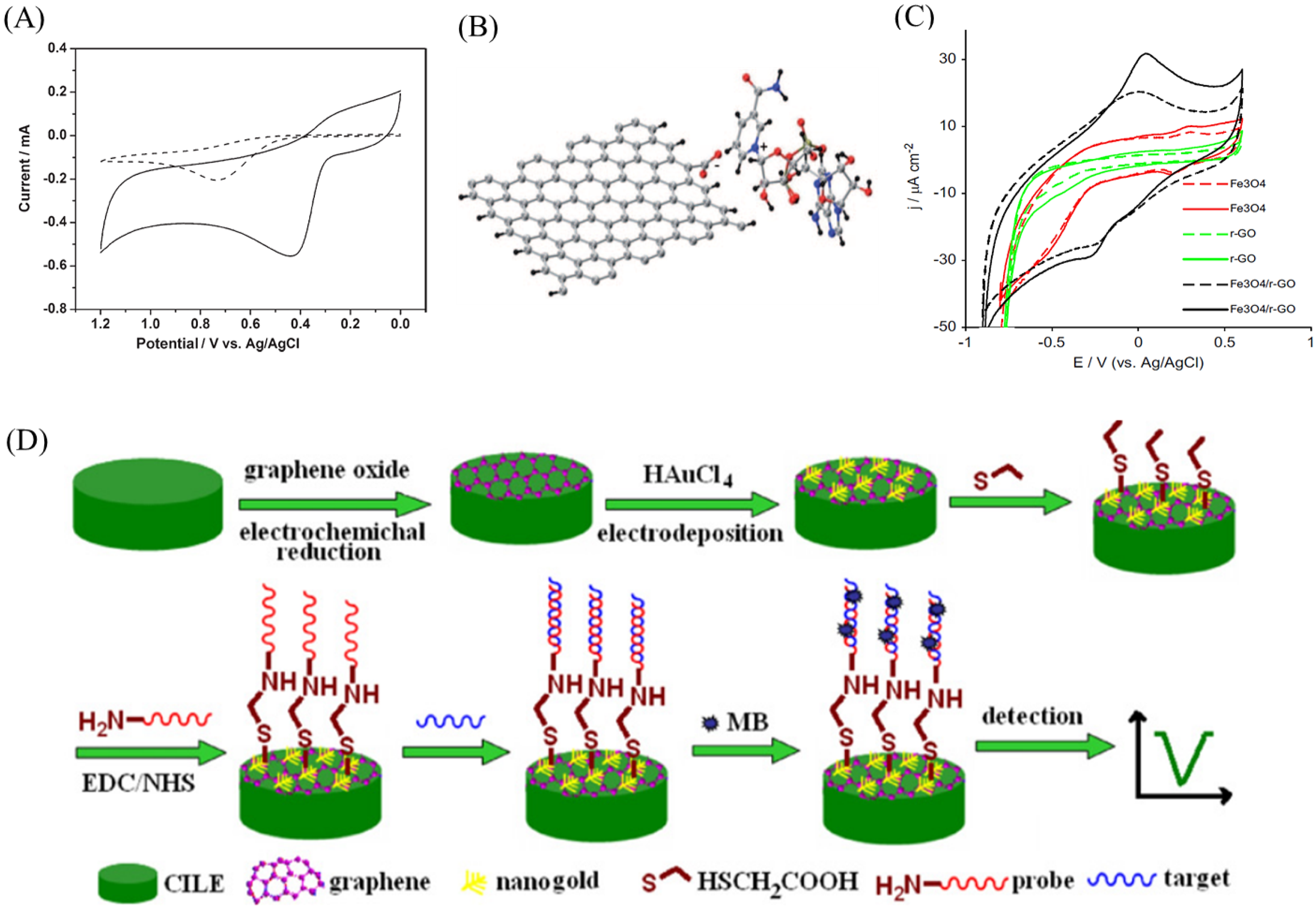
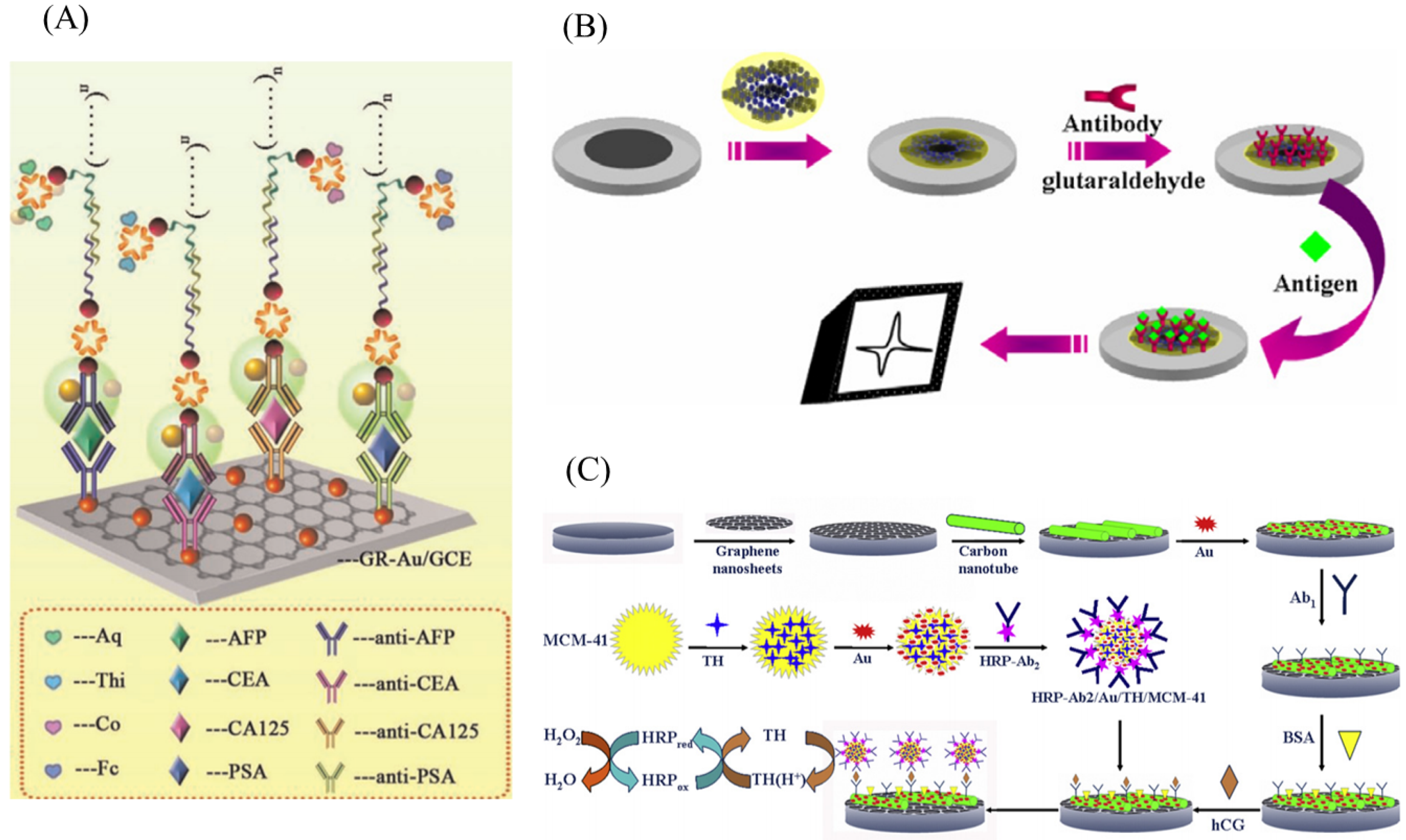
2.3. Immunosensor
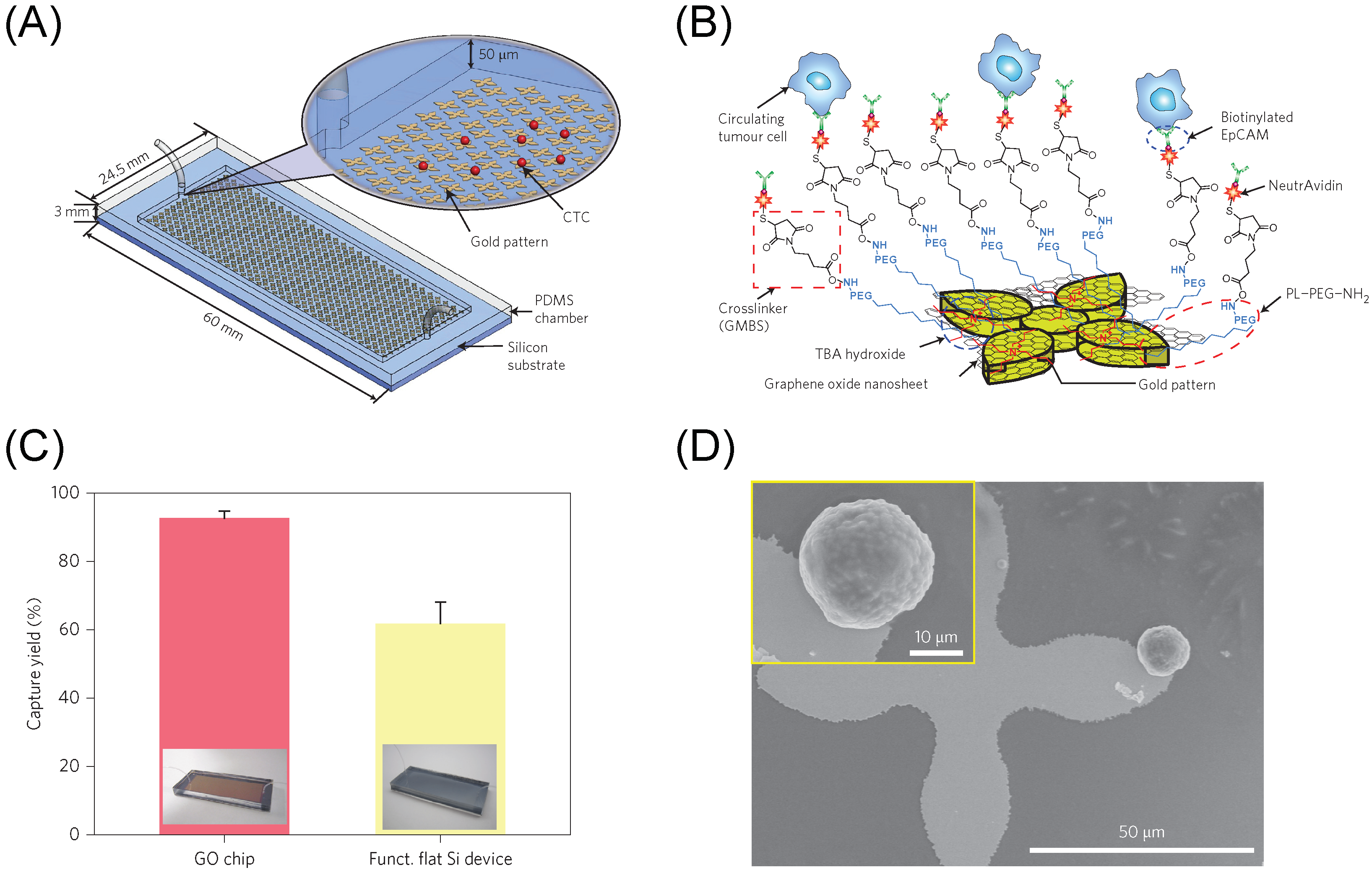
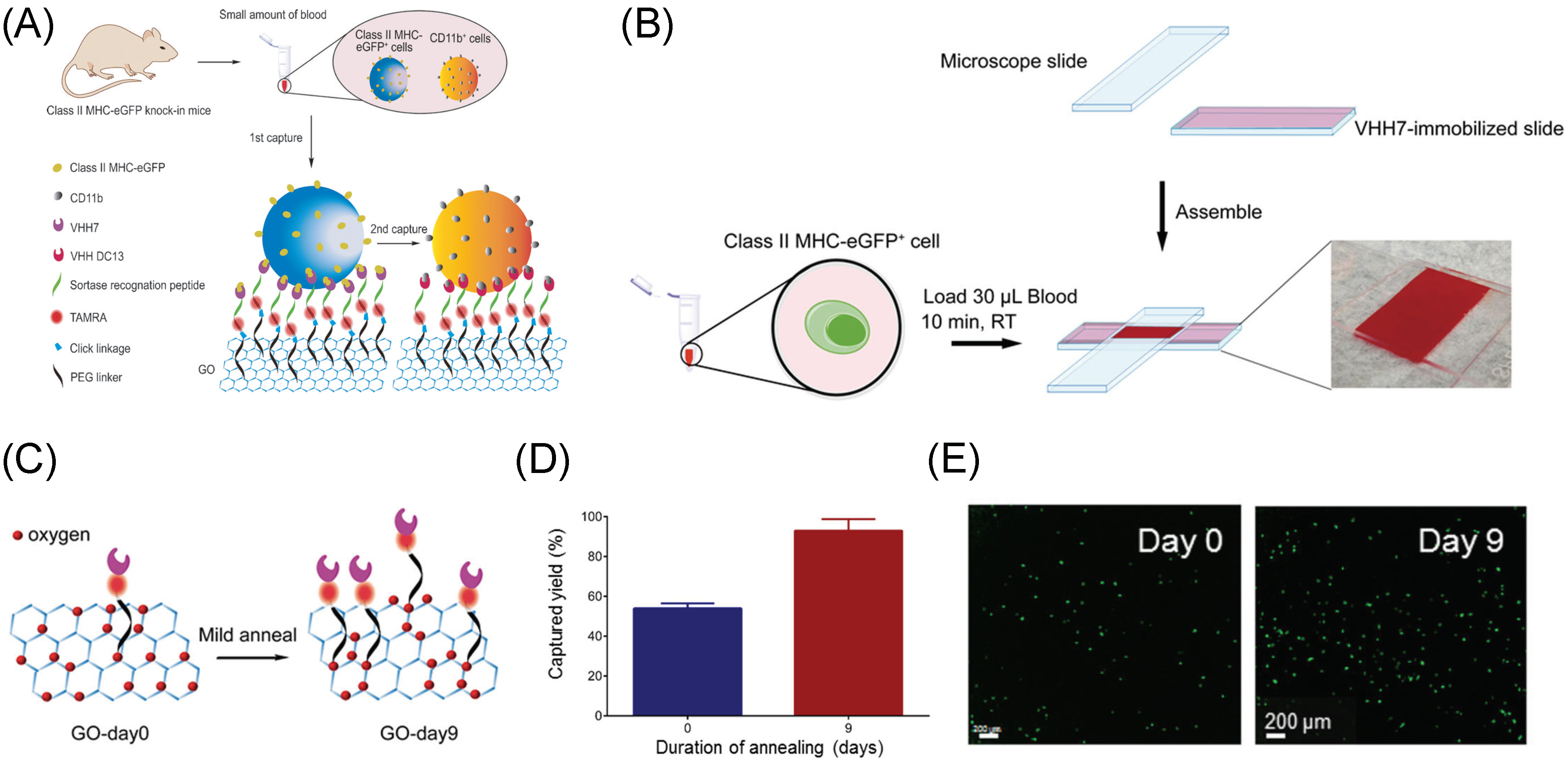
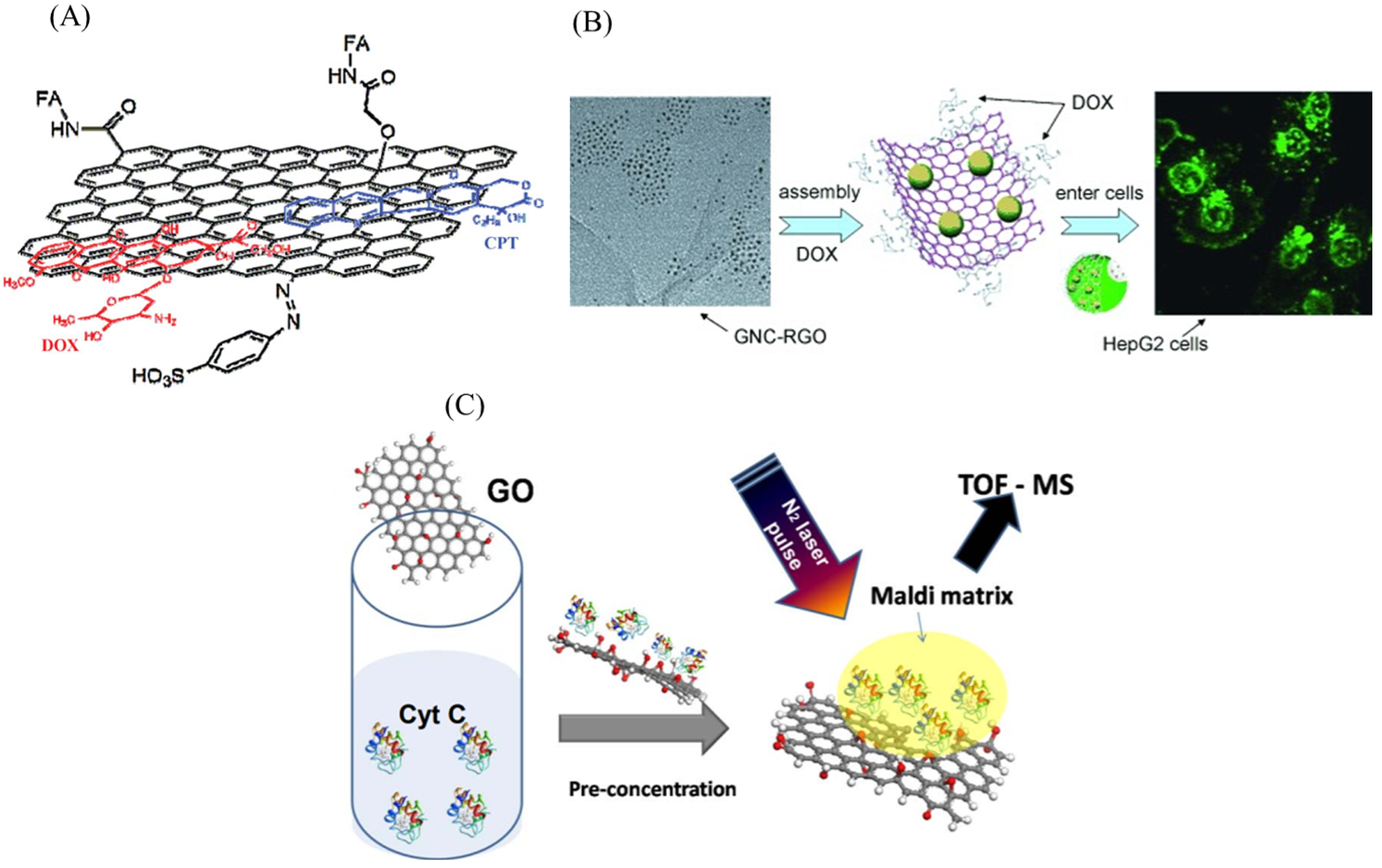
3. Graphene/Graphene Oxide Materials for Biomedical Cell Capture and Other Biomedical Applications
4. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.; Novoselov, K. The rise of graphene (editorial). Nat. Mat. 2007, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.B.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Suk, M.E.; Aluru, N.R. Water Transport through Ultrathin Graphene. J. Phys. Chem. Lett. 2010, 1, 1590–1594. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Ago, H. CVD Growth of High-Quality Single-Layer Graphene. In Frontiers of Graphene and Carbon Nanotubes: Devices and Applications; Matsumoto, K., Ed.; Springer: Tokyo, Japan, 2015; pp. 3–20. [Google Scholar]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of Graphene Films on Copper Foils by Chemical Vapor Deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Banszerus, L.; Schmitz, M.; Engels, S.; Dauber, J.; Oellers, M.; Haupt, F.; Watanabe, K.; Taniguchi, T.; Beschoten, B.; Stampfer, C. Ultrahigh-mobility graphene devices from chemical vapor deposition on reusable copper. Sci. Adv. 2015, 1, e1500222. [Google Scholar] [CrossRef]
- Lee, G.H.; Cooper, R.C.; An, S.J.; Lee, S.; van der Zande, A.; Petrone, N.; Hammerberg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-Strength Chemical-Vapor–Deposited Graphene and Grain Boundaries. Science 2013, 340, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Compos. Mater. Nat. 2006, 442, 282–286. [Google Scholar]
- Stankovich, S.; Dikin, D.; Piner, R.; Kohlhaas, K.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.; Ruoff, R. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wobkenberg, P.H.; Eda, G.; Leem, D.S.; De Mello, J.C.; Bradley, D.D.C.; Chhowalla, M.; Anthopoulos, T.D. Reduced graphene oxide electrodes for large area organic electronics. Adv. Mater. Deerfield Beach Fla 2011, 23, 1558–1562. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Bagri, A.; Grantab, R.; Medhekar, N.V.; Shenoy, V.B. Stability and Formation Mechanisms of Carbonyl- and Hydroxyl-Decorated Holes in Graphene Oxide. J. Phys. Chem. 2010, 114, 12053–12061. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and Thermal Reduction of Graphene Oxide: Reaction Mechanisms, Product Structures, and Reaction Design. J. Phys. Chem. 2010, 114, 832–842. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of Electrical, Chemical, and Structural Properties of Transparent and Conducting Chemically Derived Graphene Thin Films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Larciprete, R.; Fabris, S.; Sun, T.; Lacovig, P.; Baraldi, A.; Lizzit, S. Dual Path Mechanism in the Thermal Reduction of Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 17315–17321. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Fabris, S.; Baroni, S. Surface Precursors and Reaction Mechanisms for the Thermal Reduction of Graphene Basal Surfaces Oxidized by Atomic Oxygen. J. Phys. Chem. 2011, 115, 4730–4737. [Google Scholar] [CrossRef]
- Sun, T.; Fabris, S. Mechanisms for Oxidative Unzipping and Cutting of Graphene. Nano Lett. 2012, 12, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Bardhan, N.M.; Chen, G.Y.; Li, Z.; Belcher, A.M.; Grossman, J.C. New insights into the thermal reduction of graphene oxide: Impact of oxygen clustering. Carbon 2016, 100, 90–98. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Silva, K.D.; Huang, H.H.; Joshi, R.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Ambrosi, A.; Bonanni, A.; Chng, E.L.K.; Poh, H.L. Graphene for electrochemical sensing and biosensing. TrAC Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bernardi, M.; Grossman, J.C. The Impact of Functionalization on the Stability, Work Function, and Photoluminescence of Reduced Graphene Oxide. ACS Nano 2013, 7, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, N.M.; Kumar, P.V.; Li, Z.; Ploegh, H.L.; Grossman, J.C.; Belcher, A.M.; Chen, G.Y. Enhanced Cell Capture on Functionalized Graphene Oxide Nanosheets through Oxygen Clustering. ACS Nano 2017, 11, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Hossain, Z.; Johns, J.E.; Bevan, K.H.; Karmel, H.J.; Liang, Y.T.; Yoshimoto, S.; Mukai, K.; Koitaya, T.; Yoshinobu, J.; Kawai, M.; et al. Chemically Homogeneous and Thermally Reversible Oxidation of Epitaxial Graphene. Nat. Chem. 2012, 4, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Johns, J.E.; Hersam, M.C. Atomic Covalent Functionalization of Graphene. Accounts Chem. Res. 2013, 46, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Zhou, S.; Hu, Y.; Acik, M.; Chabal, Y.J.; Berger, C.; De Heer, W.; Bongiorno, A.; Riedo, E. Room-Temperature Metastability of Multilayer Graphene Oxide Films. Nat. Mater. 2012, 11, 544–549. [Google Scholar] [CrossRef]
- Eda, G.; Mattevi, C.; Yamaguchi, H.; Kim, H.; Chhowalla, M. Insulator to Semimetal Transition in Graphene Oxide. J. Phys. Chem. C 2009, 113, 15768–15771. [Google Scholar] [CrossRef]
- Alwarappan, S.; Erdem, A.; Liu, C.; Li, C.Z. Probing the Electrochemical Properties of Graphene Nanosheets for Biosensing Applications. J. Phys. Chem. C 2009, 113, 8853–8857. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Gabriel, W.; Santschi, C.; Martin, O. Electrochemical sensor for bilirubin detection using screen printed electrodes functionalized with carbon nanotubes and graphene. Sensors 2018, 18, 800. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Wen, W.; Song, Y.; Yan, X.; Zhu, C.; Du, D.; Wang, S.; Asiri, A.M.; Lin, Y. Recent advances in emerging 2D nanomaterials for biosensing and bioimaging applications. Mater. Today 2018, 21, 164–177. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-based electrochemical biosensors. Analyst 2005, 130, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Chua, C.K.; Bonanni, A.; Pumera, M. Electrochemistry of graphene and related materials. Chem. Rev. 2014, 114, 7150–7188. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, Q.; Tan, C.; Wang, Y.; Zhang, H. Graphene-based electrochemical sensors. Small 2013, 9, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Madasamy, T.; Santschi, C.; Martin, O.J. A miniaturized electrochemical assay for homocysteine using screen-printed electrodes with cytochrome c anchored gold nanoparticles. Analyst 2015, 140, 6071–6078. [Google Scholar] [CrossRef]
- Madasamy, T.; Pandiaraj, M.; Balamurugan, M.; Karnewar, S.; Benjamin, A.R.; Venkatesh, K.A.; Vairamani, K.; Kotamraju, S.; Karunakaran, C. Virtual electrochemical nitric oxide analyzer using copper, zinc superoxide dismutase immobilized on carbon nanotubes in polypyrrole matrix. Talanta 2012, 100, 168–174. [Google Scholar] [CrossRef]
- Madasamy, T.; Pandiaraj, M.; Balamurugan, M.; Bhargava, K.; Sethy, N.K.; Karunakaran, C. Copper, zinc superoxide dismutase and nitrate reductase coimmobilized bienzymatic biosensor for the simultaneous determination of nitrite and nitrate. Biosens. Bioelectron. 2014, 52, 209–215. [Google Scholar] [CrossRef]
- Pandiaraj, M.; Madasamy, T.; Gollavilli, P.N.; Balamurugan, M.; Kotamraju, S.; Rao, V.K.; Bhargava, K.; Karunakaran, C. Nanomaterial-based electrochemical biosensors for cytochrome c using cytochrome c reductase. Bioelectrochemistry 2013, 91, 1–7. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, S.J.; Choi, J.W. Electrical Property of Graphene and Its Application to Electrochemical Biosensing. Nanomaterials 2019, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Muthurasu, A.; Ganesh, V. Horseradish peroxidase enzyme immobilized graphene quantum dots as electrochemical biosensors. Appl. Biochem. Biotechnol. 2014, 174, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Yang, S. Hydrogen peroxide: A signaling messenger. Antioxidants Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Liao, C.S.; Jhang, J.H.; Tsai, Y.C. Graphene modified basal and edge plane pyrolytic graphite electrodes for electrocatalytic oxidation of hydrogen peroxide and β-nicotinamide adenine dinucleotide. Electrochem. Commun. 2009, 11, 2153–2156. [Google Scholar] [CrossRef]
- Zou, N.; Wei, X.; Zong, Z.; Li, X.; Wang, Z.; Wang, X. A novel enzymatic biosensor for detection of intracellular hydrogen peroxide based on 1-aminopyrene and reduced graphene oxides. J. Chem. Sci. 2019, 131, 28. [Google Scholar] [CrossRef]
- Vilian, A.E.; Chen, S.M. Simple approach for the immobilization of horseradish peroxidase on poly-l-histidine modified reduced graphene oxide for amperometric determination of dopamine and H2O2. RSC Adv. 2014, 4, 55867–55876. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kim, S.J. An enzymatic biosensor for hydrogen peroxide based on one-pot preparation of CeO2-reduced graphene oxide nanocomposite. RSC Adv. 2015, 5, 12937–12943. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, H.; Meng, X.; Xu, Z.; Fu, Y.; Ai, S. Direct electrochemistry of sarcosine oxidase on graphene, chitosan and silver nanoparticles modified glassy carbon electrode and its biosensing for hydrogen peroxide. Electrochim. Acta 2012, 71, 294–301. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, S.; Engelhard, M.H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Roh, K.M.; Choi, J.W.; Huang, J. A glucose biosensor based on TiO2–graphene composite. Biosens. Bioelectron. 2012, 38, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Sumaryada, T.; Sandy Gunawan, M.; Perdana, S.; Arjo, S.; Maddu, A. A Molecular Interaction Analysis Reveals the Possible Roles of Graphene Oxide in a Glucose Biosensor. Biosensors 2019, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, K.; Hong, S.W.; Jin, S.H.; Chang, S.C.; Park, D.S. Fabrication of a novel disposable glucose biosensor using an electrochemically reduced graphene oxide–glucose oxidase biocomposite. Anal. Methods 2016, 8, 6974–6981. [Google Scholar] [CrossRef]
- Mohd Yazid, S.N.A.; Md Isa, I.; Abu Bakar, S.; Hashim, N.; Ab Ghani, S. A review of glucose biosensors based on graphene/metal oxide nanomaterials. Anal. Lett. 2014, 47, 1821–1834. [Google Scholar] [CrossRef]
- Casero, E.; Parra-Alfambra, A.; Petit-Domínguez, M.; Pariente, F.; Lorenzo, E.; Alonso, C. Differentiation between graphene oxide and reduced graphene by electrochemical impedance spectroscopy (EIS). Electrochem. Commun. 2012, 20, 63–66. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. A hybrid functional nanoscaffold based on reduced graphene oxide–ZnO for the development of an amperometric biosensing platform. RSC Adv. 2013, 3, 25858–25864. [Google Scholar] [CrossRef]
- Pakapongpan, S.; Poo-Arporn, R.P. Self-assembly of glucose oxidase on reduced graphene oxide-magnetic nanoparticles nanocomposite-based direct electrochemistry for reagentless glucose biosensor. Mater. Sci. Eng. C 2017, 76, 398–405. [Google Scholar] [CrossRef]
- Vilian, A.E.; Chen, S.M.; Ali, M.A.; Al-Hemaid, F.M. Direct electrochemistry of glucose oxidase immobilized on ZrO2 nanoparticles-decorated reduced graphene oxide sheets for a glucose biosensor. RSC Adv. 2014, 4, 30358–30367. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct electrochemistry of glucose oxidase and biosensing for glucose based on graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxidants Redox Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.; Li, Y.; Feng, H.; Lu, J.; Li, J. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Pumera, M.; Scipioni, R.; Iwai, H.; Ohno, T.; Miyahara, Y.; Boero, M. A Mechanism of Adsorption of β-Nicotinamide Adenine Dinucleotide on Graphene Sheets: Experiment and Theory. Chem. Eur. J. 2009, 15, 10851–10856. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qi, X.; Zhang, Y.; Yang, H.; Gao, H.; Chen, Y.; Sun, Z. Electrochemical DNA biosensor for the detection of Listeria monocytogenes with dendritic nanogold and electrochemical reduced graphene modified carbon ionic liquid electrode. Electrochim. Acta 2012, 85, 145–151. [Google Scholar] [CrossRef]
- Liu, H.; Gao, J.; Xue, M.; Zhu, N.; Zhang, M.; Cao, T. Processing of graphene for electrochemical application: noncovalently functionalize graphene sheets with water-soluble electroactive methylene green. Langmuir 2009, 25, 12006–12010. [Google Scholar] [CrossRef]
- Gasnier, A.; Pedano, M.L.; Rubianes, M.D.; Rivas, G.A. Graphene paste electrode: Electrochemical behavior and analytical applications for the quantification of NADH. Sens. Actuators Chem. 2013, 176, 921–926. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Jia, Y.; Yin, X.B.; Zhang, J.; Zhou, S.; Song, M.; Xing, K.L. Graphene oxide modified light addressable potentiometric sensor and its application for ssDNA monitoring. Analyst 2012, 137, 5866–5873. [Google Scholar] [CrossRef] [PubMed]
- Ranade, V. Cholesterol detection, diagnosis and evaluation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1993, 31, 313–321. [Google Scholar] [PubMed]
- Pramanik, K.; Sarkar, P.; Bhattacharyay, D.; Majumdar, P. One Step Electrode Fabrication for Direct Electron Transfer Cholesterol Biosensor Based on Composite of Polypyrrole, Green Reduced Graphene Oxide and Cholesterol Oxidase. Electroanalysis 2018, 30, 2719–2730. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. Enzyme-integrated cholesterol biosensing scaffold based on in situ synthesized reduced graphene oxide and dendritic Pd nanostructure. Biosens. Bioelectron. 2014, 62, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.S.; Raj, C.R. Development of an amperometric cholesterol biosensor based on graphene- Pt nanoparticle hybrid material. J. Phys. Chem. C 2010, 114, 21427–21433. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Le, H.D.; Nguyen, V.Q.; Ngo, T.T.T.; Do, Q.P.; Nguyen, X.N.; Phan, N.M. Development of the layer-by-layer biosensor using graphene films: application for cholesterol determination. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 015013. [Google Scholar] [CrossRef]
- Nikoleli, G.P.; Ibupoto, Z.H.; Nikolelis, D.P.; Likodimos, V.; Psaroudakis, N.; Tzamtzis, N.; Willander, M.; Hianik, T. Potentiometric cholesterol biosensing application of graphene electrode with stabilized polymeric lipid membrane. Cent. Eur. J. Chem. 2013, 11, 1554–1561. [Google Scholar] [CrossRef]
- Palanisamy, S.; Karuppiah, C.; Chen, S.M. Direct electrochemistry and electrocatalysis of glucose oxidase immobilized on reduced graphene oxide and silver nanoparticles nanocomposite modified electrode. Colloids Surf. Biointerfaces 2014, 114, 164–169. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.M.; Huang, S.T.; Huang, T.T.; Lin, C.M.; Hwa, K.Y.; Chen, T.Y.; Chen, B.J. Glucose biosensor based on glucose oxidase immobilized at gold nanoparticles decorated graphene-carbon nanotubes. Enzym. Microb. Technol. 2015, 78, 40–45. [Google Scholar] [CrossRef]
- Palanisamy, S.; Devasenathipathy, R.; Chen, S.M.; Ajmal Ali, M.; Karuppiah, C.; Balakumar, V.; Prakash, P.; Elshikh, M.S.; Al-Hemaid, F.M. Direct Electrochemistry of Glucose Oxidase at Reduced Graphene Oxide and β-Cyclodextrin Composite Modified Electrode and Application for Glucose Biosensing. Electroanalysis 2015, 27, 2412–2420. [Google Scholar] [CrossRef]
- Wu, S.; Su, F.; Dong, X.; Ma, C.; Pang, L.; Peng, D.; Wang, M.; He, L.; Zhang, Z. Development of glucose biosensors based on plasma polymerization-assisted nanocomposites of polyaniline, tin oxide, and three-dimensional reduced graphene oxide. Appl. Surf. Sci. 2017, 401, 262–270. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.M.; Yang, C.Y.; Periakaruppan, P.; Lou, B.S. Direct electrochemistry of glucose oxidase and sensing of glucose at a glassy carbon electrode modified with a reduced graphene oxide/fullerene-C60 composite. RSC Adv. 2015, 5, 77651–77657. [Google Scholar] [CrossRef]
- Istrate, O.M.; Rotariu, L.; Marinescu, V.E.; Bala, C. NADH sensing platform based on electrochemically generated reduced graphene oxide–gold nanoparticles composite stabilized with poly (allylamine hydrochloride). Sens. Actuators B Chem. 2016, 223, 697–704. [Google Scholar] [CrossRef]
- Tığ, G.A. Highly sensitive amperometric biosensor for determination of NADH and ethanol based on Au-Ag nanoparticles/poly (L-cysteine)/reduced graphene oxide nanocomposite. Talanta 2017, 175, 382–389. [Google Scholar]
- Tabrizi, M.A.; Zand, Z. A Facile One-Step Method for the Synthesis of Reduced Graphene Oxide Nanocomposites by NADH as Reducing Agent and Its Application in NADH Sensing. Electroanalysis 2014, 26, 171–177. [Google Scholar] [CrossRef]
- Singh, A.; Sinsinbar, G.; Choudhary, M.; Kumar, V.; Pasricha, R.; Verma, H.; Singh, S.P.; Arora, K. Graphene oxide-chitosan nanocomposite based electrochemical DNA biosensor for detection of typhoid. Sens. Actuators Chem. 2013, 185, 675–684. [Google Scholar] [CrossRef]
- Li, B.; Pan, G.; Avent, N.D.; Lowry, R.B.; Madgett, T.E.; Waines, P.L. Graphene electrode modified with electrochemically reduced graphene oxide for label-free DNA detection. Biosens. Bioelectron. 2015, 72, 313–319. [Google Scholar] [CrossRef]
- Wang, J.; Shi, A.; Fang, X.; Han, X.; Zhang, Y. An ultrasensitive supersandwich electrochemical DNA biosensor based on gold nanoparticles decorated reduced graphene oxide. Anal. Biochem. 2015, 469, 71–75. [Google Scholar] [CrossRef]
- Li, Z.; Xie, C.; Wang, J.; Meng, A.; Zhang, F. Direct electrochemistry of cholesterol oxidase immobilized on chitosan–graphene and cholesterol sensing. Sens. Actuators Chem. 2015, 208, 505–511. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, L.; Chai, Y.; Yuan, R. Electrochemistry of cholesterol biosensor based on a novel Pt–Pd bimetallic nanoparticle decorated graphene catalyst. Talanta 2013, 109, 167–172. [Google Scholar] [CrossRef]
- Ruecha, N.; Rangkupan, R.; Rodthongkum, N.; Chailapakul, O. Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosens. Bioelectron. 2014, 52, 13–19. [Google Scholar] [CrossRef]
- Komathi, S.; Muthuchamy, N.; Lee, K.; Gopalan, A. Fabrication of a novel dual mode cholesterol biosensor using titanium dioxide nanowire bridged 3D graphene nanostacks. Biosens. Bioelectron. 2016, 84, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Jing, Q.S.; Wu, Z.W.; Wang, L.; Wei, C.Y. Enhanced sensing of dopamine in the present of ascorbic acid based on graphene/poly (p-aminobenzoic acid) composite film. Colloids Surf. Biointerfaces 2011, 88, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.G.; Papakonstantinou, P.; McMullan, M.; Chu, M.; Stamboulis, A.; Potenza, A.; Dhesi, S.S.; Marchetto, H. Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv. Funct. Mater. 2008, 18, 3506–3514. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Gu, Y.e.; Wang, Z.; Wang, C. One-pot solvothermal synthesis of a Cu2O/graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim. Acta 2011, 173, 103–109. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.; Zan, X.; Liao, K.; Xu, R.; Duan, H. Growth of metal–metal oxide nanostructures on freestanding graphene paper for flexible biosensors. Adv. Funct. Mater. 2012, 22, 2487–2494. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Szunerits, S.; Boukherroub, R. Environmentally friendly reduction of graphene oxide using tyrosine for nonenzymatic amperometric H2O2 detection. Electroanalysis 2014, 26, 156–163. [Google Scholar] [CrossRef]
- Gao, W.; Tjiu, W.W.; Wei, J.; Liu, T. Highly sensitive nonenzymatic glucose and H2O2 sensor based on Ni(OH)2/electroreduced graphene oxide- Multiwalled carbon nanotube film modified glass carbon electrode. Talanta 2014, 120, 484–490. [Google Scholar] [CrossRef]
- Zor, E.; Saglam, M.E.; Akin, I.; Saf, A.O.; Bingol, H.; Ersoz, M. Green synthesis of reduced graphene oxide/nanopolypyrrole composite: characterization and H 2 O 2 determination in urine. RSC Adv. 2014, 4, 12457–12466. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, F.; Ching, C.B.; Duan, H. One-step electrochemical synthesis of PtNi nanoparticle-graphene nanocomposites for nonenzymatic amperometric glucose detection. ACS Appl. Mater. Interfaces 2011, 3, 3049–3057. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, T.K.; Sun, C.L.; Nien, Y.T.; Pu, N.W.; Ger, M.D. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Song, J.; Xu, L.; Zhou, C.; Xing, R.; Dai, Q.; Liu, D.; Song, H. Synthesis of graphene oxide based CuO nanoparticles composite electrode for highly enhanced nonenzymatic glucose detection. ACS Appl. Mater. Interfaces 2013, 5, 12928–12934. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Niedziolka-Jonsson, J.; Lesniewski, A.; Wang, Q.; Li, M.; Boukherroub, R.; Szunerits, S. Preparation of reduced graphene oxide–Ni(OH)2 composites by electrophoretic deposition: application for non-enzymatic glucose sensing. J. Mater. Chem. A 2014, 2, 5525–5533. [Google Scholar] [CrossRef]
- Dhara, K.; Ramachandran, T.; Nair, B.G.; Babu, T.S. Single step synthesis of Au–CuO nanoparticles decorated reduced graphene oxide for high performance disposable nonenzymatic glucose sensor. J. Electroanal. Chem. 2015, 743, 1–9. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Wang, G.; Li, G.; Deng, D.; Qu, L. One-pot synthesis of Mn3O4 nanoparticles decorated with nitrogen-doped reduced graphene oxide for sensitive nonenzymatic glucose sensing. J. Electroanal. Chem. 2015, 755, 15–21. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Wen, C.; Xie, Y.; Miao, L.; Chen, S.; Li, H.; Li, P.; Song, Y. One-step synthesis of Pt–NiO nanoplate array/reduced graphene oxide nanocomposites for nonenzymatic glucose sensing. J. Mater. Chem. A 2015, 3, 608–616. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Yu, X.; Ma, D.; Zheng, J.; Dou, P.; Cao, Z.; Xu, X. Ag–Pt hollow nanoparticles anchored reduced graphene oxide composites for non-enzymatic glucose biosensor. J. Mater. Sci. Mater. Electron. 2016, 27, 9370–9378. [Google Scholar] [CrossRef]
- Rengaraj, A.; Haldorai, Y.; Kwak, C.H.; Ahn, S.; Jeon, K.J.; Park, S.H.; Han, Y.K.; Huh, Y.S. Electrodeposition of flower-like nickel oxide on CVD-grown graphene to develop an electrochemical non-enzymatic biosensor. J. Mater. Chem. B 2015, 3, 6301–6309. [Google Scholar] [CrossRef]
- Lakshmi, G.; Sharma, A.; Solanki, P.R.; Avasthi, D. Mesoporous polyaniline nanofiber decorated graphene micro-flowers for enzyme-less cholesterol biosensors. Nanotechnology 2016, 27, 345101. [Google Scholar] [CrossRef]
- Agnihotri, N.; Chowdhury, A.D.; De, A. Non-enzymatic electrochemical detection of cholesterol using β-cyclodextrin functionalized graphene. Biosens. Bioelectron. 2015, 63, 212–217. [Google Scholar] [CrossRef]
- Alexander, S.; Baraneedharan, P.; Balasubrahmanyan, S.; Ramaprabhu, S. Modified graphene based molecular imprinted polymer for electrochemical non-enzymatic cholesterol biosensor. Eur. Polym. J. 2017, 86, 106–116. [Google Scholar] [CrossRef]
- Saeed, A.A.; Sánchez, J.L.A.; O’Sullivan, C.K.; Abbas, M.N. DNA biosensors based on gold nanoparticles- modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Bioelectrochemistry 2017, 118, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Tang, H.; Gao, Z.; He, S.; Li, J.; Han, S. Ultrasensitive electrochemical detection of tumor cells based on multiple layer CdS quantum dots-functionalized polystyrene microspheres and graphene oxide–polyaniline composite. Biosens. Bioelectron. 2018, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sens. Actuators Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Zhu, Q.; Chai, Y.; Zhuo, Y.; Yuan, R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin–streptavidin signal amplification strategy. Biosens. Bioelectron. 2015, 68, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Wu, D.; Li, Y.; Ma, H.; Ni, Z.; Yu, H.; Luo, C.; Wei, Q.; Du, B. Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal. Biochem. 2012, 422, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Javadi, A.; Gong, S. Sensitive electrochemical immunosensor for the detection of cancer biomarker using quantum dot functionalized graphene sheets as labels. Sens. Actuators Chem. 2011, 155, 357–360. [Google Scholar] [CrossRef]
- Lu, J.; Liu, S.; Ge, S.; Yan, M.; Yu, J.; Hu, X. Ultrasensitive electrochemical immunosensor based on Au nanoparticles dotted carbon nanotube–graphene composite and functionalized mesoporous materials. Biosens. Bioelectron. 2012, 33, 29–35. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef]
- Song, C.; Xie, G.; Wang, L.; Liu, L.; Tian, G.; Xiang, H. DNA-based hybridization chain reaction for an ultrasensitive cancer marker EBNA-1 electrochemical immunosensor. Biosens. Bioelectron. 2014, 58, 68–74. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Choi, J.W. 3D label-free prostate specific antigen (PSA) immunosensor based on graphene–gold composites. Biosens. Bioelectron. 2015, 63, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Mondal, K.; Jiao, Y.; Oren, S.; Xu, Z.; Sharma, A.; Dong, L. Microfluidic immuno-biochip for detection of breast cancer biomarkers using hierarchical composite of porous graphene and titanium dioxide nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 20570–20582. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Y.; Li, M.; Li, F.; Han, J.; Dong, Y.; Chen, Z.; Wang, P.; Liu, H.; Wei, Q. A novel sandwich-type electrochemical immunosensor for PSA detection based on PtCu bimetallic hybrid (2D/2D) rGO/g-C3N4. Biosens. Bioelectron. 2017, 91, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ma, H.; Zhang, T.; Zhang, Y.; Yan, T.; Du, B.; Wei, Q. Sulfur-doped graphene-based immunological biosensing platform for multianalysis of cancer biomarkers. ACS Appl. Mater. Interfaces 2017, 9, 37637–37644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Z.; Fan, L.; Guo, Y. Electrochemical prostate specific antigen aptasensor based on hemin functionalized graphene-conjugated palladium nanocomposites. Microchim. Acta 2018, 185, 159. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Salimian, R. Ultrasensitive detection of cancer biomarkers using conducting polymer/electrochemically reduced graphene oxide-based biosensor: Application toward BRCA1 sensing. Sens. Actuators Chem. 2018, 266, 160–169. [Google Scholar] [CrossRef]
- Eda, G.; Lin, Y.Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.A.; Chen, I.S.; Chen, C.W.; Chhowalla, M. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef]
- Chien, C.T.; Li, S.S.; Lai, W.J.; Yeh, Y.C.; Chen, H.A.; Chen, I.S.; Chen, L.C.; Chen, K.H.; Nemoto, T.; Isoda, S.; et al. Tunable Photoluminescence from Graphene Oxide. Angew. Chem. Int. Ed. 2012, 51, 6662–6666. [Google Scholar] [CrossRef]
- Mei, Q.; Chen, J.; Zhao, J.; Yang, L.; Liu, B.; Liu, R.; Zhang, Z. Atomic Oxygen Tailored Graphene Oxide Nanosheets Emissions for Multicolor Cellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 7390–7395. [Google Scholar] [CrossRef]
- Cheng, S.J.; Chiu, H.Y.; Kumar, P.V.; Hsieh, K.Y.; Yang, J.W.; Lin, Y.R.; Shen, Y.C.; Chen, G.Y. Simultaneous drug delivery and cellular imaging using graphene oxide. Biomater. Sci. 2018, 6, 813–819. [Google Scholar] [CrossRef]
- Yang, J.W.; Tseng, M.L.; Fu, Y.M.; Kang, C.H.; Cheng, Y.T.; Kuo, P.H.; Tzeng, C.K.; Chiou, S.H.; Wu, C.Y.; Chen, G.Y. Printable Graphene Oxide Micropatterns for a Bio-Subretinal Chip. Adv. Healthc. Mater. 2018, 7, 1800365. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Hsieh, K.Y.; Kumar, P.V.; Cheng, S.J.; Lin, Y.R.; Shen, Y.C.; Chen, G.Y. Enhanced Osteogenic Differentiation of Stem Cells on Phase-Engineered Graphene Oxide. ACS Appl. Mater. Interfaces 2018, 10, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Cheon, D.; Liu, F.; Lee, K.; Seo, T. A Graphene Oxide Based Immuno-biosensor for Pathogen Detection. Angew. Chem. Int. Ed. 2010, 49, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Cheng, D.; Song, Y.; Jiang, M.; Yu, J.; Wang, Y. A graphene oxide-based FRET sensor for rapid and sensitive detection of matrix metalloproteinase 2 in human serum sample. Biosens. Bioelectron. 2013, 47, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, J.; Li, L.; Ren, L.; Zhang, X.; Chaudhuri, J.; Wang, S. A reduced graphene oxide based electrochemical biosensor for tyrosine detection. Nanotechnology 2012, 23, 335707. [Google Scholar] [CrossRef]
- Zhang, N.; Hou, J.; Chen, S.; Xiong, C.; Liu, H.; Jin, Y.; Wang, J.; He, Q.; Zhao, R.; Nie, Z. Rapidly Probing Antibacterial Activity of Graphene Oxide by Mass Spectrometry-based Metabolite Fingerprinting. Sci. Rep. 2016, 6, 28045. [Google Scholar] [CrossRef]
- Kim, Y.K.; Na, H.K.; Kwack, S.J.; Ryoo, S.R.; Lee, Y.; Hong, S.; Hong, S.; Jeong, Y.; Min, D.H. Synergistic Effect of Graphene Oxide/MWCNT Films in Laser Desorption/Ionization Mass Spectrometry of Small Molecules and Tissue Imaging. ACS Nano 2011, 5, 4550–4561. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, T.H.; Zhang, Z.; Azizi, E.; Pham, T.M.; Paoletti, C.; Lin, J.; Ramnath, N.; Wicha, M.S.; Hayes, D.F.; et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat. Nanotechnol. 2013, 8, 735–741. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, X.; Dai, H. Chemical self-assembly of graphene sheets. Nano Res. 2009, 2, 336–342. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shanker, A.; Wang, Y.; Kozminsky, M.; Jin, Q.; Palanisamy, N.; Burness, M.L.; Azizi, E.; Simeone, D.M.; Wicha, M.S.; et al. Tunable Thermal-Sensitive Polymer–Graphene Oxide Composite for Efficient Capture and Release of Viable Circulating Tumor Cells. Adv. Mater. 2016, 28, 4891–4897. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Li, Z.; Theile, C.S.; Bardhan, N.M.; Kumar, P.V.; Duarte, J.N.; Maruyama, T.; Rashidfarrokh, A.; Belcher, A.M.; Ploegh, H.L. Graphene Oxide Nanosheets Modified with Single-Domain Antibodies for Rapid and Efficient Capture of Cells. Chem. Eur. J. 2015, 21, 17178–17183. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity study of polyethylene glycol derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
- Viraka Nellore, B.P.; Kanchanapally, R.; Pramanik, A.; Sinha, S.S.; Chavva, S.R.; Hamme, A.; Ray, P.C. Aptamer-Conjugated Graphene Oxide Membranes for Highly Efficient Capture and Accurate Identification of Multiple Types of Circulating Tumor Cells. Bioconjugate Chem. 2015, 26, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, Y.; Zhang, X.; Yu, J.; Li, Y.; Yang, X.; Dai, Z.; Li, M. The clinical evaluation of Iressa first-line treatment of senium advanced-stage non-small cell lung cancer. Chin. Ger. J. Clin. Oncol. 2008, 7, 203–206. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X.M. Gold nanoclusters and graphene nanocomposites for drug delivery and imaging of cancer cells. Angew. Chem. Int. Ed. 2011, 50, 11644–11648. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.A.L.; Wang, J.; Loh, K.P. Graphene-based SELDI probe with ultrahigh extraction and sensitivity for DNA oligomer. J. Am. Chem. Soc. 2010, 132, 10976–10977. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Cheng, J.; Li, J.; Wang, Y. Graphene as a novel matrix for the analysis of small molecules by MALDI-TOF MS. Anal. Chem. 2010, 82, 6208–6214. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, Y.; He, Q.; Boey, F.; Zhang, Q.; Zhang, H. Reduced graphene oxide films used as matrix of MALDI-TOF-MS for detection of octachlorodibenzo-p-dioxin. Chem. Commun. 2010, 46, 6974–6976. [Google Scholar] [CrossRef] [PubMed]
- Colli, A. Transparent Photodetector for Mobile Devices. U.S. Patent 9,130,085, 8 September 2015. [Google Scholar]
- Zhamu, A.; Shi, J.; Chen, G.; Fang, Q.; Jang, B.Z. Graphene-Enhanced Anode Particulates for Lithium Ion Batteries. U.S. Patent 9,558,860, 31 January 2017. [Google Scholar]
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Möbius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef]
| Sensing Electrode | Detected Element | Detection Range | Detection Limit | Reference |
|---|---|---|---|---|
| P-L-His–rGO | HO | 0.2 M to 5 mM | 0.05 M | [59] |
| rGO | HO | 1.5–28.5 M | 0.5 M | [58] |
| CeO–rGO | HO | 0.1–500 M | 0.021 M | [60] |
| rGO–ZnO | Glucose | 0.2–6.6 mM | 0.2 mM | [68] |
| rGO–AgNPs | Glucose | 0.5–12.5 mM | 0.16 mM | [89] |
| AuNPs–GR–CNTs | Glucose | 10 mM to 2 mM | 4.1 mM | [90] |
| rGO–cyclodextrin | Glucose | 50 M to 3.0 mM | 59.74 mM | [91] |
| PANI-modifed SnO–rGO | Glucose | 0.1 nM to 5 mM | 0.26 nM | [92] |
| FeO–rGO | Glucose | 50 M to 1 mM | 0.1 M | [69] |
| rGO–Fullerene–C60 | Glucose | 100 uM to 12.5 mM | 35 M | [93] |
| ZrO–rGO | Glucose | 14–290 M | 130 M | [70] |
| AuNPs–ErGO–PAH | NADH | 0.01 to 5 mM | 3.5 M | [94] |
| Au–AgNPs–P(L-Cys)–ErGO | NADH | 0.017 to 1.84 M | 5 M | [95] |
| rGO | NADH | 0–500 M | 0.6 M | [96] |
| Chitosan–GO | DNA | 10 fM to 50 nM | 10 fM | [97] |
| GR–ErGO | DNA | 10 pM to 0.1 M | 0.15 fM | [98] |
| AuNPs–rGO | DNA | 0.1 fM to 0.1 M | 35 aM | [99] |
| PPy–grGO | Cholesterol | 0.01 to 6 mM | 3.78 M | [84] |
| Chitosan–GR | Cholesterol | 0.005 to 1 mM | 17.39 M | [100] |
| PtNPs–GR | Cholesterol | 0.035 to 12 mM | 0.2 M | [86] |
| Pd-Pt NPs–GR | Cholesterol | 2.2 M to 0.52 mM | 0.75 mM | [101] |
| GR–PVP–PANI | Cholesterol | 50 M to 10 mM | 1 M | [102] |
| rGO–dendritic Pd | Cholesterol | 0.005–0.014 mM | 0.05 M | [85] |
| CS–GR | Cholesterol | 0.005–1.0 mM | 0.715 M | [100] |
| TiO nanowires–3D GR | Cholesterol | 0.05–8.0 mM | 6 M | [103] |
| Sensing Electrode | Detected Element | Detection Range | Detection Limit | Reference |
|---|---|---|---|---|
| PtNPs–MnO–rGO | HO | 2 M to 133 mM | 1 M | [107] |
| rGO–tyrosine | HO | 0.1–2.1 mM | 80 M | [108] |
| Ni(OH)–rGO–MWCNTs | HO | 10–9050 M | 4 M | [109] |
| rGO–nPPY | HO | 1–4 M | 34 nM | [110] |
| GR–PtNiNPs | Glucose | 0.5–35 mM | 10 M | [111] |
| GR–CuO NPs | Glucose | 1 M to 8 mM | 1 M | [112] |
| GO–CuONPs | Glucose | 2.79 M to 2.03 mM | 0.69 M | [113] |
| rGO–Ni(OH) | Glucose | 15 M to 30 mM | 15 M | [114] |
| rGO–Au-CuO NPs | Glucose | 1 M to 12 mM | 0.01 M | [115] |
| N-rGO–MnO NPs | Glucose | 1.0–329.5 M | 0.5 M | [116] |
| rGO–Pt–NiO | Glucose | 0.008–14.5 mM | 2.67 M | [117] |
| AgPt–rGO | Glucose | 0.003–7.72 mM | 1.8 M | [118] |
| NiO–CVD-GR | Cholesterol | 2–40 M | 0.13 M | [119] |
| PANInf–GMF | Cholesterol | 1.93 to 464.04 mg dL | 1.93 mg dL | [120] |
| GR–-CD | Cholesterol | 1–100 M | 1 M | [121] |
| GO–MIP | Cholesterol | 0.1 M–1 nM | 0.1 nM | [122] |
| Sensing Electrode | Detected Element | Detection Range | Detection Limit | Reference |
|---|---|---|---|---|
| GR–CS–AuNPs | CEA | 0.5–60 ng mL | 0.1 ng mL | [130] |
| GR–MWCNTs–CS–AuNPs | EBNA-1 | 0.05–6.4 ng mL | 0.7 pg·mL | [131] |
| GR–AuNPs | PSA | 0–10 ng mL | 0.59 ng mL | [132] |
| GR–TiO | ErbB2 | 1 fM–0.1 M | 0.06 ng mL | [133] |
| PtCu@rGO–g-CN | PSA | 50 fg mL–40 ng mL | 16.6 fg mL | [134] |
| AuNPs–GO | ErbB2 | 0.37–10 nM mL | 0.16 nM | [123] |
| S-doped GR–PANI | CEA | 0.1 pg·mL to 0.3 ng mL | 30 fg mL | [135] |
| CdS QDs@PS–GO–PANI | K562 cells | 10 ×10 cells per mL | 3 cells per mL | [124] |
| Hemin-GR–PdNPs | PSA | 0.025–205 ng mL | 8 pg·mL | [136] |
| PPy–ErGO | BRCA1 gene | 10 fM to 0.1 M | 3fM | [137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangamuthu, M.; Hsieh, K.Y.; Kumar, P.V.; Chen, G.-Y. Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications. Int. J. Mol. Sci. 2019, 20, 2975. https://doi.org/10.3390/ijms20122975
Thangamuthu M, Hsieh KY, Kumar PV, Chen G-Y. Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications. International Journal of Molecular Sciences. 2019; 20(12):2975. https://doi.org/10.3390/ijms20122975
Chicago/Turabian StyleThangamuthu, Madasamy, Kuan Yu Hsieh, Priyank V. Kumar, and Guan-Yu Chen. 2019. "Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications" International Journal of Molecular Sciences 20, no. 12: 2975. https://doi.org/10.3390/ijms20122975
APA StyleThangamuthu, M., Hsieh, K. Y., Kumar, P. V., & Chen, G.-Y. (2019). Graphene- and Graphene Oxide-Based Nanocomposite Platforms for Electrochemical Biosensing Applications. International Journal of Molecular Sciences, 20(12), 2975. https://doi.org/10.3390/ijms20122975





