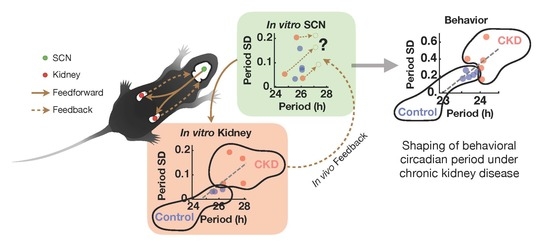
The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock
Abstract
1. Introduction
2. Results
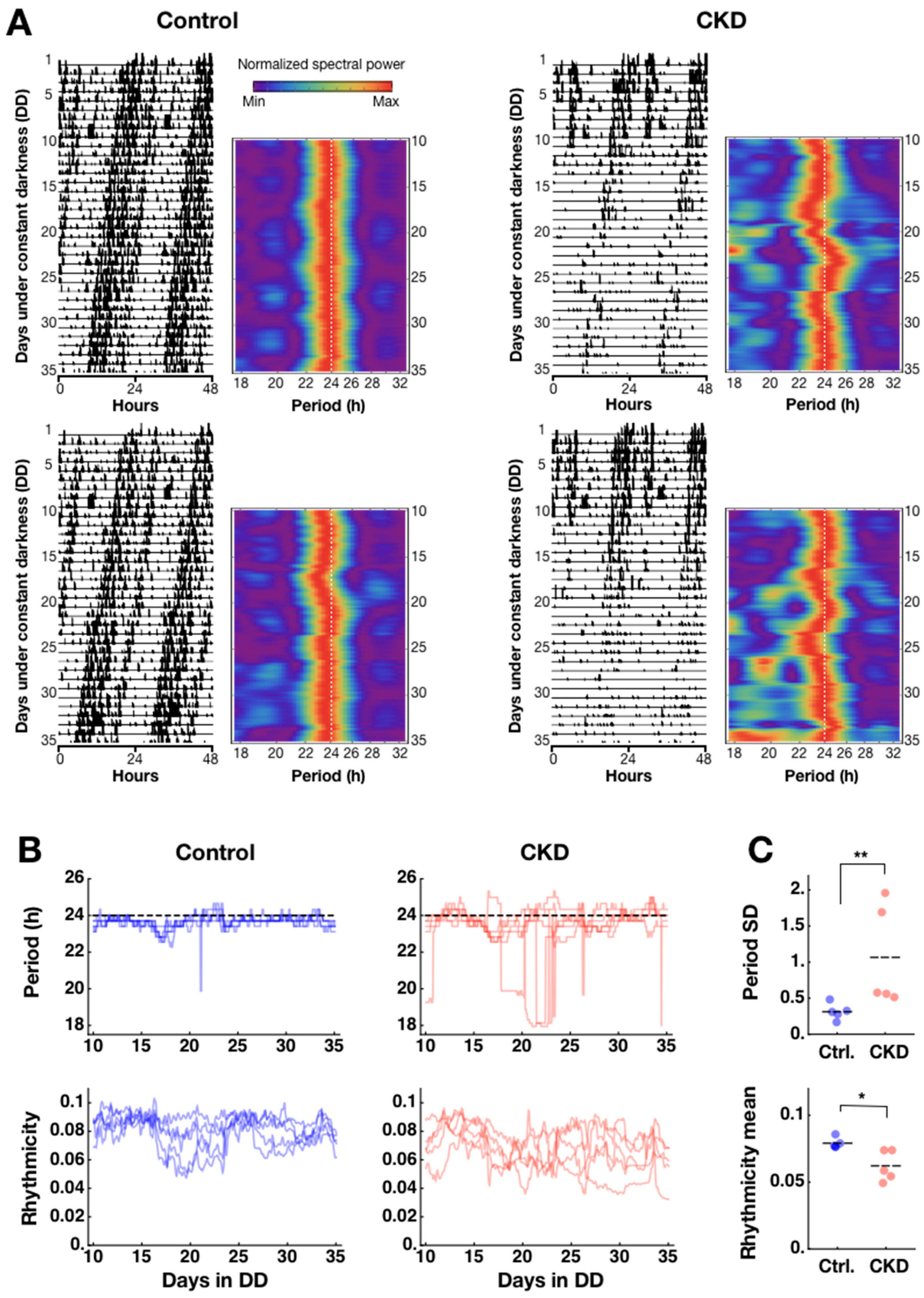
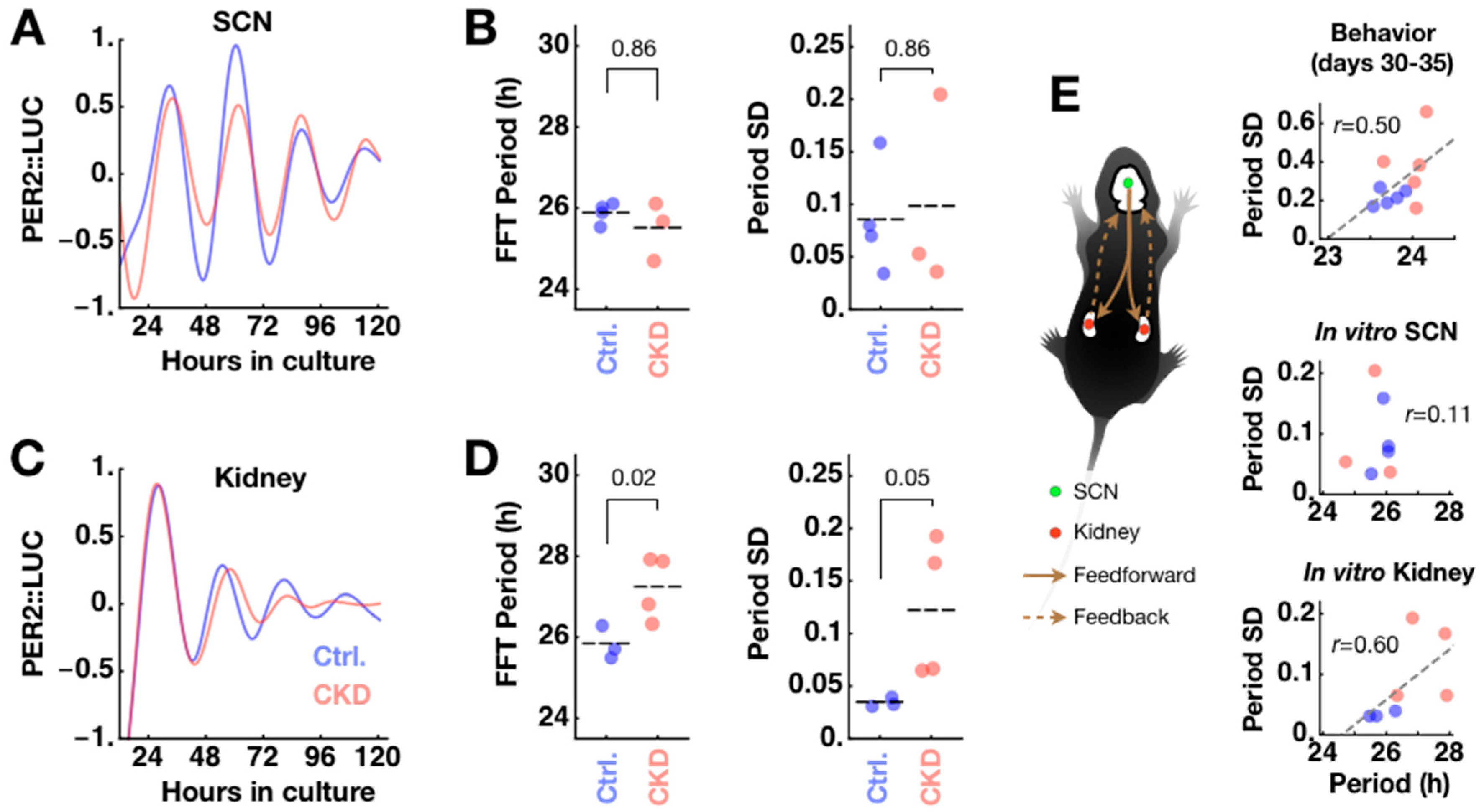
2.1. Weak and Disorganized Circadian Rhythmicity in CKD Mice
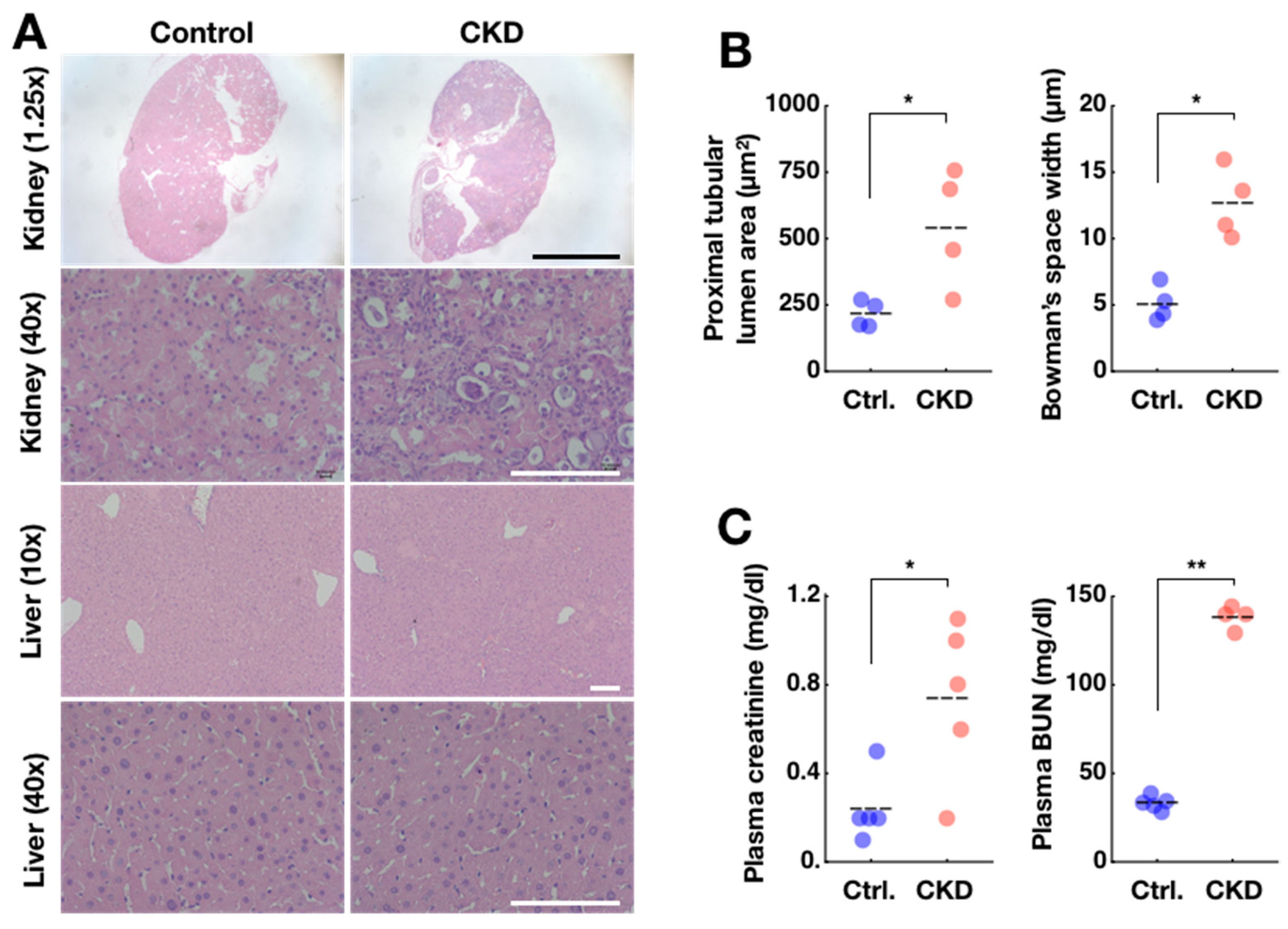
2.2. Confirmation of Adenine-Induced CKD Model
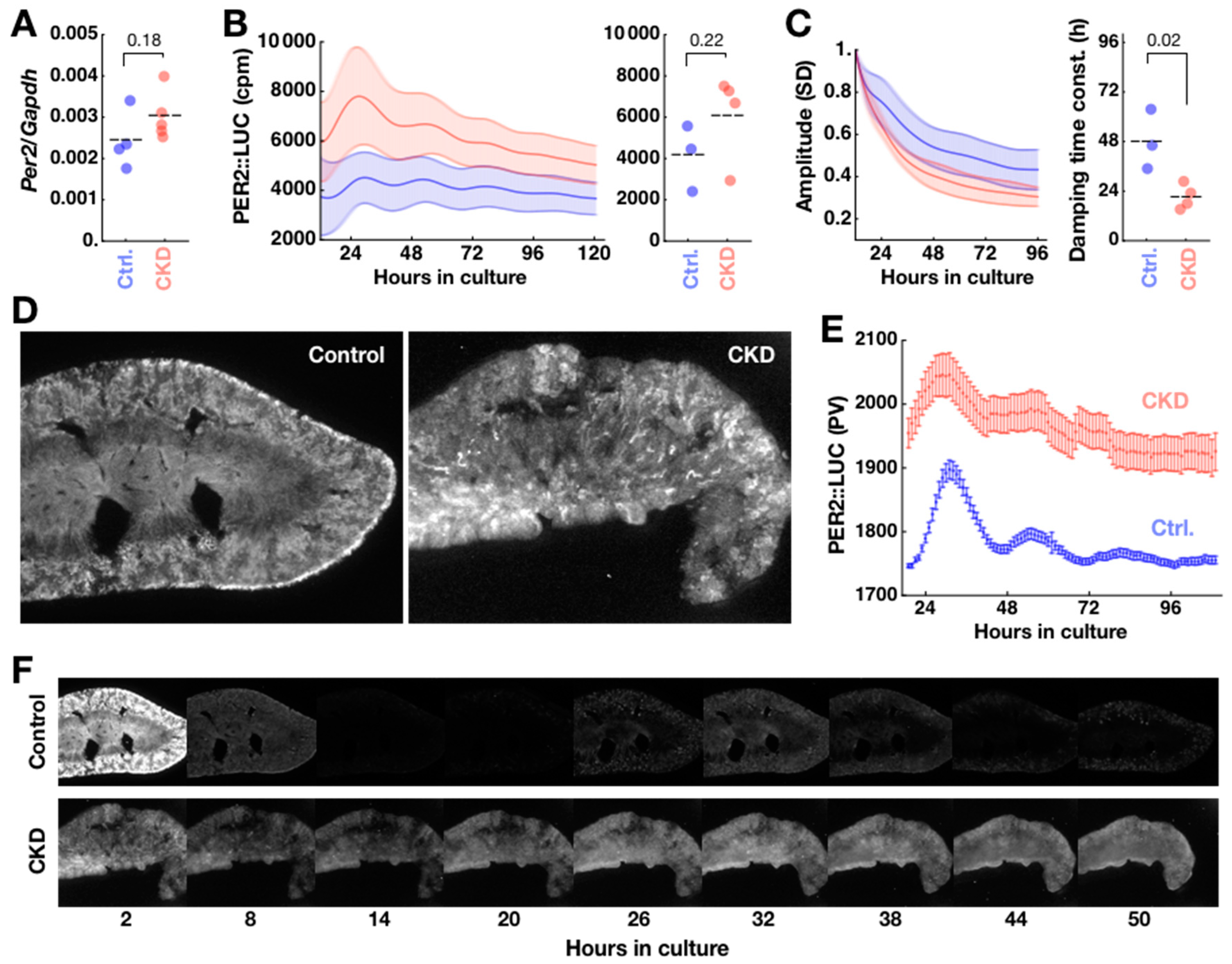
2.3. Disorganization of PER2 Expression in CKD Kidney
2.4. CKD Alters Kidney Clock but not SCN Clock
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Locomotor Activity Measurement
4.3. Spectral Analysis of Locomotor Activities and Circadian Heatmap
4.4. Thin-Section Micrographs and Histological Analysis
4.5. Sampling and Analysis of Blood Serum
4.6. Total RNA Isolation
4.7. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.8. Explant Culture
4.9. Bioluminescence Imaging and Luminometry
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCN | suprachiasmatic nucleus |
| CP | choroid plexus |
| ESRD | end-stage renal disease |
| CKD | chronic kidney disease |
| FFT | Fast Fourier Transform |
| PER2 | PERIOD2 |
| WT | wild type |
| CSF | cerebrospinal fluid |
| CT | circadian time |
References
- Moore, R.Y.; Reppert, S.M. Suprachiasmatic Nucleus: The Mind’s Clock; Klein, D.C., Moore, R.Y., Reppert, S.M., Eds.; Oxford University Press: New York, NY, USA, 1991; ISBN 978-0-19-506250-2. [Google Scholar]
- Silver, R.; LeSauter, J.; Tresco, P.A.; Lehman, M.N. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 1996, 382, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.K.; Logothetis, D.E.; Meister, M.; Reppert, S.M. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995, 14, 697–706. [Google Scholar] [CrossRef]
- Balsalobre, A.; Damiola, F.; Schibler, U. A Serum Shock Induces Circadian Gene Expression in Mammalian Tissue Culture Cells. Cell 1998, 93, 929–937. [Google Scholar] [CrossRef]
- Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Effects of aging on central and peripheral mammalian clocks. Proc. Natl. Acad. Sci. USA 2002, 99, 10801–10806. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [PubMed]
- Guilding, C.; Hughes, A.T.L.; Brown, T.M.; Namvar, S.; Piggins, H.D. A riot of rhythms: Neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol. Brain 2009, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Schmal, C.; Hong, S.; Tsukizawa, Y.; Rose, P.; Zhang, Y.; Holtzman, M.J.; De Schutter, E.; Herzel, H.; Bordyugov, G.; et al. The choroid plexus is an important circadian clock component. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.W.; Takao, M.; Berson, D.M.; Yau, K.W. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Antle, M.C.; Foley, D.K.; Foley, N.C.; Silver, R. Gates and Oscillators: A Network Model of the Brain Clock. J. Biol. Rhythm. 2003, 18, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and Peripheral Circadian Clocks in Mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Stokkan, K.-A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the Circadian Clock in the Liver by Feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.G.; Pollock, D.M. Circadian regulation of renal function. Free. Radic. Biol. Med. 2018, 119, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Zuber, A.M.; Centeno, G.; Pradervand, S.; Nikolaeva, S.; Maquelin, L.; Cardinaux, L.; Bonny, O.; Firsov, D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. USA 2009, 106, 16523–16528. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Minami, Y.; Ohashi, M.; Tsuchiya, Y.; Kusaba, T.; Tamagaki, K.; Koike, N.; Umemura, Y.; Inokawa, H.; Yagita, K. Robust Circadian Clock Oscillation and Osmotic Rhythms in Inner Medulla Reflecting Cortico-Medullary Osmotic Gradient Rhythm in Rodent Kidney. Sci. Rep. 2017, 7, 7306. [Google Scholar] [CrossRef]
- Nishi, E.E.; Bergamaschi, C.T.; Campos, R.R. The crosstalk between the kidney and the central nervous system: The role of renal nerves in blood pressure regulation: Crosstalk between the kidney and the central nervous system. Exp. Physiol. 2015, 100, 479–484. [Google Scholar] [CrossRef]
- Stow, L.R.; Gumz, M.L. The Circadian Clock in the Kidney. J. Am. Soc. Nephrol. 2011, 22, 598–604. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chang, F.C.; Ng, H.Y.; Kuo, C.C.; Lee, Y.T.; Lu, C.Y.; Lee, C.T. Disrupted circadian rhythm in rats with nephrectomy-induced chronic kidney disease. Life Sci. 2012, 91, 127–131. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Za’abi, M.; Waly, M.I.; Ramkumar, A.; Beegam, S.; Al-Lawati, I.; Adham, S.A.; Nemmar, A. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: Comparison with rats. J. Pharmacol. Toxicol. Methods 2013, 68, 384–393. [Google Scholar] [CrossRef]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats: Models of kidney diseases. Nephrology 2018, 23, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Hong, S.; Hatanaka, F.; Nakajima, Y.; De Schutter, E.; Takumi, T. Period Coding of Bmal1 Oscillators in the Suprachiasmatic Nucleus. J. Neurosci. 2012, 32, 8900–8918. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.T.L.; Croft, C.L.; Samuels, R.E.; Myung, J.; Takumi, T.; Piggins, H.D. Constant Light Enhances Synchrony among Circadian Clock Cells and Promotes Behavioral Rhythms in VPAC2-Signaling Deficient Mice. Sci. Rep. 2015, 5, 14044. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; De la Garza, M.; Fang, R.C.; Hong, S.J.; Galiano, R.D. Excisional Wound Healing Is Delayed in a Murine Model of Chronic Kidney Disease. PLoS ONE 2013, 8, e59979. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Hong, S.; DeWoskin, D.; De Schutter, E.; Forger, D.B.; Takumi, T. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proc. Natl. Acad. Sci. USA 2015, 112, E3920–E3929. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Pauls, S.D. Encoding seasonal information in a two-oscillator model of the multi-oscillator circadian clock. Eur. J. Neurosci. 2018, 48, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.C.P.; Nagtegaal, J.E.; Kerkhof, G.A.; ter Wee, P.M. Circadian sleep–wake rhythm disturbances in end-stage renal disease. Nat. Rev. Nephrol. 2009, 5, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.M.; Chan, C.T.; Bradley, T.D. Altered sleep structure in patients with end-stage renal disease. Sleep Med. 2016, 20, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hanly, P.J.; Ahmed, S.B. Sleep Apnea and the Kidney. Chest 2014, 146, 1114–1122. [Google Scholar] [CrossRef]
- Goto, N.; Uchida, K.; Morozumi, K.; Ueki, T.; Matsuoka, S.; Katayama, A.; Haba, T.; Tominaga, Y.; Fukuda, M.; Nakao, A.; et al. Circadian Blood Pressure Rhythm Is Disturbed by Nephrectomy. Hypertens. Res. 2005, 28, 301–306. [Google Scholar] [CrossRef]
- Rubinger, D.; Sapoznikov, D.; Pollak, A.; Popovtzer, M.M.; Luria, M.H. Heart rate variability during chronic hemodialysis and after renal transplantation: Studies in patients without and with systemic amyloidosis. J. Am. Soc. Nephrol. 1999, 10, 1972–1981. [Google Scholar] [PubMed]
- Dos Santos, I.F.; Sheriff, S.; Amlal, S.; Ahmed, R.P.H.; Thakar, C.V.; Amlal, H. Adenine acts in the kidney as a signaling factor and causes salt- and water-losing nephropathy: Early mechanism of adenine-induced renal injury. Am. J. Physiol. Ren. Physiol. 2019, 316, F743–F757. [Google Scholar] [CrossRef] [PubMed]
- Pett, J.P.; Kondoff, M.; Bordyugov, G.; Kramer, A.; Herzel, H. Co-existing feedback loops generate tissue-specific circadian rhythms. Life Sci. Alliance 2018, 1, e201800078. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.S.; Emoto, N.; Nonaka, H.; Okura, R.; Nishimura, M.; Yagita, K.; Van Der Horst, G.T.J.; Matsuo, M.; Okamura, H.; Yokoyama, M. Circadian clock genes directly regulate expression of the Na+/H+ exchanger NHE3 in the kidney. Kidney Int. 2005, 67, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L.; Stow, L.R.; Lynch, I.J.; Greenlee, M.M.; Rudin, A.; Cain, B.D.; Weaver, D.R.; Wingo, C.S. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Investig. 2009, 119, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Tokonami, N.; Mordasini, D.; Pradervand, S.; Centeno, G.; Jouffe, C.; Maillard, M.; Bonny, O.; Gachon, F.; Gomez, R.A.; Sequeira-Lopez, M.L.S.; et al. Local Renal Circadian Clocks Control Fluid–Electrolyte Homeostasis and BP. J. Am. Soc. Nephrol. 2014, 25, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Merlino, G.; Piani, A.; Dolso, P.; Adorati, M.; Cancelli, I.; Valente, M.; Gigli, G.L. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol. Dial. Transpl. 2006, 21, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, A.; Butler, R.; Godinho, S.I.H.; Couch, Y.; Brown, L.A.; Vasudevan, S.R.; Flanagan, K.C.; Anthony, D.; Churchill, G.C.; Wood, M.J.A.; et al. The CRTC1-SIK1 Pathway Regulates Entrainment of the Circadian Clock. Cell 2013, 154, 1100–1111. [Google Scholar] [CrossRef]
- Hayasaka, N.; Hirano, A.; Miyoshi, Y.; Tokuda, I.T.; Yoshitane, H.; Matsuda, J.; Fukada, Y. Salt-inducible kinase 3 regulates the mammalian circadian clock by destabilizing PER2 protein. eLife 2017, 6. [Google Scholar] [CrossRef]
- Greco, C.M.; Sassone–Corsi, P. Circadian blueprint of metabolic pathways in the brain. Nat. Rev. Neurosci. 2019, 20, 71–82. [Google Scholar] [CrossRef]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting Central and Peripheral Circadian Oscillators in Transgenic Rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Pezuk, P.; Mohawk, J.A.; Yoshikawa, T.; Sellix, M.T.; Menaker, M. Circadian Organization Is Governed by Extra-SCN Pacemakers. J. Biol. Rhythm. 2010, 25, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.A.; Oudit, G.Y.; Herzenberg, A.M.; Tata, N.; Koletar, M.M.; Kabir, G.M.; Belsham, D.D.; Backx, P.H.; Ralph, M.R.; Sole, M.J. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1675–R1683. [Google Scholar] [CrossRef] [PubMed]
- Mescher, A.L.; Junqueira, L.C.U. Junqueira’s Basic Histology: Text and Atlas, 14th ed.; McGraw Hill Education: New York, NY, USA, 2016; ISBN 978-0-07-184270-9. [Google Scholar]
- Keppler, A.; Gretz, N.; Schmidt, R.; Kloetzer, H.M.; Groene, H.J.; Lelongt, B.; Meyer, M.; Sadick, M.; Pill, J. Plasma creatinine determination in mice and rats: An enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007, 71, 74–78. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myung, J.; Wu, M.-Y.; Lee, C.-Y.; Rahim, A.R.; Truong, V.H.; Wu, D.; Piggins, H.D.; Wu, M.-S. The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock. Int. J. Mol. Sci. 2019, 20, 2765. https://doi.org/10.3390/ijms20112765
Myung J, Wu M-Y, Lee C-Y, Rahim AR, Truong VH, Wu D, Piggins HD, Wu M-S. The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock. International Journal of Molecular Sciences. 2019; 20(11):2765. https://doi.org/10.3390/ijms20112765
Chicago/Turabian StyleMyung, Jihwan, Mei-Yi Wu, Chun-Ya Lee, Amalia Ridla Rahim, Vuong Hung Truong, Dean Wu, Hugh David Piggins, and Mai-Szu Wu. 2019. "The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock" International Journal of Molecular Sciences 20, no. 11: 2765. https://doi.org/10.3390/ijms20112765
APA StyleMyung, J., Wu, M.-Y., Lee, C.-Y., Rahim, A. R., Truong, V. H., Wu, D., Piggins, H. D., & Wu, M.-S. (2019). The Kidney Clock Contributes to Timekeeping by the Master Circadian Clock. International Journal of Molecular Sciences, 20(11), 2765. https://doi.org/10.3390/ijms20112765