Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus
Abstract
1. Introduction
2. Results
2.1. Early Life Stress Selectively Increases Anxiety-Like Behavior in rTgFKBP5 Mice
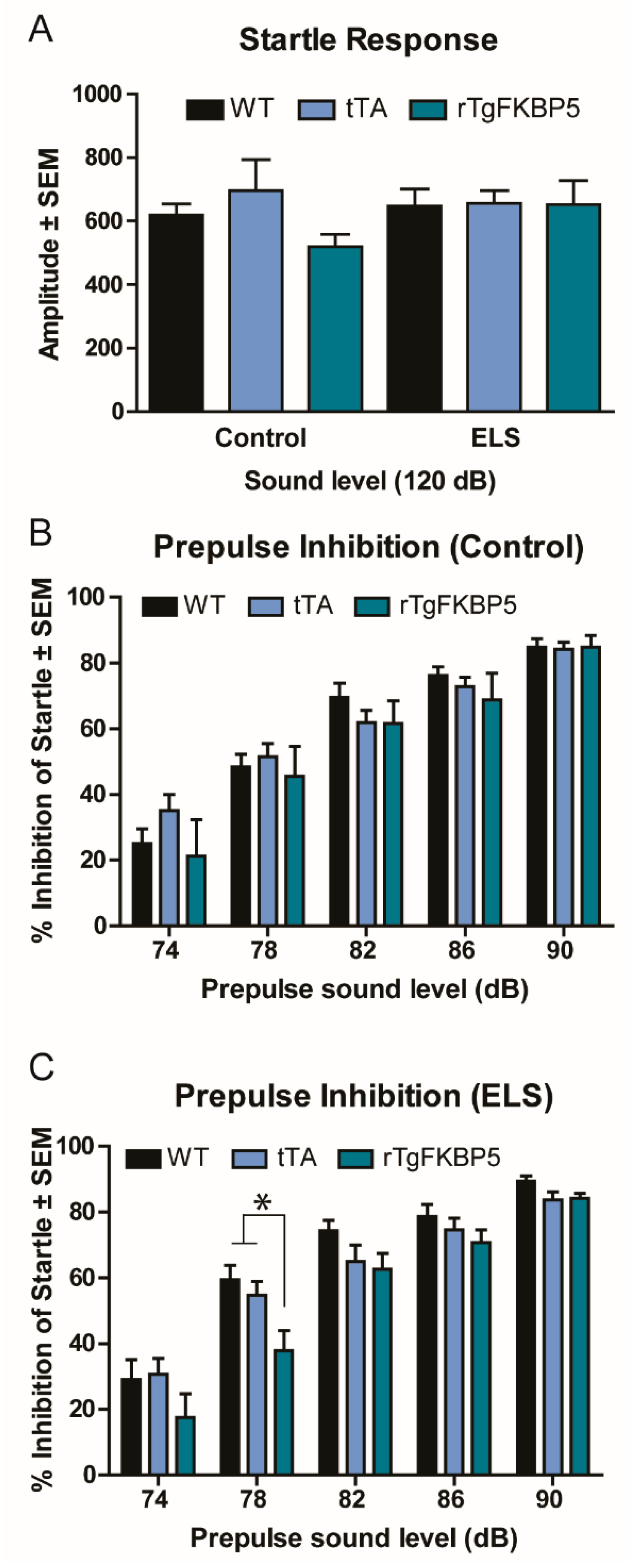
2.2. Sex and Genotype Modestly Affect the Prepulse Inhibition Response
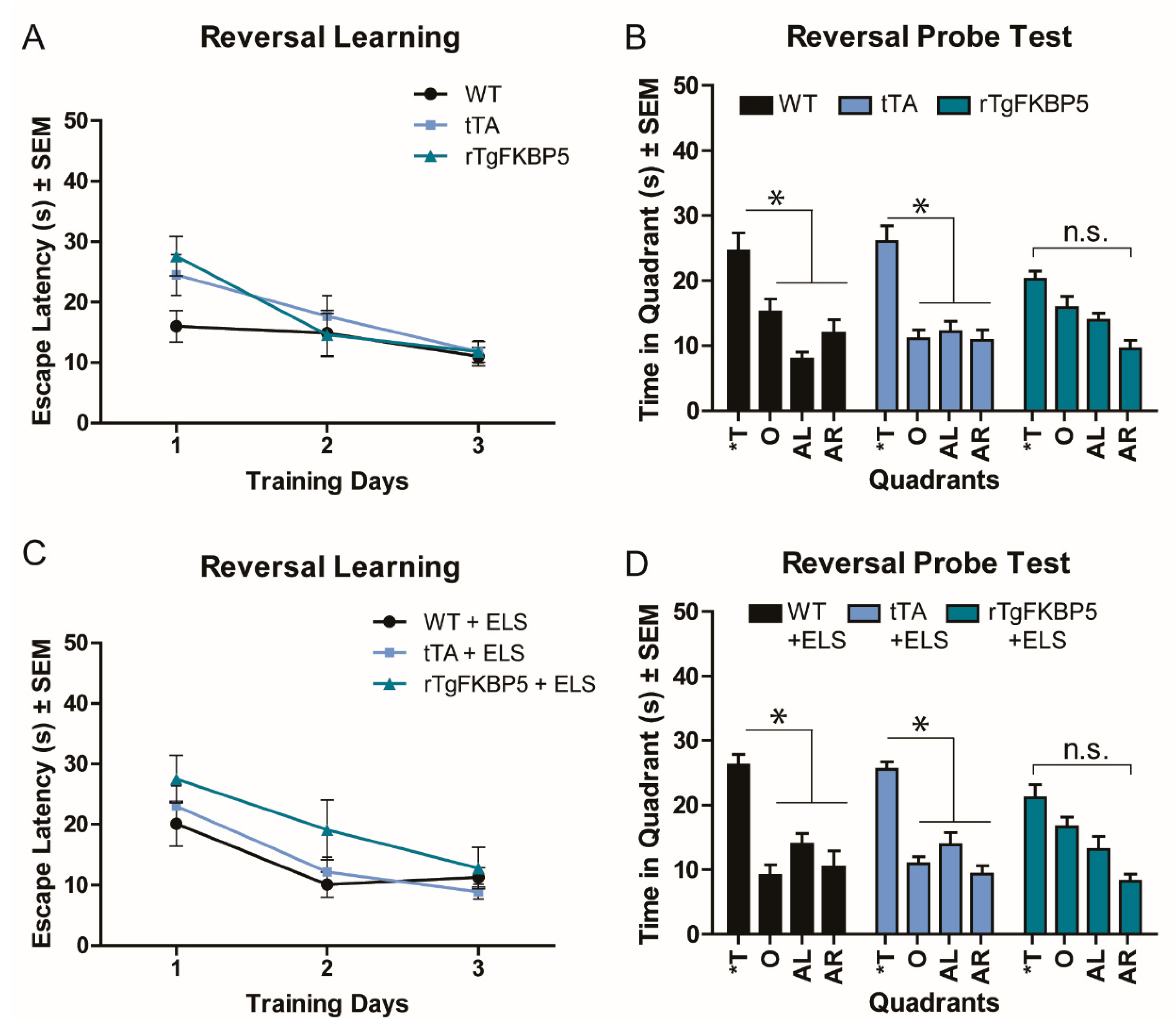
2.3. FKBP5 Induces Spatial Reversal Learning Deficits in the Morris Water Maze (MWM) Test
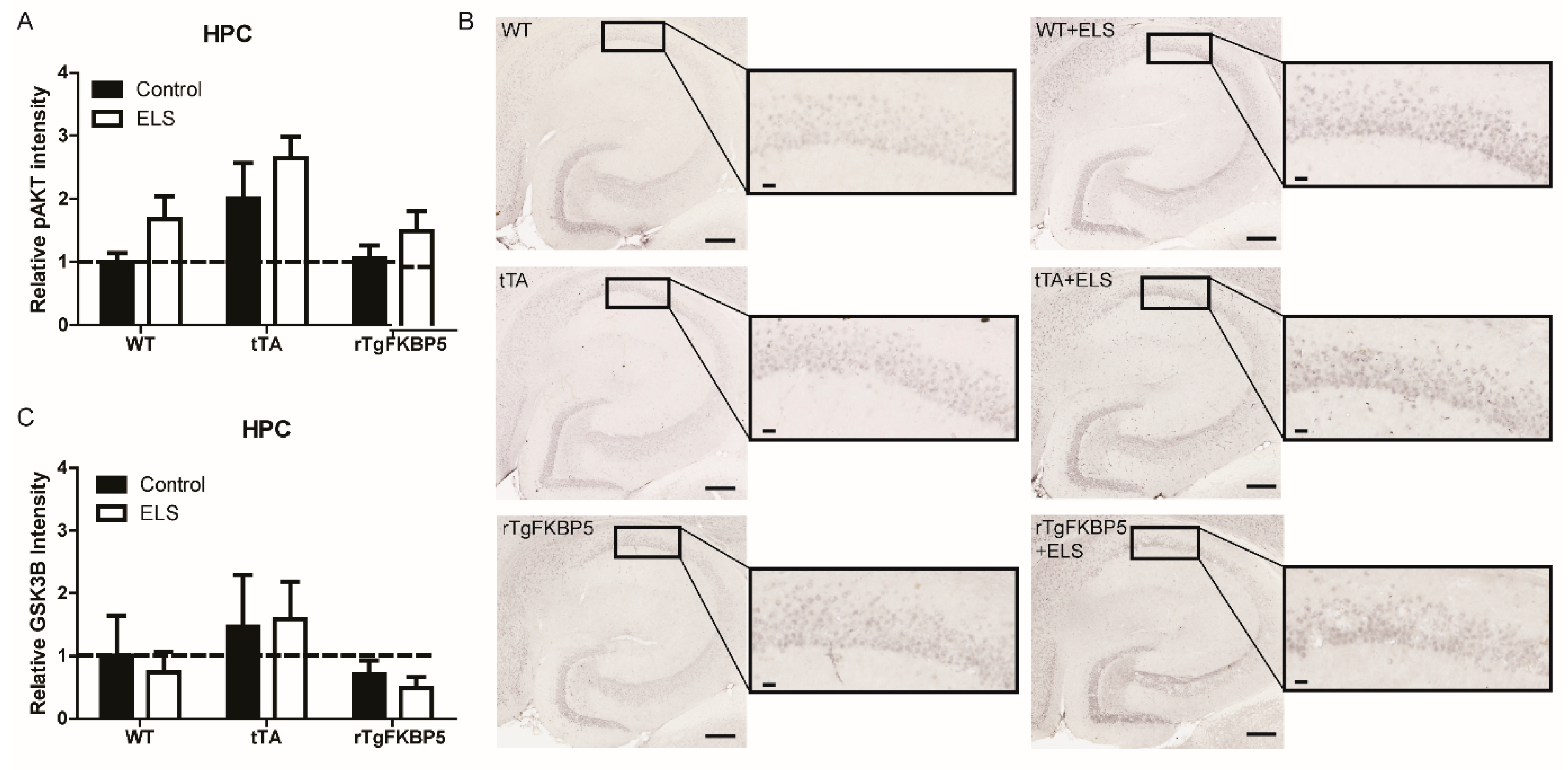
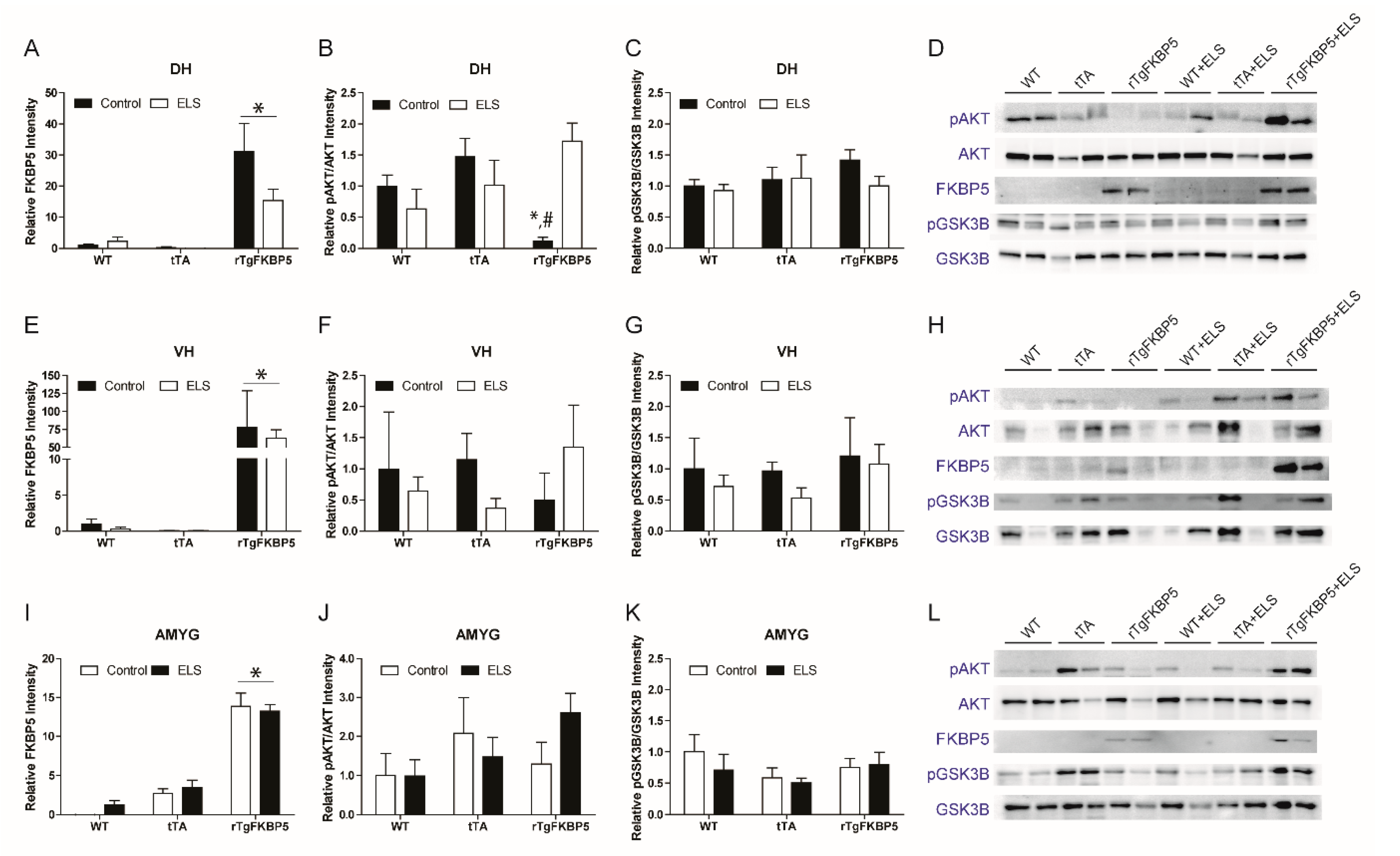
2.4. Early Life Stress Affects the pAKT/AKT Ratio in the Hippocampus
2.5. FKBP5 Differentially Regulates AKT Phosphorylation in the Dorsal Hippocampus in the Presence Or Absence of ELS
2.6. AKT and GSK-3β Expression in the VH and Amygdala (AMYG) is not Affected by the FKBP5 x ELS Interaction
3. Discussion
3.1. Sex as a Confounding Factor that Effects Stress Susceptibility
3.2. Early Life Stress Modestly Interacted with Genotype
3.3. Genotype Influences Learning Processes at the Behavioral and Molecular Levels
3.4. FKBP5 Overexpression Modulates AKT in Selective Brain Structures
3.5. Concluding Remarks
4. Materials and Methods
4.1. Animal Subjects
4.2. Early Life Stress (ELS) Model
4.3. Elevated Plus Maze (EPM)
4.4. Prepulse Inhibition (PPI)
4.5. Morris Water Maze (MWM)
4.6. Tissue Collection, IHC, and Western Blot
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AMYG | Amygdala |
| ANOVA | Analysis of variance |
| BLA and CeA | Basolateral and central nuclei of the amygdala |
| CAMKII | Ca2+/calmodulin-dependent protein kinase II |
| DH | Dorsal hippocampus |
| ELS | Early life stress |
| EPM | Elevated plus maze |
| FKBP5 | FK506-binding protein 5/FK506-binding protein 51 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GR | Glucocorticoid receptor |
| GSK-3β | Glycogen synthase kinase 3 |
| HPA | Hypothalamic-pituitary-adrenal axis |
| HPC | Hippocampus |
| MWM | Morris water maze |
| pAKTSer473 | Phosphorylated AKT at Serine 473 |
| pGSK-3βSer9 | Phosphorylated GSK-3β phosphorylation at Serine 9 |
| P1 | Postnatal Day 1 |
| P21 | Postnatal Day 2 |
| PPI | Prepulse inhibition |
| PTSD | Post-traumatic stress disorder |
| PVDF | Polyvinylidene fluoride or polyvinylidene difluoride |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SEM | Standard error of the mean |
| VH | Ventral hippocampus |
References
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Available online: https://www.samhsa.gov/data/ (accessed on 12 January 2019).
- Merikangas, K.R.; He, J.-P.; Burstein, M.; Swanson, S.A.; Avenevoli, S.; Cui, L.; Benjet, C.; Georgiades, K.; Swendsen, J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Avenevoli, S.; Jane Costello, E.; Georgiades, K.; Greif Green, J.; Gruber, M.J.; He, J.; Koretz, D.; McLaughlin, K.A.; Petukhova, M.; et al. Prevalence, Persistence, and Sociodemographic Correlates of DSM-IV Disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch. Gen. Psychiatry 2012, 69, 372–380. [Google Scholar] [PubMed]
- Green, J.G.; McLaughlin, K.A.; Berglund, P.A.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry 2010, 67, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Binder, E.B. Epigenetics of Stress-Related Psychiatric Disorders and Gene 3 Environment Interactions. Neuron 2015, 86, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Alhamzawi, A.O.; Alonso, J.; Angermeyer, M.; et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Kessler, R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication II: Associations with persistence of DSM-IV disorders. Arch. Gen. Psychiatry 2010, 67, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Keers, R.; Lester, K.J.; Coleman, J.R.; Breen, G.; Arendt, K.; Blatter-Meunier, J.; Cooper, P.; Creswell, C.; Fjermestad, K.; et al. HPA Axis Related Genes and Response to Psychological Therapies: Genetics and Epigenetics. Depress. Anxiety 2015, 32, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Golier, J.A.; Yang, R.-K.; Tischler, L.; Alonso, S.; Damas, C.; Navarra, E.; Altemus, M.; Cloitre, M.; Dhabhar, F.; et al. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol. Psychiatry 2004, 55, 1110–1116. [Google Scholar] [CrossRef]
- Hori, H.; Ozeki, Y.; Teraishi, T.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Suto, S.; Terada, S.; Higuchi, T.; Kunugi, H. Relationships between psychological distress, coping styles, and HPA axis reactivity in healthy adults. J. Psychiatr. Res. 2010, 44, 865–873. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Solomon, M.B.; Carvalho-Netto, E.; Myers, B. Neural regulation of the stress response: Glucocorticoid feedback mechanisms. Braz. J. Med. Biol. Res. 2012, 45, 292–298. [Google Scholar] [CrossRef]
- Arango-Lievano, M.; Jeanneteau, F. Timing and crosstalk of glucocorticoid signaling with cytokines, neurotransmitters and growth factors. Pharmacol. Res. 2016, 113, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Klengel, T.; Rubel, J.; Brückl, T.; Pfister, H.; Lucae, S.; Uhr, M.; Holsboer, F.; Binder, E.B. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes. Brain. Behav. 2013, 12, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Brückl, T.; Nocon, A.; Pfister, H.; Binder, E.B.; Uhr, M.; Lieb, R.; Moffitt, T.E.; Caspi, A.; Holsboer, F.; et al. Interaction of FKBP5 Gene Variants and Adverse Life Events in Predicting Depression Onset: Results From a 10-Year Prospective Community Study. Am. J. Psychiatry 2011, 168, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, S.; Ising, M.; Uhr, M.; Otto, Y.; von Klitzing, K.; Klein, A.M. FKBP5 polymorphisms moderate the influence of adverse life events on the risk of anxiety and depressive disorders in preschool children. J. Psychiatr. Res. 2016, 72, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-haffner, M.; Jens, C.; Pariante, C.M.; Pace, T.W.W.; Mercer, K.B.; Helen, S.; Ressler, K.J.; et al. Allele-specific FKBP5 DNA demethylation mediates gene– childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Criado-Marrero, M.; Zheng, D.; Wang, X.; Kamath, S.; Nordhues, B.A.; Weeber, E.J.; Dickey, C.A. The disease-associated chaperone FKBP51 impairs cognitive function by accelerating AMPA receptor recycling. eNeuro 2019. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Schmidt, M.V.; Rein, T. FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy 2015, 11, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Fridley, B.L.; Jenkins, G.D.; Kalari, K.R.; Lingle, W.; Petersen, G.; Lou, Z.; Wang, L. FKBP51 Affects Cancer Cell Response to Chemotherapy by Negatively Regulating Akt. Cancer Cell 2009, 16, 259–266. [Google Scholar] [CrossRef]
- Yang, P.-C.; Yang, C.-H.; Huang, C.-C.; Hsu, K.-S. Phosphatidylinositol 3-Kinase Activation Is Required for Stress Protocol-induced Modification of Hippocampal Synaptic Plasticity. J. Biol. Chem. 2008, 283, 2631–2643. [Google Scholar] [CrossRef]
- Kohl, S.; Heekeren, K.; Klosterkötter, J.; Kuhn, J. Prepulse inhibition in psychiatric disorders—Apart from schizophrenia. J. Psychiatr. Res. 2013, 47, 445–452. [Google Scholar] [CrossRef]
- Pinto, V.; Costa, J.C.; Morgado, P.; Mota, C.; Miranda, A.; Bravo, F.V.; Oliveira, T.G.; Cerqueira, J.J.; Sousa, N. Differential impact of chronic stress along the hippocampal dorsal–ventral axis. Brain Struct. Funct. 2015, 220, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Zannas, A.; Kretzschmar, A.; Zschocke, J.; Maccarrone, G.; Hafner, K.; Zellner, A.; Kollmannsberger, L.; Wagner, K.; et al. FKBP51 inhibits GSK3β and augments the effects of distinct psychotropic medications. Mol. Psychiatry 2016, 21, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Uhelski, M.L.; Fuchs, P.N. Maternal separation stress leads to enhanced emotional responses to noxious stimuli in adult rats. Behav. Brain Res. 2010, 212, 208–212. [Google Scholar] [CrossRef] [PubMed]
- de Castro-Catala, M.; Peña, E.; Kwapil, T.R.; Papiol, S.; Sheinbaum, T.; Cristóbal-Narváez, P.; Ballespí, S.; Barrantes-Vidal, N.; Rosa, A. Interaction between FKBP5 gene and childhood trauma on psychosis, depression and anxiety symptoms in a non-clinical sample. Psychoneuroendocrinology 2017, 85, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016, 41, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Maeng, L.Y.; Milad, M.R. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm. Behav. 2015, 76, 106–117. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Scharf, S.H.; Liebl, C.; Binder, E.B.; Schmidt, M.V.; Müller, M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS ONE 2011, 6, e16883. [Google Scholar] [CrossRef]
- O’Leary, J.C.; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J.; et al. A new anti-depressive strategy for the elderly: Ablation of FKBP5/FKBP51. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Sabbagh, J.J.; O’Leary, J.C.; Blair, L.J.; Klengel, T.; Nordhues, B.A.; Fontaine, S.N.; Binder, E.B.; Dickey, C.A. Age-Associated Epigenetic Upregulation of the FKBP5 Gene Selectively Impairs Stress Resiliency. PLoS ONE 2014, 9, e107241. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, J.; Comasco, E.; Åslund, C.; Rehn, M.; Tuvblad, C.; Andershed, H.; Nilsson, K.W. Associations between the FKBP5 haplotype, exposure to violence and anxiety in females. Psychoneuroendocrinology 2016, 72, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Fani, N.; King, T.Z.; Shin, J.; Srivastava, A.; Brewster, R.C.; Jovanovic, T.; Bradley, B.; Ressler, K.J. Structural and Functional Connectivity in Posttraumatic Stress Disorder: Associations with FKBP5. Depress. Anxiety 2016, 33, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Glover, E.M.; Jovanovic, T.; Norrholm, S.D. Estrogen and extinction of fear memories: Implications for posttraumatic stress disorder treatment. Biol. Psychiatry 2015, 78, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Jerram, M.; Abbs, B.; Whitfield-Gabrieli, S.; Makris, N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010, 30, 431–438. [Google Scholar] [CrossRef]
- Fernandes, C.; González, M..; Wilson, C.; File, S. Factor Analysis Shows That Female Rat Behaviour Is Characterized Primarily by Activity, Male Rats Are Driven by Sex and Anxiety. Pharmacol. Biochem. Behav. 1999, 64, 731–736. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R.; Peters, J.J. The three-hit concept of vulnerability and resilience: Towards understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef]
- Gassen, N.C.; Hartmann, J.; Zschocke, J.; Stepan, J.; Hafner, K.; Zellner, A.; Kirmeier, T.; Kollmannsberger, L.; Wagner, K.V.; Dedic, N.; et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: Evidence in cells, mice, and humans. PLoS Med. 2014, 11. [Google Scholar] [CrossRef]
- Bouallegue, A.; Pandey, N.R.; Srivastava, A.K. CaMKII knockdown attenuates H2O2-induced phosphorylation of ERK1/2, PKB/Akt, and IGF-1R in vascular smooth muscle cells. Free Radic. Biol. Med. 2009, 47, 858–866. [Google Scholar] [CrossRef]
- Hartmann, J.; Wagner, K.V.; Gaali, S.; Kirschner, A.; Kozany, C.; Ru, G.; Dedic, N.; Ha, A.S.; Hoeijmakers, L.; Namendorf, C.; et al. Pharmacological Inhibition of the Psychiatric Risk Factor FKBP51 Has Anxiolytic Properties. J. Neurosci. 2015, 35, 9007–9016. [Google Scholar] [CrossRef]
- Hernández, F.; Borrell, J.; Guaza, C.; Avila, J.; Lucas, J.J. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3β in the brain but do not form tau filaments. J. Neurochem. 2002, 83, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Patel, P.D.; Akil, H.; Watson, S.J. Localization and Regulation of Glucocorticoid and Mineralocorticoid Receptor Messenger RNAs in the Hippocampal Formation of the Rat. Mol. Endocrinol. 1989, 3, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Ding, J.; Shi, Y. Expression of amygdala mineralocorticoid receptor and glucocorticoid receptor in the single-prolonged stress rats. BMC Neurosci. 2014, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Prakash, R.; Kim, S.-Y.; Fenno, L.E.; Grosenick, L.; Zarabi, H.; Thompson, K.R.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011, 471, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Mahan, A.L.; Ressler, K.J. Fear conditioning, synaptic plasticity and the amygdala: Implications for posttraumatic stress disorder. Trends Neurosci. 2012, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Chocyk, A.; Bobula, B.; Dudys, D.; Przyborowska, A.; Majcher-Maślanka, I.; Hess, G.; Wędzony, K. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur. J. Neurosci. 2013, 38, 2089–2107. [Google Scholar] [CrossRef] [PubMed]
- Banqueri, M.; Méndez, M.; Arias, J.L. Behavioral effects in adolescence and early adulthood in two length models of maternal separation in male rats. Behav. Brain Res. 2017, 324, 77–86. [Google Scholar] [CrossRef] [PubMed]
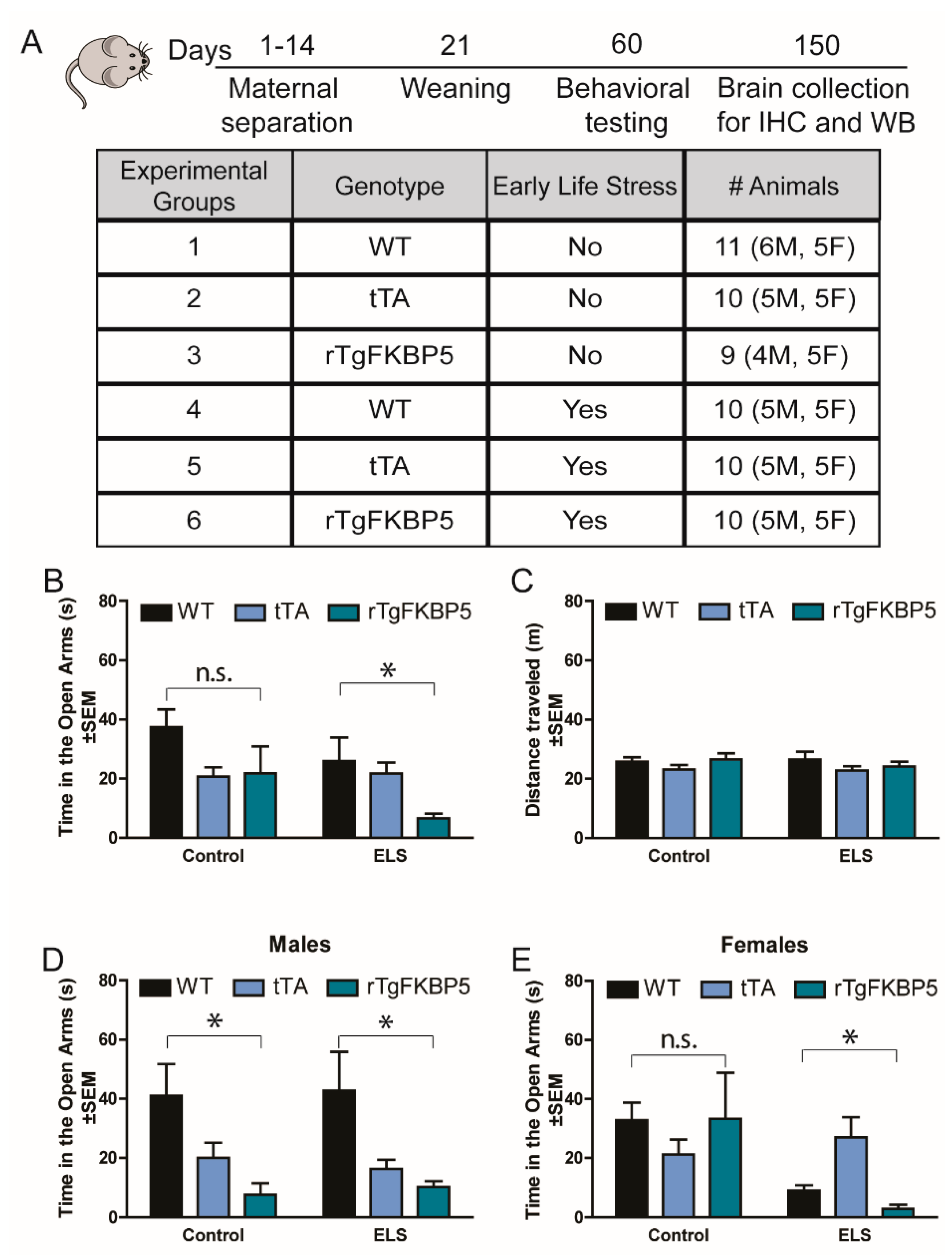
| Figure | Measured Condition | Groups Analyzed | Gen. x ELS | Factor | F Statistic and p-Value |
|---|---|---|---|---|---|
| 1B | EPM: Anxiety levels (open arms) See 1D-E for sex differences | 3-way ANOVA, All groups | F(2, 48) = 1.078, p = 0.347 | Genotype | F(2, 48) = 4.610, p = 0.014 |
| ELS | F(1, 48) = 3.28, p = 0.075 | ||||
| Sex | F(1, 48) = 0.308, p = 0.582 | ||||
| Sex/Gen/ELS | F(2, 48) = 1.968, p = 0.151 | ||||
| 2-way ANOVA, Non-stressed | F(2, 48) = 1.548, p = 0.233 | Genotype | F(2, 48) = 1.442, p = 0.256 | ||
| Sex | F(1, 48) = 0.617, p = 0.439 | ||||
| 2-way ANOVA, ELS | F(2, 48) = 3.089, p = 0.06 | Genotype | F(2, 48) = 4.015, p = 0.031 | ||
| Sex | F(1, 48) = 4.003, p = 0.05 | ||||
| Number entries to open arms | 3-way ANOVA, All groups | F(2, 48) = 0.351, p = 0.705 | Genotype | F(2, 48) = 1.283, p = 0.285 | |
| ELS | F(1, 48) = 0.049, p = 0.824 | ||||
| Sex | F(1, 48) = 1.478, p = 0.230 | ||||
| Sex/Gen/ELS | F(1, 48) = 0.541, p = 0.585 | ||||
| Anxiety levels (closed arms) | 3-way ANOVA, All groups | F(2, 48) = 1.181, p = 0.316 | Genotype | F(2, 48) = 0.010, p = 0.989 | |
| ELS | F(1, 48) = 0.850, p = 0.360 | ||||
| Sex | F(1, 48) = 0.638, p = 0.428 | ||||
| Sex/Gen/ELS | F(2, 48) = 1.096, p = 0.342 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.228, p = 0.797 | Genotype | F(2,24) = 3.509, p = 0.049 | ||
| ELS | F(1,24) = 0.002, p = 0.958 | ||||
| 2-way ANOVA, Females | F(2,24) = 1.865, p = 0.176 | Genotype | F(2,24) = 1.649, p = 0.213 | ||
| ELS | F(1,24) = 0.354, p = 0.557 | ||||
| 1C | EPM: Locomotion | 3-way ANOVA, All groups | F(2,48) = 0.353, p = 0.704 | Genotype | F(2,48) = 1.657, p = 0.200 |
| ELS | F(1,48) = 0.205 p = 0.652 | ||||
| Sex | F(1,48) = 0.399, p = 0.673 | ||||
| Sex/Gen/ELS | F(2,24) = 1.649, p = 0.213 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.137, p = 0.879 | Genotype | F(2,24) = 0.714, p = 0.499 | ||
| ELS | F(1,24) = 0.465 p = 0.501 | ||||
| 2-way ANOVA, Females | F(2,24) = 0.336, p = 0.717 | Genotype | F(2,24) = 1.327, p = 0.284 | ||
| ELS | F(1,24) = 0.0 p = 1 | ||||
| 1D | Anxiety levels (open arms) | 2-way ANOVA, Males | F(2,24) = 0.089, p = 0.914 | Genotype | F(2,24) = 8.900, p = 0.001 |
| ELS | F(1,24) = 0.001, p = 0.971 | ||||
| 1E | 2-way ANOVA, Females | F(2,24) = 3.069, p = 0.065 | Genotype | F(2,24) = 0.747 p = 0.294 | |
| ELS | F(1,24) = 6.449, p = 0.018 | ||||
| 2A | Startle response | 3-way ANOVA, All groups | F(2, 48) = 0.997, p = 0.377 | Genotype | F(2, 48) = 1.058 p = 0.355 |
| ELS | F(1, 48) = 0.609, p = 0.439 | ||||
| Sex | F(1, 48) = 0.388, p = 0.536 | ||||
| Sex/Gen/ELS | F(1, 48) = 0.395, p = 0.676 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.691, p = 0.511 | Genotype | F(2,24) = 0.770, p = 0.475 | ||
| ELS | F(1,24) = 0.199, p = 0.660 | ||||
| 2-way ANOVA, Females | F(2,24) = 0.464, p = 0.634 | Genotype | F(2,24) = 0.791, p = 0.464 | ||
| ELS | F(1,24) = 0.789, p = 0.383 | ||||
| 2B,C | PPI | 3-way RM-ANOVA, All groups | F(2, 48) = 0.629, p = 0.537 | Genotype | F(2, 48) = 7.658, p = 0.001 |
| ELS | F(1, 48) = 0.272, p = 0.605 | ||||
| Sex | F(1, 48) = 4.532, p = 0.038 | ||||
| Sex/Gen/ELS | F(1, 48) = 0.620, p = 0.542 | ||||
| 3A,C | MWM, Reversal training | 3-way RM-ANOVA, All groups | F(2, 54) = 0.688, p = 0.507 | Genotype | F(2, 54) = 2.685, p = 0.078 |
| ELS | F(1, 54) = 0.123, p = 0.727 | ||||
| Sex | F(1, 54) = 0.021, p = 0.886 | ||||
| Sex/Gen/ELS | F(2, 54) = 0.385, p = 0.683 | ||||
| 2-way RM-ANOVA, Non-Stressed | Genotype | F(2, 24) = 1.456, p = 0.253 | |||
| Sex | F(1, 24) = 0.101, p = 0.753 | ||||
| Training | F(2, 23) = 28.02, p < 0.001 | ||||
| 2-way RM-ANOVA, ELS | Genotype | F(2, 24) = 1.913, p = 0.169 | |||
| Sex | F(1, 24) = 0.006, p = 0.941 | ||||
| Training | F(2, 23) = 17.53, p < 0.001 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.812, p = 0.456 | Genotype | F(2,24) = 3.454, p = 0.048 | ||
| ELS | F(1,24) = 0.003, p = 0.456 | ||||
| 2-way ANOVA, Females | F(2,24) = 0.054, p = 0.947 | Genotype | F(2,24) = 0.091, p = 0.913 | ||
| ELS | F(1,24) = 0.245, p = 0.625 | ||||
| 3B,D | MWM, Reversal Probe | 3-way RM-ANOVA, All groups | F(2, 54) = 0.135, p = 0.873 | Genotype | F(2, 54) = 3.827, p = 0.027 |
| ELS | F(1, 54) = 0.170, p = 0.681 | ||||
| Sex | F(1, 54) = 0.183, p = 0.670 | ||||
| Sex/Gen/ELS | F(2, 54) = 0.244, p = 0.784 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.076, p = 0.927 | Genotype | F(2,24) = 1.208, p = 0.316 | ||
| ELS | F(1,24) = 0.712, p = 0.407 | ||||
| 2-way ANOVA, Females | F(2,24) = 0.289, p = 0.752 | Genotype | F(2,24) = 0.012, p = 0.988 | ||
| ELS | F(1,24) = 2.547, p = 0.124 | ||||
| S1A | Percent of open arm entries | 3-way ANOVA, All groups | F(2, 48) = 1.560, p = 0.219 | Genotype | F(2, 48) = 3.956, p = 0.026 |
| ELS | F(1, 48) = 0.000, p = 0.998 | ||||
| Sex | F(1, 48) = 0.348, p = 0.558 | ||||
| Sex/Gen/ELS | F(1, 48) = 1.720, p = 0.097 | ||||
| S1B | Percent of open arm entries | 2-way ANOVA, Males | F(2, 48) = 2.753, p = 0.042 | Genotype | F(2, 48) = 5.423, p = 0.01 |
| ELS | F(1, 48) = 1.493, p = 0.234 | ||||
| S1C | Percent of open arm entries | 2-way ANOVA, Females | F(2, 48) =0.854, p = 0.526 | Genotype | F(2, 48) = 0.199, p = 0.821 |
| ELS | F(1, 48) = 1.627, p = 0.214 | ||||
| S2A | 2-way RM-ANOVA, Males | F(2,24) = 0.412, p = 0.668 | Genotype | F(2,24) = 0.614, p = 0.552 | |
| ELS | F(1,24) = 0.879, p = 0.360 | ||||
| S2B | 2-way RM-ANOVA, Females | F(2,24) = 7.092, p = 0.004 | Genotype | F(2,24) = 0.692, p = 0.511 | |
| ELS | F(1,24) = 3.903, p = 0.061 | ||||
| S3A | MWM, Visible | 3-way ANOVA, All groups | F(2, 54) = 0.107, p = 0.898 | Genotype | F(2, 54) = 2.062, p= 0.137 |
| ELS | F(1, 54) = 0.110, p= 0.740 | ||||
| Sex | F(1, 54) = 1.289, p = 0.262 | ||||
| Sex/Gen/ELS | F(2, 54) = 0.099, p = 0.906 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.352, p = 0.707 | Genotype | F(2,24) = 5.372, p = 0.012 | ||
| ELS | F(1,24) = 0.488, p = 0.492 | ||||
| 2-way ANOVA, Females | F(2,24) = 0.008, p = 0.992 | Genotype | F(2,24) = 0.370, p = 0.694 | ||
| ELS | F(1,24) = 0.002, p = 0.961 | ||||
| S3B,D | MWM, Training | 3-way RM-ANOVA, All groups | F(2, 54) = 0.407, p = 0.668 | Genotype | F(2, 54) = 0.434, p = 0.650 |
| ELS | F(1, 54) = 1.231, p = 0.272 | ||||
| Sex | F(1, 54) = 0.003, p = 0.954 | ||||
| Sex/Gen/ELS | F(2, 54) = 0.195, p = 0.823 | ||||
| 2-way RM-ANOVA, Non-Stressed | Genotype | F(2, 24) = 0.020, p = 0.981 | |||
| Sex | F(1, 24) = 0.053, p = 0.821 | ||||
| Training | F(2, 23) = 14.27, p < 0.001 | ||||
| 2-way RM-ANOVA, ELS | Genotype | F(2, 24) = 0.847, p = 0.441 | |||
| Sex | F(1, 24) = 0.028, p = 0.868 | ||||
| Training | F(2, 23) = 26.49, p < 0.001 | ||||
| 2-way ANOVA, Males | F(2,24) = 0.174, p = 0.841 | Genotype | F(2,24) = 0.092, p = 0.912 | ||
| ELS | F(1,24) = 0.838, p = 0.369 | ||||
| 2-way ANOVA, Females | F(2,24) = 0.402, p = 0.673 | Genotype | F(2,24) = 0.534, p = 0.593 | ||
| ELS | F(1,24) = 0.313, p = 0.581 | ||||
| S3C,E | MWM, Probe | 3-way ANOVA, All groups | F(2, 54) = 7.047, p = 0.002 | Genotype | F(2, 54) = 7.018, p = 0.002 |
| ELS | F(1, 54) = 9.118, p = 0.004 | ||||
| Sex | F(1, 54) = 0.057, p = 0.813 | ||||
| Sex/Gen/ELS | F(1, 54) = 0.362, p = 0.698 |
| Figure | Brain Area | Protein | Groups | Gen. x ELS | Gen. | ELS |
|---|---|---|---|---|---|---|
| Figure 4A | HPC | pAKTSer473 | All groups | F(2, 24) = 0.077, p = 0.926 | F(2, 24) = 5.903, p = 0.007 | F(1, 24) = 4.373, p = 0.046 |
| Figure 4C | HPC | GSK-3β | All groups | F(2, 24) = 0.079, p = 0.923 | F(2, 24) = 1.639, p = 0.215 | F(1, 24) = 0.083, p = 0.774 |
| Figure 5A | DH | FKBP5 | All groups | F(2, 24) = 3.563, p = 0.045 | F(2, 24) = 27.23, p < 0.001 | F(1, 24) = 3.068, p = 0.093 |
| Figure 5B | DH | pAKTSer473/AKT ratio | All groups | F(2, 22) = 6.304, p = 0.006 | F(2, 22) = 1.110, p < 0.347 | F(1, 22) = 0.965, p = 0.336 |
| Figure 5C | DH | pGSK-3 βSer9/GSK-3β | All groups | F(2, 22) = 0.575 p = 0.570 | F(2, 22) = 0.703, p < 0.505 | F(1, 22) = 0.812, p = 0.376 |
| Figure 5E | VH | FKBP5 | All groups | F(2, 22) = 0.898, p = 0.421 | F(2, 22) = 15.51, p < 0.001 | F(1, 22) = 1.041, p = 0.318 |
| Figure 5F | VH | pAKTSer473/AKT ratio | All groups | F(2, 22) = 0.746, p = 0.487 | F(2, 22) = 0.057, p < 0.944 | F(1, 22) = 0.159, p = 0.694) |
| Figure 5G | VH | pGSK-3βSer9/GSK-3β | All groups | F(2, 22) = 0.185, p = 0.832 | F(2, 22) = 1.175, p < 0.329 | F(1, 22) = 1.219, p = 0.286 |
| Figure 5I | AMYG | FKBP5 | All groups | F(2,18) = 1.485, p = 0.253 | F(2, 18) = 118.6, p < 0.001 | F(1, 18) = 0.645, p = 0.432 |
| Figure 5J | AMYG | pAKTSer473/AKT ratio | All groups | F(2, 18) = 1.446, p = 0.263 | F(2, 18) = 2.502, p < 0.115 | F(1, 18) = 0.774, p = 0.391 |
| Figure 5K | AMYG | pGSK-3βSer9/GSK-3β | All groups | F(2, 18) = 0.374, p = 0.693 | F(2, 18) = 1.322, p < 0.291 | F(1, 18) = 0.462, p = 0.505 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criado-Marrero, M.; Gebru, N.T.; Gould, L.A.; Smith, T.M.; Kim, S.; Blackburn, R.J.; Dickey, C.A.; Blair, L.J. Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus. Int. J. Mol. Sci. 2019, 20, 2738. https://doi.org/10.3390/ijms20112738
Criado-Marrero M, Gebru NT, Gould LA, Smith TM, Kim S, Blackburn RJ, Dickey CA, Blair LJ. Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus. International Journal of Molecular Sciences. 2019; 20(11):2738. https://doi.org/10.3390/ijms20112738
Chicago/Turabian StyleCriado-Marrero, Marangelie, Niat T. Gebru, Lauren A. Gould, Taylor M. Smith, Sojeong Kim, Roy J. Blackburn, Chad A. Dickey, and Laura J. Blair. 2019. "Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus" International Journal of Molecular Sciences 20, no. 11: 2738. https://doi.org/10.3390/ijms20112738
APA StyleCriado-Marrero, M., Gebru, N. T., Gould, L. A., Smith, T. M., Kim, S., Blackburn, R. J., Dickey, C. A., & Blair, L. J. (2019). Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus. International Journal of Molecular Sciences, 20(11), 2738. https://doi.org/10.3390/ijms20112738





