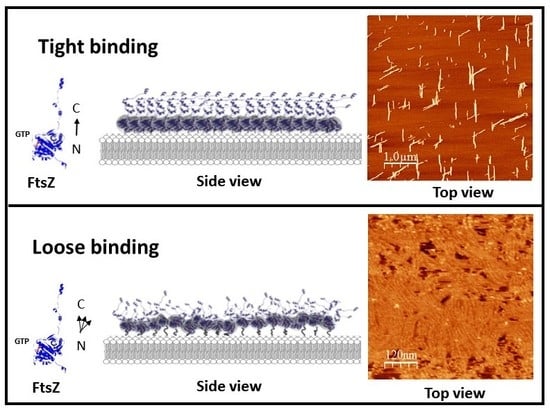
Surface Orientation and Binding Strength Modulate Shape of FtsZ on Lipid Surfaces
Abstract
1. Introduction
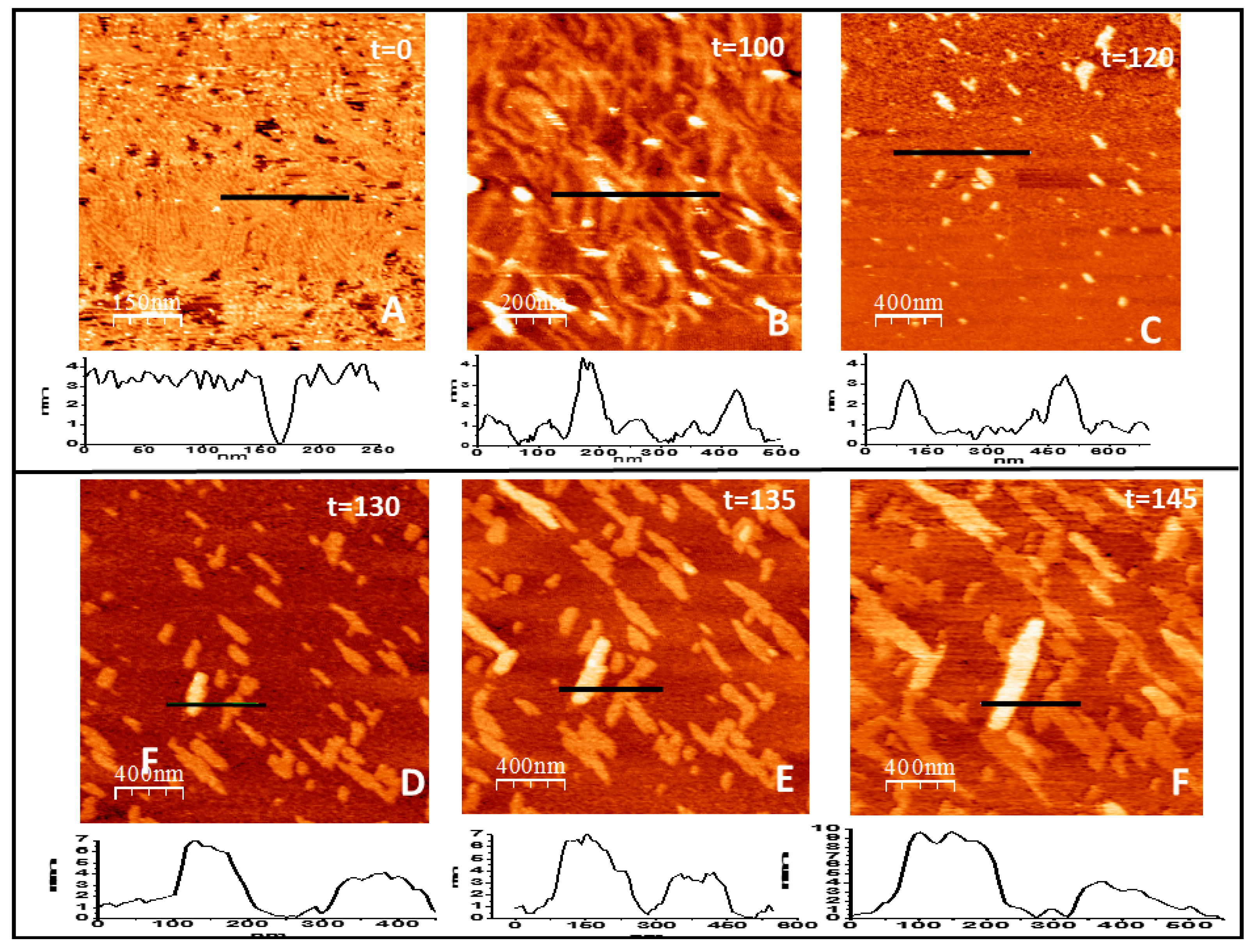
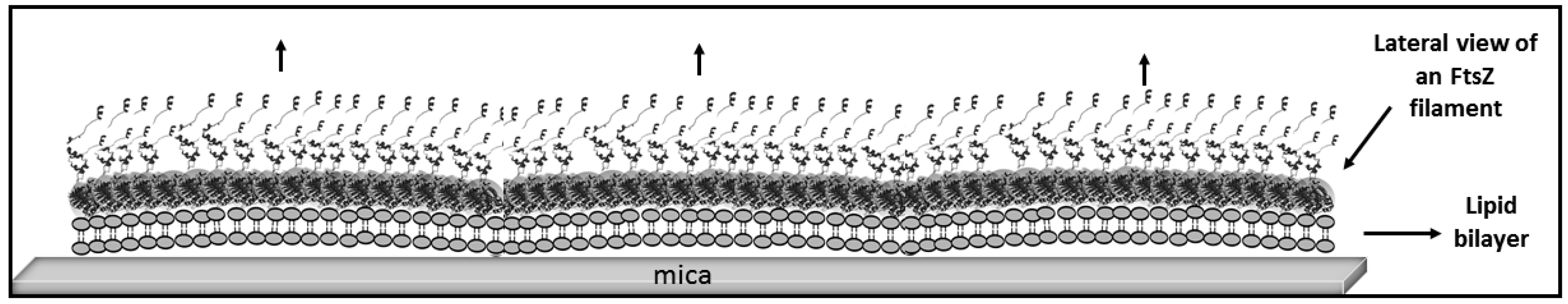
2. Results
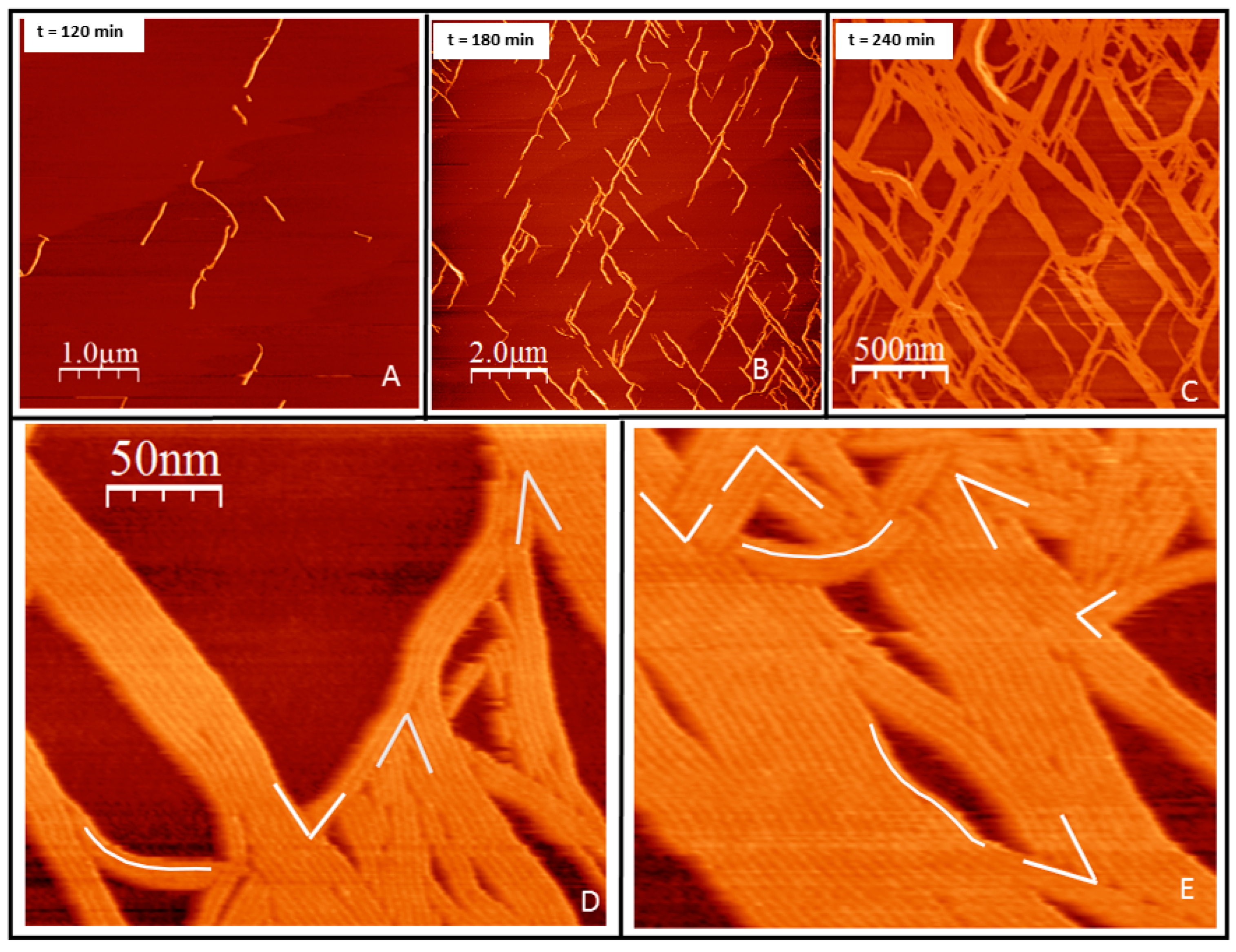
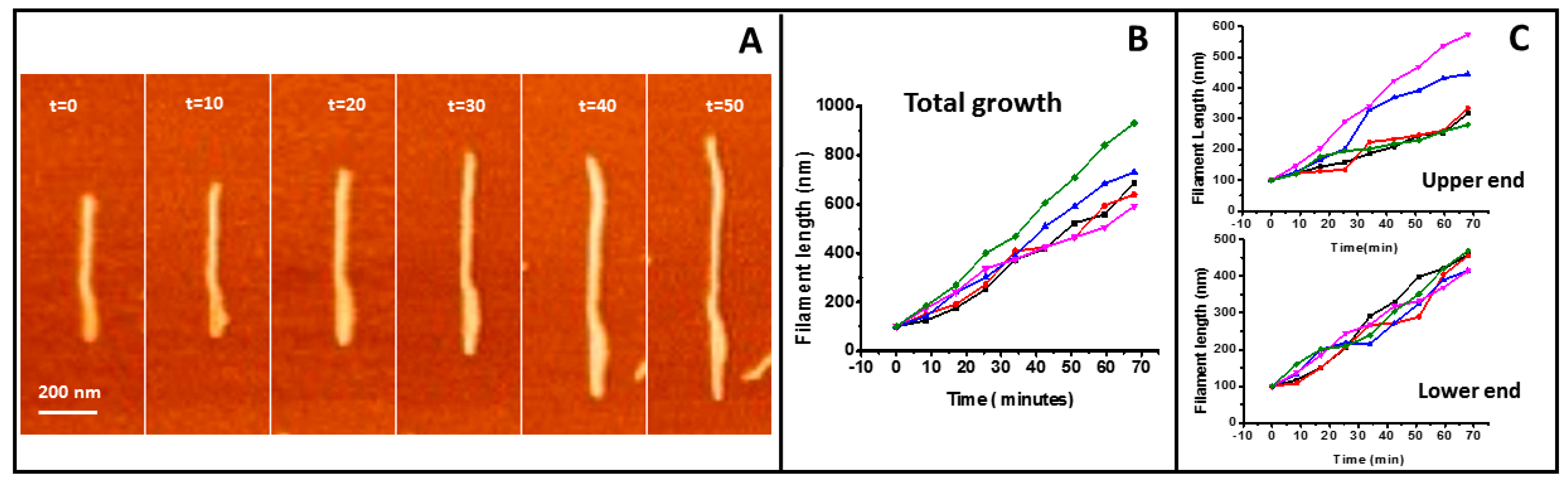
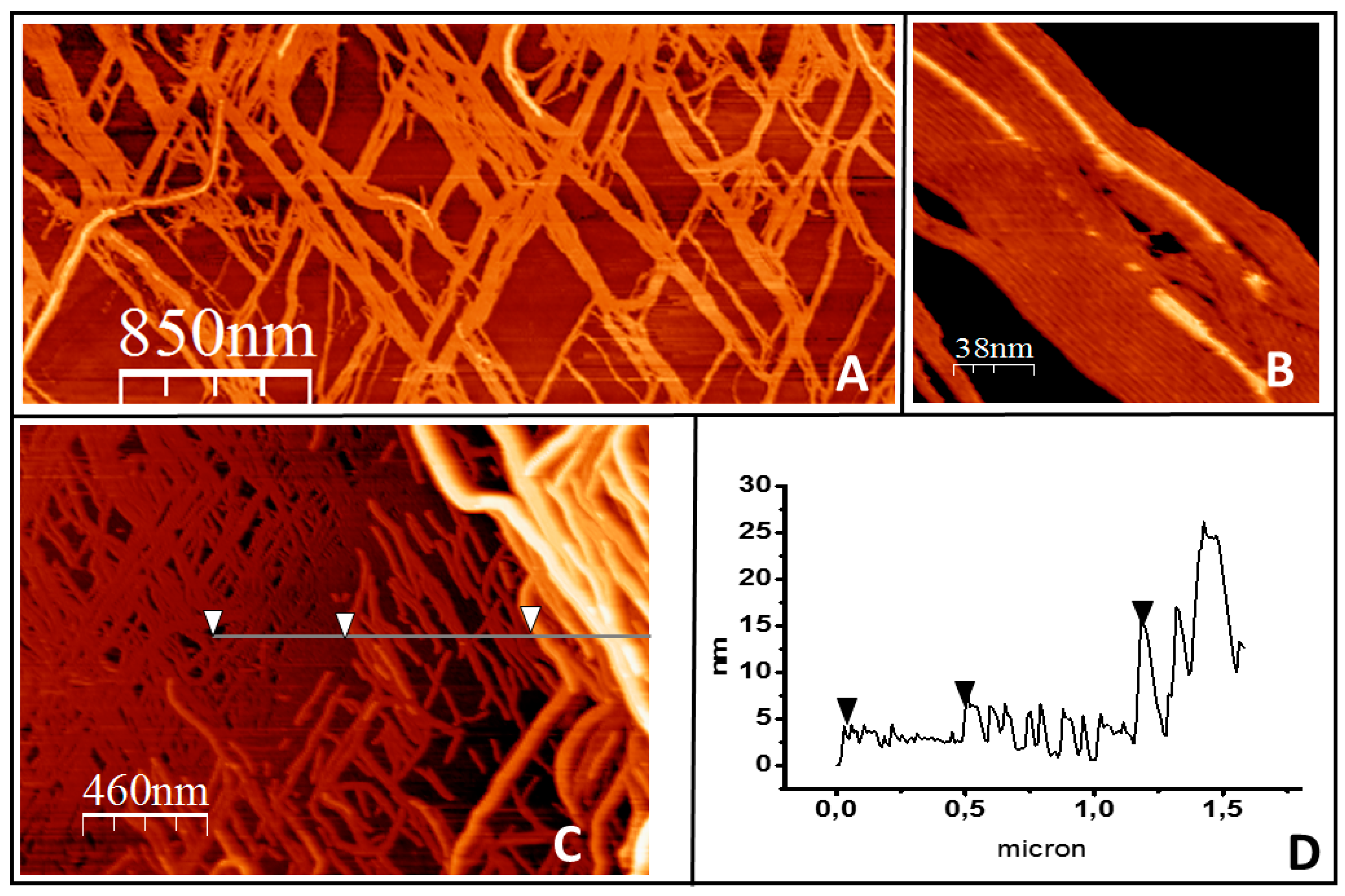
2.1. Tight Binding
2.2. Loose Binding
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Lutkenhaus, J. Ftsz ring: The eubacterial division apparatus conserved in archaebacteria. Mol. Microbiol. 1996, 21, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.R.; Beech, P.L. Cell division protein ftsz: Running rings around bacteria, chloroplasts and mitochondria. Res. Microbiol. 2001, 152, 3–10. [Google Scholar] [CrossRef]
- Mingorance, J.; Rivas, G.; Vélez, M.; Gómez-Puertas, P.; Vicente, M. Strong ftsz is with the force: Mechanisms to constrict bacteria. Trends Microbiol. 2010, 18, 348–356. [Google Scholar] [CrossRef]
- Mateos-Gil, P.; Tarazona, P.; Vélez, M. Bacterial cell division: Modeling ftsz assembly and force generation from single filament experimental data. FEMS Microbiol. Rev. 2019, 43, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Yamane, J.; Mogi, N.; Yamaguchi, H.; Takemoto, H.; Yao, M.; Tanaka, I. Structural reorganization of the bacterial cell-division protein ftsz from staphylococcus aureus. Acta Crystall. Sect. D 2012, 68, 1175–1188. [Google Scholar] [CrossRef]
- Huecas, S.; Ramírez-Aportela, E.; Vergoñós, A.; Núñez-Ramírez, R.; Llorca, O.; Díaz, J.F.; Juan-Rodríguez, D.; Oliva, M.A.; Castellen, P.; Andreu, J.M. Self-organization of ftsz polymers in solution reveals spacer role of the disordered c-terminal tail. Biophys. J. 2017, 113, 1831–1844. [Google Scholar] [CrossRef]
- Nogales, E.; Wolf, S.G.; Downing, K.H. Structure of the αβ tubulin dimer by electron crystallography. Nature 1998, 391. [Google Scholar] [CrossRef]
- Oliva, M.A.; Cordell, S.C.; Lowe, J. Structural insights into ftsz protofilament formation. Nat. Struct. Mol. Biol. 2004, 11, 1243–1250. [Google Scholar] [CrossRef]
- Scheffers, D.J.; de Wit, J.G.; den Blaauwen, T.; Driessen, A.J. Gtp hydrolysis of cell division protein ftsz: Evidence that the active site is formed by the association of monomers. Biochemistry 2002, 41. [Google Scholar] [CrossRef]
- Martín-Galiano, A.J.; Buey, R.M.; Cabezas, M.; Andreu, J.M. Mapping flexibility and the assembly switch of cell division protein ftsz by computational and mutational approaches. J. Biol. Chem. 2010, 285, 22554–22565. [Google Scholar] [CrossRef]
- Wagstaff, J.M.; Tsim, M.; Oliva, M.A.; García-Sanchez, A.; Kureisaite-Ciziene, D.; Andreu, J.M.; Löwe, J. A polymerization-associated structural switch in ftsz that enables treadmilling of model filaments. mBio 2017, 8. [Google Scholar] [CrossRef]
- Buske, P.J.; Levin, P.A. A flexible c-terminal linker is required for proper ftsz assembly in vitro and cytokinetic ring formation in vivo. Mol. Microbiol. 2013, 89, 249–263. [Google Scholar] [CrossRef]
- Hernández-Rocamora, V.M.; Reija, B.; García, C.; Natale, P.; Alfonso, C.; Minton, A.P.; Zorrilla, S.; Rivas, G.; Vicente, M. Dynamic interaction of the escherichia coli cell division zipa and ftsz proteins evidenced in nanodiscs. J. Biol. Chem. 2012, 287, 30097–30104. [Google Scholar] [CrossRef]
- Márquez, I.F.; Mateos-Gil, P.; Shin, J.Y.; Lagos, R.; Monasterio, O.; Vélez, M. Mutations on ftsz lateral helix h3 that disrupt cell viability hamper reorganization of polymers on lipid surfaces. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1815–1827. [Google Scholar] [CrossRef]
- Pichoff, S.; Lutkenhaus, J. Tethering the z ring to the membrane through a conserved membrane targeting sequence in ftsa. Mol. Microbiol. 2005, 55, 1722–1734. [Google Scholar] [CrossRef]
- Hale, C.A.; de Boer, P.A.J. Direct binding of ftsz to zipa, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 1997, 88, 175–185. [Google Scholar] [CrossRef]
- Ohashi, T.; Hale, C.A.; de Boer, P.A.J.; Erickson, H.P. Structural evidence that the p/q domain of zipa is an unstructured, flexible tether between the membrane and the c-terminal ftsz-binding domain. J. Bacteriol. 2002, 184, 4313–4315. [Google Scholar] [CrossRef]
- Buske, P.J.; Mittal, A.; Pappu, R.V.; Levin, P.A. An intrinsically disordered linker plays a critical role in bacterial cell division. Semin. Cell Dev. Biol. 2015, 37, 3–10. [Google Scholar] [CrossRef]
- Sundararajan, K.; Miguel, A.; Desmarais, S.M.; Meier, E.L.; Casey Huang, K.; Goley, E.D. The bacterial tubulin ftsz requires its intrinsically disordered linker to direct robust cell wall construction. Nat. Commun. 2015, 6, 7281. [Google Scholar] [CrossRef]
- Sundararajan, K.; Goley, E.D. The intrinsically disordered c-terminal linker of ftsz regulates protofilament dynamics and superstructure in vitro. J. Biol. Chem. 2017, 292, 20509–20527. [Google Scholar] [CrossRef]
- Mateos-Gil, P.; Marquez, I.; Lopez-Navajas, P.; Jimenez, M.; Vicente, M.; Mingorance, J.; Rivas, G.; Velez, M. Ftsz polymers bound to lipid bilayers through zipa form dynamic two dimensional networks. Biochim. Biophys. Acta 2012, 1818, 806–813. [Google Scholar] [CrossRef]
- Encinar, M.; Kralicek, A.V.; Martos, A.; Krupka, M.; Cid, S.; Alonso, A.; Rico, A.I.; Jiménez, M.; Vélez, M. Polymorphism of ftsz filaments on lipid surfaces: Role of monomer orientation. Langmuir 2013, 29, 9436–9446. [Google Scholar] [CrossRef] [PubMed]
- Loose, M.; Mitchison, T.J. The bacterial cell division proteins ftsa and ftsz self-organize into dynamic cytoskeletal patterns. Nat. Cell Biol. 2013, 16, 38–46. [Google Scholar] [CrossRef]
- Ramirez-Diaz, D.A.; García-Soriano, D.A.; Raso, A.; Mücksch, J.; Feingold, M.; Rivas, G.; Schwille, P. Treadmilling analysis reveals new insights into dynamic ftsz ring architecture. PLoS Biol. 2018, 16, e2004845. [Google Scholar] [CrossRef]
- Sundararajan, K.; Vecchiarelli, A.; Mizuuchi, K.; Goley, E.D. Species- and c-terminal linker-dependent variations in the dynamic behavior of ftsz on membranes in vitro. Mol. Microbiol. 2018, 110, 47–63. [Google Scholar] [CrossRef]
- Milam, S.L.; Osawa, M.; Erickson, H. Negative-stain electron microscopy of inside-out ftsz rings reconstituted on artificial membrane tubules show ribbons of protofilaments. Biophys. J. 2012, 103, 59–68. [Google Scholar] [CrossRef][Green Version]
- Osawa, M.; Anderson, D.E.; Erickson, H.P. Reconstitution of contractile ftsz rings in liposomes. Science 2008, 320, 792–794. [Google Scholar] [CrossRef]
- Osawa, M.; Anderson, D.E.; Erickson, H.P. Curved ftsz protofilaments generate bending forces on liposome membranes. EMBO J. 2009, 28, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Prado Salas, P.; Encinar, M.; Velez, M.; Tarazona, P. Ftsz protein on bilayer membranes: Effects of specific lateral bonds. Soft Matter 2013, 9, 6072–6079. [Google Scholar] [CrossRef]
- Paez, A.; Mateos-Gil, P.; Hörger, I.; Mingorance, J.; Rivas, G.; Vicente, M.; Vélez, M.; Tarazona, P. Simple modeling of ftsz polymers on flat and curved surfaces: Correlation with experimental in vitro observations. PMC Biophys. 2009, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Hörger, I.; Velasco, E.; Mingorance, J.; Rivas, G.; Tarazona, P.; Vélez, M. Langevin computer simulations of bacterial protein filaments and the force-generating mechanism during cell division. Phys. Rev. E 2008, 77, 011902. [Google Scholar] [CrossRef] [PubMed]
- Hörger, I.; Velasco, E.; Rivas, G.; Vélez, M.; Tarazona, P. Ftsz bacterial cytoskeletal polymers on curved surfaces: The importance of lateral interactions. Biophys. J. 2008, 94, L81–L83. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Prado Salas, P.; Horger, I.; Martin-Garcia, F.; Mendieta, J.; Alonso, A.; Encinar, M.; Gomez-Puertas, P.; Velez, M.; Tarazona, P. Torsion and curvature of ftsz filaments. Soft Matter 2014, 10, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- de Prado Salas, P.G.; Encinar, M.; Alonso, A.; Vélez, M.; Tarazona, P. Modeling the interplay between protein and lipid aggregation in supported membranes. Chem. Phys. Lipids 2015, 185, 141–152. [Google Scholar] [CrossRef] [PubMed]
- González de Prado Salas, P.; Tarazona, P. Collective effects of torsion in ftsz filaments. Phys. Rev. E 2016, 93, 042407. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Chwastek, G.; Fischer-Friedrich, E.; Ehrig, C.; Mönch, I.; Schwille, P. Surface topology engineering of membranes for the mechanical investigation of the tubulin homologue ftsz. Angew. Chem. Int. Ed. 2012, 51, 11858–11862. [Google Scholar] [CrossRef]
- Hsin, J.; Gopinathan, A.; Huang, K.C. Nucleotide-dependent conformations of ftsz dimers and force generation observed through molecular dynamics simulations. Proc. Natl. Acad. Sci. USA 2012, 109, 9432–9437. [Google Scholar] [CrossRef]
- Qing-Ming, Y.; Lutkenhaus, J. The nucleotide sequence of the essential cell-division gene ftsz of escherichia coli. Gene 1985, 36, 241–247. [Google Scholar] [CrossRef]
- Nogales, E.; Downing, K.H.; Amos, L.A.; Löwe, J. Tubulin and ftsz form a distinct family of gtpases. Nat. Struct. Biol. 1998, 5. [Google Scholar] [CrossRef]
- Shahinian, S.; Silvius, J.R. A novel strategy affords high-yield coupling of antibody fab´fragments to liposomes. Biochim. Biophys. Acta Biomembr. 1995, 1239, 157–167. [Google Scholar] [CrossRef]
- Huwyler, J.; Wu, D.; Pardridge, W.M. Brain drug delivery of small molecules using immunoliposomes. Proc. Natl. Acad. Sci. USA 1996, 93, 14164–14169. [Google Scholar] [CrossRef] [PubMed]
- Mingorance, J.; Tadros, M.; Vicente, M.; Gonzalez, J.M.; Rivas, G.; Velez, M. Visualization of single escherichia coli ftsz filament dynamics with atomic force microscopy. J. Biol.Chem. 2005, 280, 20909–20914. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Erickson, H.P. Rapid in vitro assembly dynamics and subunit turnover of ftsz demonstrated by fluorescence resonance energy transfer. J. Biol. Chem. 2005, 280, 22549–22554. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Stricker, J.; Erickson, H.P. Site-specific mutations of ftsz - effects on gtpase and in vitro assembly. BMC Microbiol. 2001, 1, 7. [Google Scholar] [CrossRef]
- Shin, J.Y.; Vollmer, W.; Lagos, R.; Monasterio, O. Glutamate 83 and arginine 85 of helix h3 bend are key residues for ftsz polymerization, gtpase activity and cellular viability of escherichia coli: Lateral mutations affect ftsz polymerization and E. coli viability. BMC Microbiol. 2013, 13, 26. [Google Scholar] [CrossRef]
- Jaiswal, R.; Patel, R.Y.; Asthana, J.; Jindal, B.; Balaji, P.V.; Panda, D. E93r substitution of escherichia coli ftsz induces bundling of protofilaments, reduces gtpase activity, and impairs bacterial cytokinesis. J. Biol. Chem. 2010, 285, 31796–31805. [Google Scholar] [CrossRef] [PubMed]
- Monahan, L.G.; Robinson, A.; Harry, J.E. Lateral ftsz association and the assembly of the cytokinetic z ring in bacteria. Mol. Microbiol. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Yu, J.; Yu, J.; Liu, Y.; Li, Y.; Feng, X.-H.; Huang, K.C.; Chang, Z.; Ye, S. Lateral interactions between protofilaments of the bacterial tubulin homolog ftsz are essential for cell division. eLife 2018, 7, e35578. [Google Scholar] [CrossRef]
- Fontaine, S.D.; Reid, R.; Robinson, L.; Ashley, G.W.; Santi, D.V. Long-term stabilization of maleimide–thiol conjugates. Bioconj. Chem. 2015, 26, 145–152. [Google Scholar] [CrossRef]
- Watkins, E.B.; El-khouri, R.J.; Miller, C.E.; Seaby, B.G.; Majewski, J.; Marques, C.M.; Kuhl, T.L. Structure and thermodynamics of lipid bilayers on polyethylene glycol cushions: Fact and fiction of peg cushioned membranes. Langmuir 2011, 27, 13618–13628. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Zhang, F.; Pillai, S.; Liu, J.; Li, R.; Dai, B.; Li, B.; Zhang, Y. Hierarchical ordering of amyloid fibrils on the mica surface. Nanoscale 2013, 5, 4816–4822. [Google Scholar] [CrossRef] [PubMed]
- Bisson-Filho, A.W.; Hsu, Y.-P.; Squyres, G.R.; Kuru, E.; Wu, F.; Jukes, C.; Sun, Y.; Dekker, C.; Holden, S.; VanNieuwenhze, M.S.; et al. Treadmilling by ftsz filaments drives peptidoglycan synthesis and bacterial cell division. Science 2017, 355, 739. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lyu, Z.; Miguel, A.; McQuillen, R.; Huang, K.C.; Xiao, J. Gtpase activity–coupled treadmilling of the bacterial tubulin ftsz organizes septal cell wall synthesis. Science 2017, 355, 744. [Google Scholar] [CrossRef] [PubMed]
- Torrance, L.; Ziegler, A.; Pittman, H.; Paterson, M.; Toth, R.; Eggleston, I. Oriented immobilisation of engineered single-chain antibodies to develop biosensors for virus detection. J. Virol. Methods 2006, 134, 164–170. [Google Scholar] [CrossRef]
- Rivas, G.; López, A.; Mingorance, J.; Ferrándiz, M.a.J.; Zorrilla, S.; Minton, A.P.; Vicente, M.; Andreu, J.M. Magnesium-induced linear self-association of the ftsz bacterial cell division protein monomer: The primary steps for ftsz assembly. J. Biol. Chem. 2000, 275, 11740–11749. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Herrero, F.; Pablo, P.J.D.; Fernández-Sánchez, R.; Colchero, J.; Gómez-Herrero, J.; Baró, A.M. Scanning force microscopy jumping and tapping modes in liquids. Appl. Phys. Lett. 2002, 81, 2620–2622. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez, I.; Díaz-Haro, G.; Vélez, M. Surface Orientation and Binding Strength Modulate Shape of FtsZ on Lipid Surfaces. Int. J. Mol. Sci. 2019, 20, 2545. https://doi.org/10.3390/ijms20102545
Márquez I, Díaz-Haro G, Vélez M. Surface Orientation and Binding Strength Modulate Shape of FtsZ on Lipid Surfaces. International Journal of Molecular Sciences. 2019; 20(10):2545. https://doi.org/10.3390/ijms20102545
Chicago/Turabian StyleMárquez, Ileana, Gabriel Díaz-Haro, and Marisela Vélez. 2019. "Surface Orientation and Binding Strength Modulate Shape of FtsZ on Lipid Surfaces" International Journal of Molecular Sciences 20, no. 10: 2545. https://doi.org/10.3390/ijms20102545
APA StyleMárquez, I., Díaz-Haro, G., & Vélez, M. (2019). Surface Orientation and Binding Strength Modulate Shape of FtsZ on Lipid Surfaces. International Journal of Molecular Sciences, 20(10), 2545. https://doi.org/10.3390/ijms20102545