GI inflammation Increases Sodium-Glucose Cotransporter Sglt1
Abstract
1. Introduction
2. Results
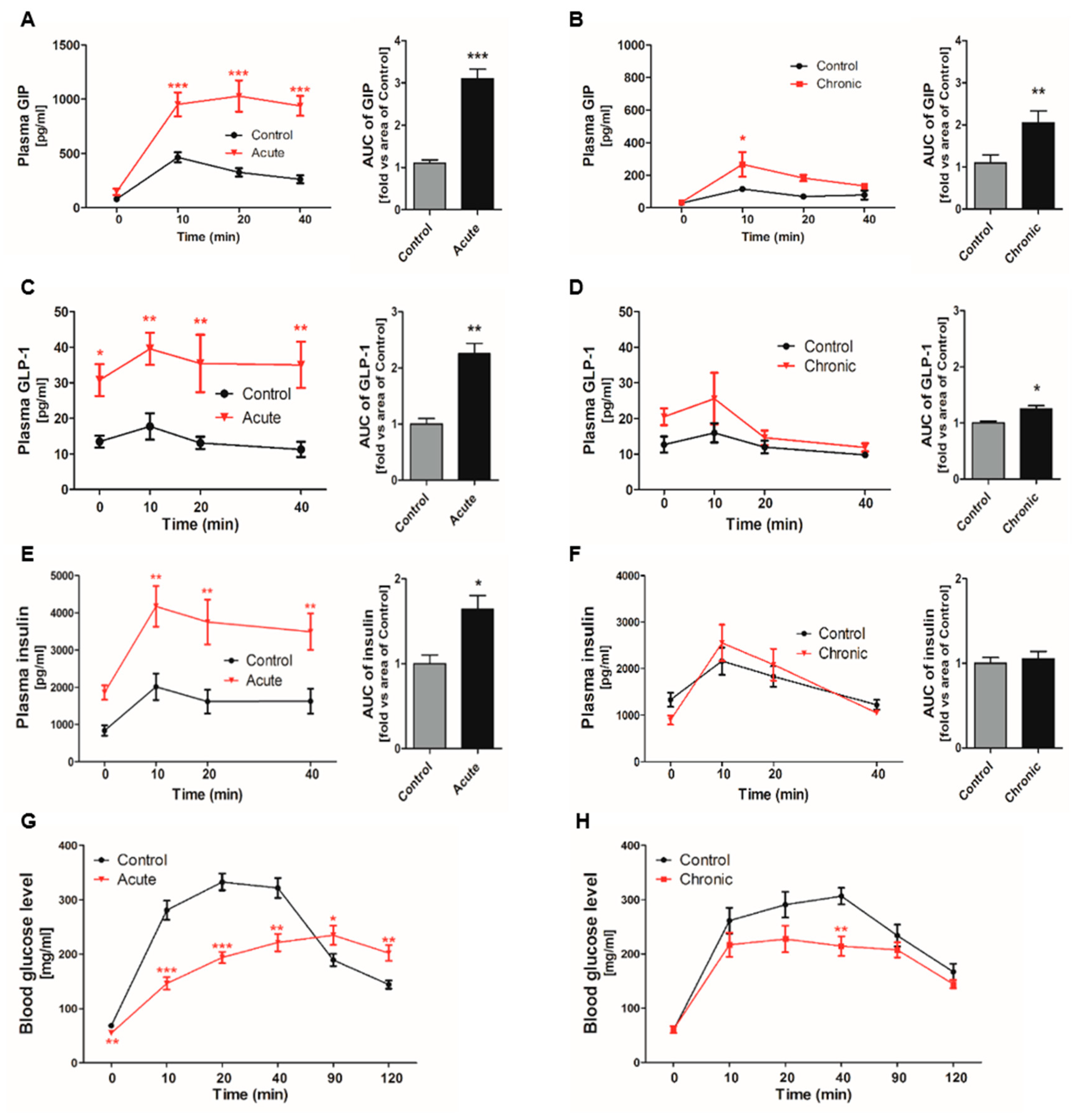
2.1. Incretin Secretion Increased by Glucose Gavage in Gastrointestinal (GI)-Inflamed Mice
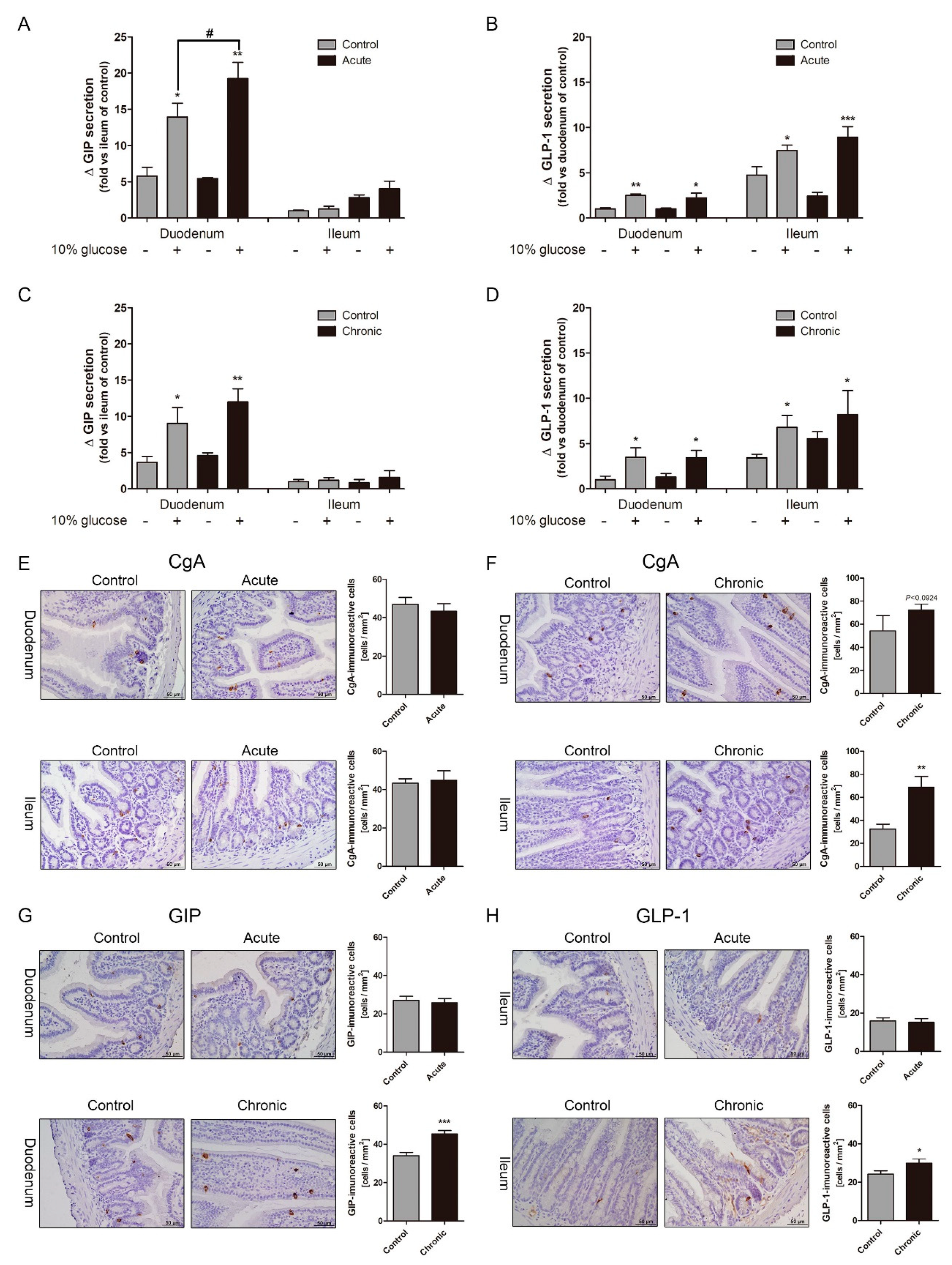
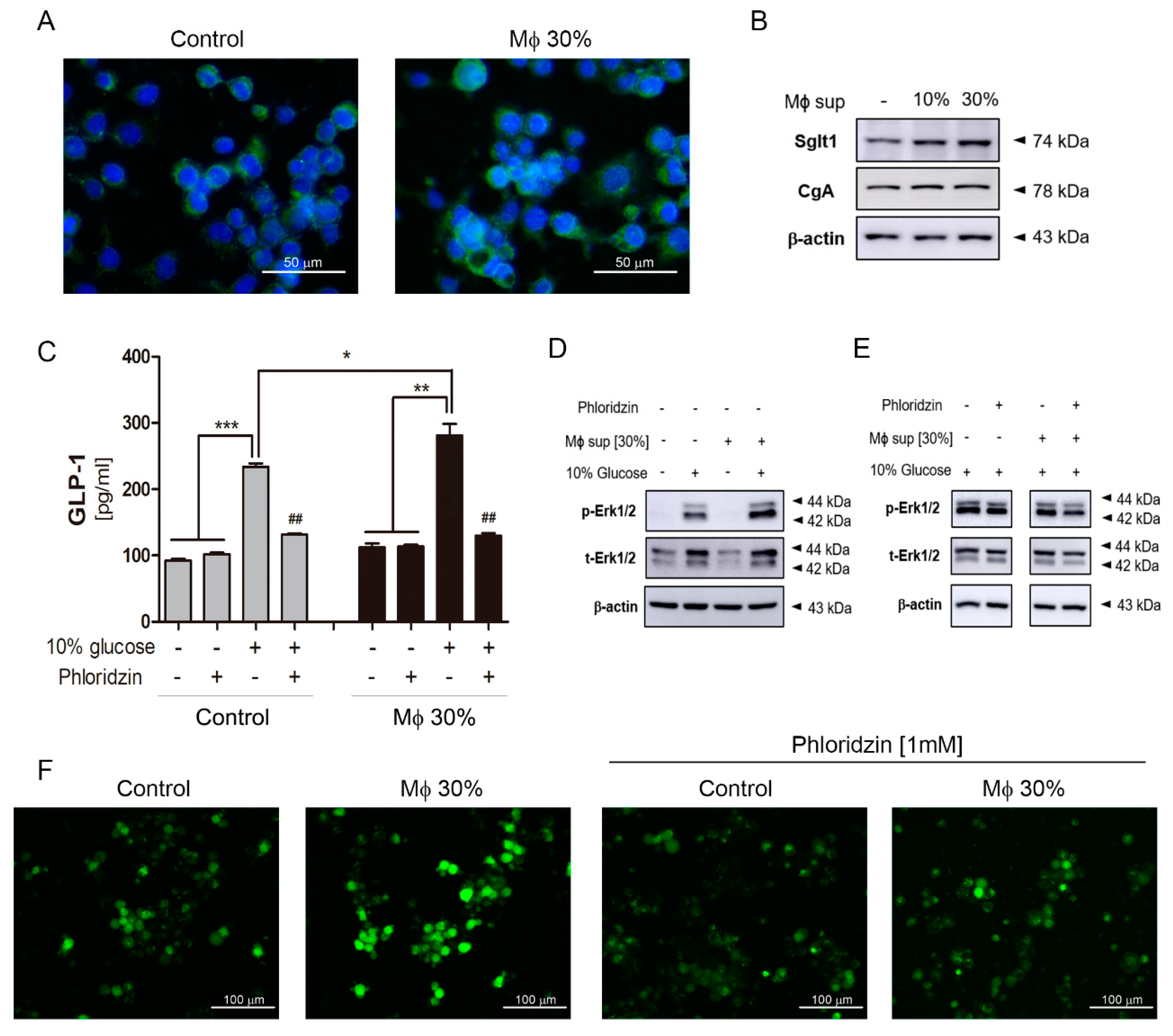
2.2. Gastrointestinal (GI) Inflammatory Conditions Stimulate Glucose-Induced Incretin Secretion
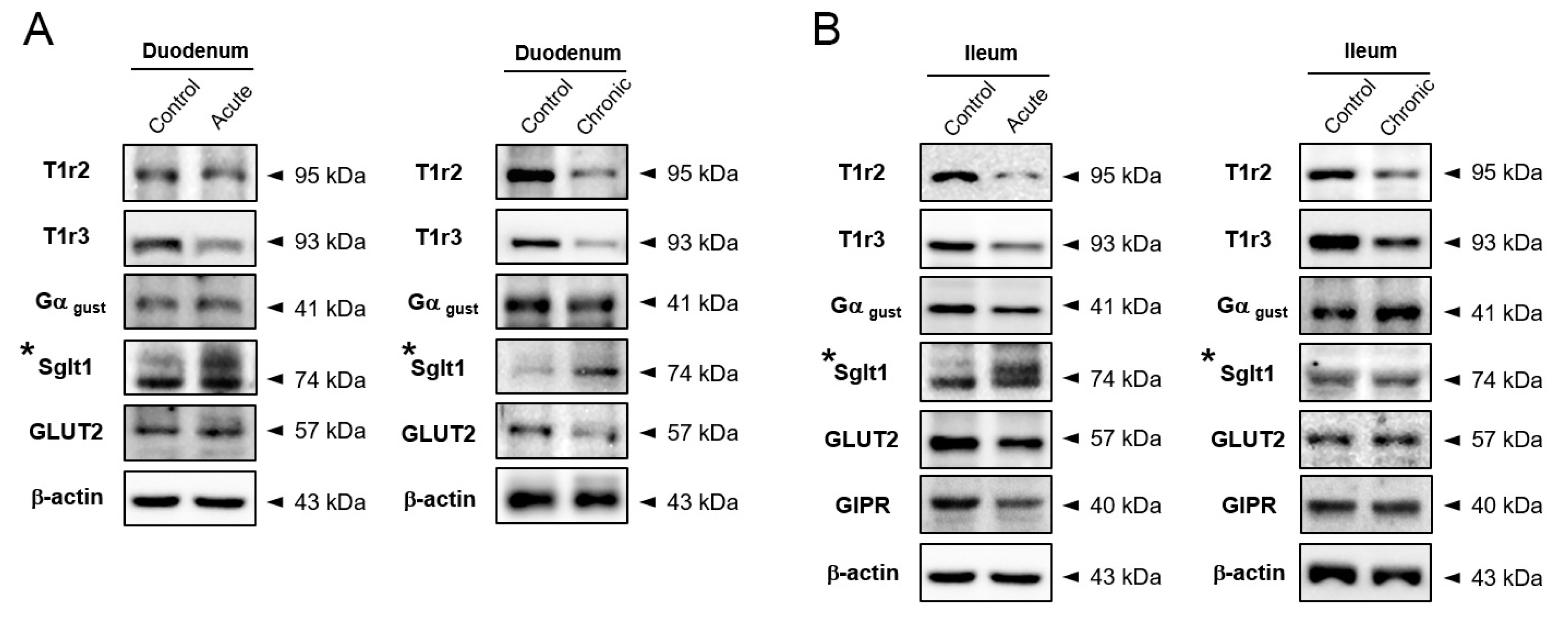
2.3. Na+-Glucose Co-Transporter Sglt1 Protein is Increased in GI-Inflamed Mice
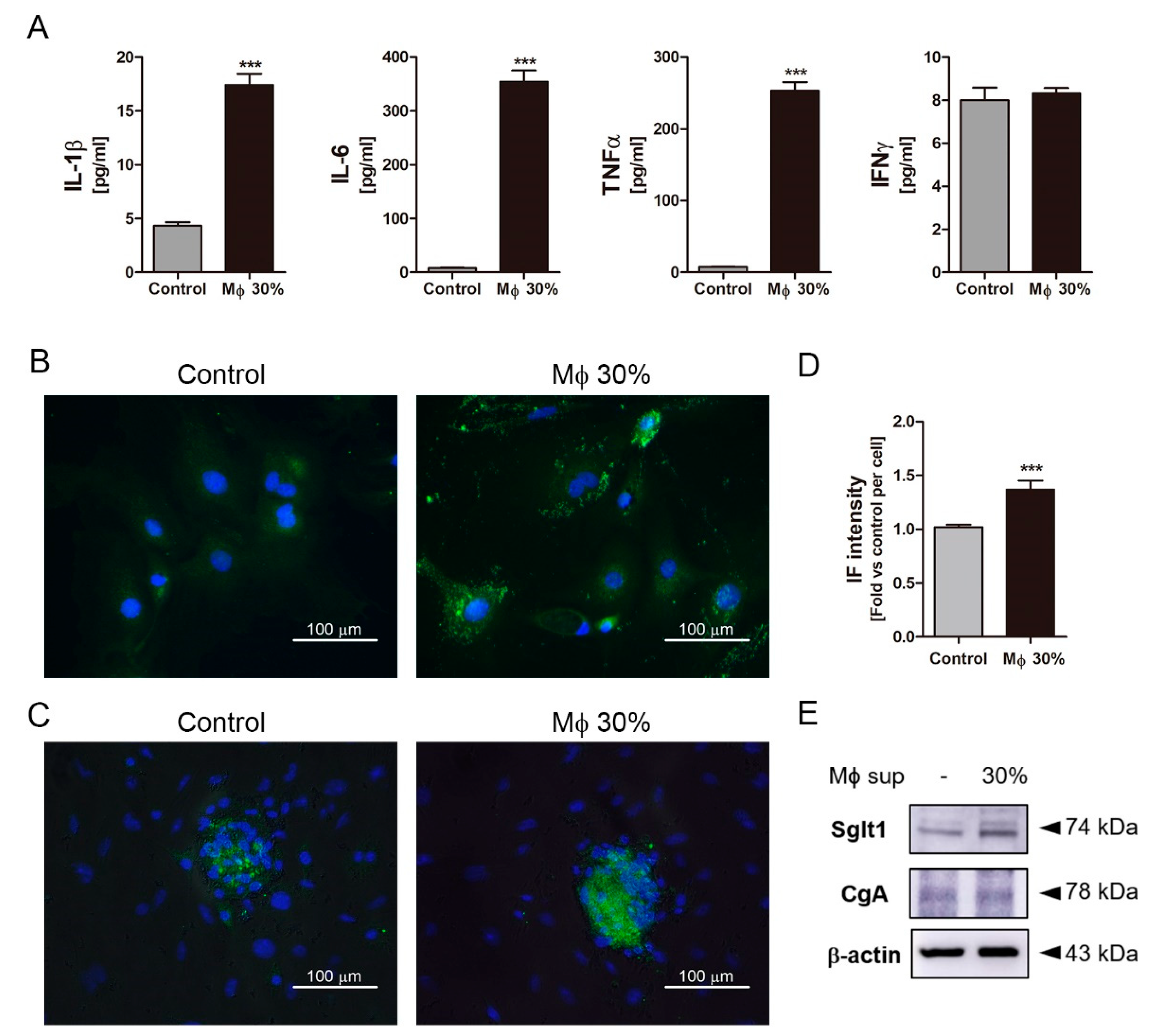
2.4. Inflammatory Condition Induces an Increment of Na+-Glucose Co-Transporter Sglt1
3. Discussion
4. Material and Methods
4.1. Mice and DSS-Induced GI Inflammation
4.2. Assessment of Clinical Score and Histological Score
4.3. Oral Glucose Tolerance Test and Blood Sampling for Multiplex Assay
4.4. Measurement of Gut Hormones and Cytokines in Blood
4.5. Immunohistochemistry and Immunofluorescence
4.6. Tissue Culture of Mouse Small Intestine
4.7. Cell Culture and Preparation of Conditioned Media
4.8. Isolation Intestinal Epithelium Cells
4.9. Immunoblotting
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GI | Gastrointestinal |
| Sglt1 | Sodium-glucose cotransporter 1 |
| GIP | Glucose-dependent insulinotropic polypeptide |
| GLP-1 | Glucagon-like peptide-1 |
| Incretin | GIP and GLP-1 |
| STR | Sweet taste receptor |
| IBD | Inflammatory bowel disease |
| IECs | Intestinal epithelium cells |
References
- Wu, T.; Rayner, C.K.; Young, R.L.; Horowitz, M. Gut motility and enteroendocrine secretion. Curr. Opin. Pharmacol. 2013, 13, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef]
- Dockray, G.J. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharm. 2013, 13, 954–958. [Google Scholar] [CrossRef]
- Doyle, M.E.; Egan, J.M. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007, 113, 546–593. [Google Scholar] [CrossRef]
- Buteau, J. GLP-1 receptor signaling: Effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 2008, 34 (Suppl. 2), S73–S77. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Tovar, A.R.; Torres, N. Diet: Friend or foe of enteroendocrine cells--how it interacts with enteroendocrine cells. Adv. Nutr. (BethesdaMd.) 2012, 3, 8–20. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Bloom, S.R. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver 2012, 6, 10–20. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Prud’homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Egan, J.M.; Jang, H.J. Denatonium induces secretion of glucagon-like peptide-1 through activation of bitter taste receptor pathways. Diabetologia 2014, 57, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kokrashvili, Z.; Yee, K.K.; Ilegems, E.; Iwatsuki, K.; Li, Y.; Mosinger, B.; Margolskee, R.F. Endocrine taste cells. Br. J. Nutr. 2014, 111 (Suppl. 1), S23–S29. [Google Scholar] [CrossRef]
- Gagnon, J.; Baggio, L.L.; Drucker, D.J.; Brubaker, P.L. Ghrelin Is a Novel Regulator of GLP-1 Secretion. Diabetes 2015, 64, 1513–1521. [Google Scholar] [CrossRef]
- Rozengurt, N.; Wu, S.V.; Chen, M.C.; Huang, C.; Sternini, C.; Rozengurt, E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G792–G802. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008, 8, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obesity 2015, 39, 424–429. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- Lennard-Jones, J.E. Classification of inflammatory bowel disease. Scand. J. Gastroenterol. Suppl. 1989, 170, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory bowel disease (2). New Engl. J. Med. 1991, 325, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef]
- Harrison, E.; Lal, S.; McLaughlin, J.T. Enteroendocrine cells in gastrointestinal pathophysiology. Curr. Opin. Pharm. 2013, 13, 941–945. [Google Scholar] [CrossRef]
- McDermott, J.R.; Leslie, F.C.; D’Amato, M.; Thompson, D.G.; Grencis, R.K.; McLaughlin, J.T. Immune control of food intake: Enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation. Gut 2006, 55, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Beglinger, C.; Holst, J.J.; Andresen, V.; Layer, P. Mechanisms of gastric emptying disturbances in chronic and acute inflammation of the distal gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G861–G868. [Google Scholar] [CrossRef]
- Kahles, F.; Meyer, C.; Mollmann, J.; Diebold, S.; Findeisen, H.M.; Lebherz, C.; Trautwein, C.; Koch, A.; Tacke, F.; Marx, N.; et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes 2014, 63, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Mandard, S.; Dray, C.; Deckert, V.; Valet, P.; Besnard, P.; Drucker, D.J.; Lagrost, L.; Grober, J. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: Involvement of the GLP-1 pathway. Diabetes 2014, 63, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar]
- Kitajima, S.; Takuma, S.; Morimoto, M. Tissue distribution of dextran sulfate sodium (DSS) in the acute phase of murine DSS-induced colitis. J. Vet. Med. Sci. 1999, 61, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.Y.; Park, J.H.; Yim, S.-V.; Son, Y.; Hong, H.S. Substance-P protects intestinal epithelium against dextran sulfate sodium-induced toxicity in vitro. Mol. Cell. Toxicol. 2016, 12, 391–398. [Google Scholar] [CrossRef]
- Roda, G.; Sartini, A.; Zambon, E.; Calafiore, A.; Marocchi, M.; Caponi, A.; Belluzzi, A.; Roda, E. Intestinal epithelial cells in inflammatory bowel diseases. World J. Gastroenterol. 2010, 16, 4264–4271. [Google Scholar] [CrossRef]
- Zietek, T.; Rath, E. Inflammation Meets Metabolic Disease: Gut Feeling Mediated by GLP-1. Front. Immunol. 2016, 7, 154. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H. Abnormalities in endocrine and immune cells are correlated in dextransulfatesodiuminduced colitis in rats. Mol. Med. Rep. 2017, 15, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Zhang, N.; Wen, S.; Zhang, J.; Sun, X.L.; Fan, X.M.; Sun, Y.H. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 459–465. [Google Scholar] [CrossRef]
- Schmidt, P.T.; Ljung, T.; Hartmann, B.; Hare, K.J.; Holst, J.J.; Hellstrom, P.M. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: Comparison with peptide YY. Eur. J. Gastroenterol. Hepatol. 2005, 17, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tigas, S.; Tsatsoulis, A. Endocrine and metabolic manifestations in inflammatory bowel disease. Ann. Gastroenterol. 2012, 25, 37–44. [Google Scholar] [PubMed]
- Martin-de-Carpi, J.; Moriczi, M.; Pujol-Muncunill, G.; Navas-Lopez, V.M. Pancreatic Involvement in Pediatric Inflammatory Bowel Disease. Front. Pediatrics 2017, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Kahles, F.; Piotrowski, K.; Vogeser, M.; Foldenauer, A.C.; Nassau, K.; Kilger, E.; Marx, N.; Parhofer, K.G.; Lehrke, M. Interleukin-6 predicts inflammation-induced increase of Glucagon-like peptide-1 in humans in response to cardiac surgery with association to parameters of glucose metabolism. Cardiovasc. Diabetol. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Grisouard, J.; Sauter, N.S.; Herzog-Radimerski, T.; Dembinski, K.; Peterli, R.; Frey, D.M.; Zulewski, H.; Keller, U.; Muller, B.; et al. Glucose-dependent insulinotropic polypeptide induces cytokine expression, lipolysis, and insulin resistance in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1–E13. [Google Scholar] [CrossRef]
- Munitz, A.; Seidu, L.; Cole, E.T.; Ahrens, R.; Hogan, S.P.; Rothenberg, M.E. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J. Immunol. 2009, 182, 2357–2363. [Google Scholar] [CrossRef]
- Gabele, E.; Dostert, K.; Hofmann, C.; Wiest, R.; Scholmerich, J.; Hellerbrand, C.; Obermeier, F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 2011, 55, 1391–1399. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Lenaerts, K.; Kiers, D.; Pais de Barros, J.P.; Le Guern, N.; Plesnik, J.; Thomas, C.; Bourgeois, T.; Dejong, C.H.C.; Kox, M.; et al. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 2017, 21, 1160–1168. [Google Scholar] [CrossRef]
- Kahles, F.; Meyer, C.; Diebold, S.; Foldenauer, A.C.; Stohr, R.; Mollmann, J.; Lebherz, C.; Findeisen, H.M.; Marx, N.; Lehrke, M. Glucose-dependent insulinotropic peptide secretion is induced by inflammatory stimuli in an interleukin-1-dependent manner in mice. Diabetes Obes. Metab. 2016, 18, 1147–1151. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schurmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.; Sauve, M.; Zhao, W.; Stacey, H.M.; Wiber, S.C.; Bolz, S.S.; Brubaker, P.L. Chronic Exposure to TNFalpha Impairs Secretion of Glucagon-Like Peptide-1. Endocrinology 2015, 156, 3950–3960. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-W.; Park, S.-H.; Lee, I.-C.; Lee, S.-M.; Shin, I.-S.; Kang, S.-S.; Moon, C.; Kim, S.-H.; Heo, J.-D.; Kim, J.-C. Protective effects of garlic oil against 1,3-dichloro-2-propanol-induced hepatotoxicity: Role of CYP2E1 and MAPKs. Mol. Cell. Toxicol. 2016, 12, 185–195. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, K.S.; Kim, K.H.; Park, J.; Jeong, H.S.; Kim, Y.; Na, Y.C.; Chung, W.S.; Ahn, K.S.; Lee, S.G.; et al. Antihyperglycemic and Antiobesity Effects of JAL2 on db/db Mice. Evid. -Based Complementary Altern. Med. Ecam 2016, 2016, 6828514. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.S.; Kim, K.H.; Lee, I.S.; Jeong, H.S.; Kim, Y.; Jang, H.J. GLP-1 secretion is stimulated by 1,10-phenanthroline via colocalized T2R5 signal transduction in human enteroendocrine L cell. Biochem. Biophys. Res. Commun. 2015, 468, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Lee, I.S.; Kim, K.H.; Kim, Y.; Na, Y.C.; Lee, J.H.; Jang, H.J. The therapeutic effects of Yongdamsagan-tang on autoimmune hepatitis models. Biomed. Pharm. 2017, 94, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Park, J.Y.; Lee, I.-S.; Kim, Y.; Jang, H.-J. Proteins derived from Prunus armeniaca kernel are possible to cause Immunoglobulin E reactivity in human sera. Mol. Cell. Toxicol. 2017, 13, 213–220. [Google Scholar] [CrossRef]
- Lee, I.S.; Kim, K.S.; Kim, K.H.; Park, J.; Jeong, H.S.; Kim, Y.; Na, Y.C.; Lee, S.G.; Ahn, K.S.; Lee, J.H.; et al. Anti-diabetic and anti-obesitic effects of aqueous extracts of Yangkyuksanhwa-tang and its two major compositions on db/db mice. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 83, 431–438. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, I.-S.; Kim, K.-H.; Kim, Y.; An, E.-J.; Jang, H.-J. GI inflammation Increases Sodium-Glucose Cotransporter Sglt1. Int. J. Mol. Sci. 2019, 20, 2537. https://doi.org/10.3390/ijms20102537
Park J, Lee I-S, Kim K-H, Kim Y, An E-J, Jang H-J. GI inflammation Increases Sodium-Glucose Cotransporter Sglt1. International Journal of Molecular Sciences. 2019; 20(10):2537. https://doi.org/10.3390/ijms20102537
Chicago/Turabian StylePark, Jiyoung, In-Seung Lee, Kang-Hoon Kim, Yumi Kim, Eun-Jin An, and Hyeung-Jin Jang. 2019. "GI inflammation Increases Sodium-Glucose Cotransporter Sglt1" International Journal of Molecular Sciences 20, no. 10: 2537. https://doi.org/10.3390/ijms20102537
APA StylePark, J., Lee, I.-S., Kim, K.-H., Kim, Y., An, E.-J., & Jang, H.-J. (2019). GI inflammation Increases Sodium-Glucose Cotransporter Sglt1. International Journal of Molecular Sciences, 20(10), 2537. https://doi.org/10.3390/ijms20102537





