Kinase-Inactivated EGFR Is Required for the Survival of Wild-Type EGFR-Expressing Cancer Cells Treated with Tyrosine Kinase Inhibitors
Abstract
1. Introduction
2. Results
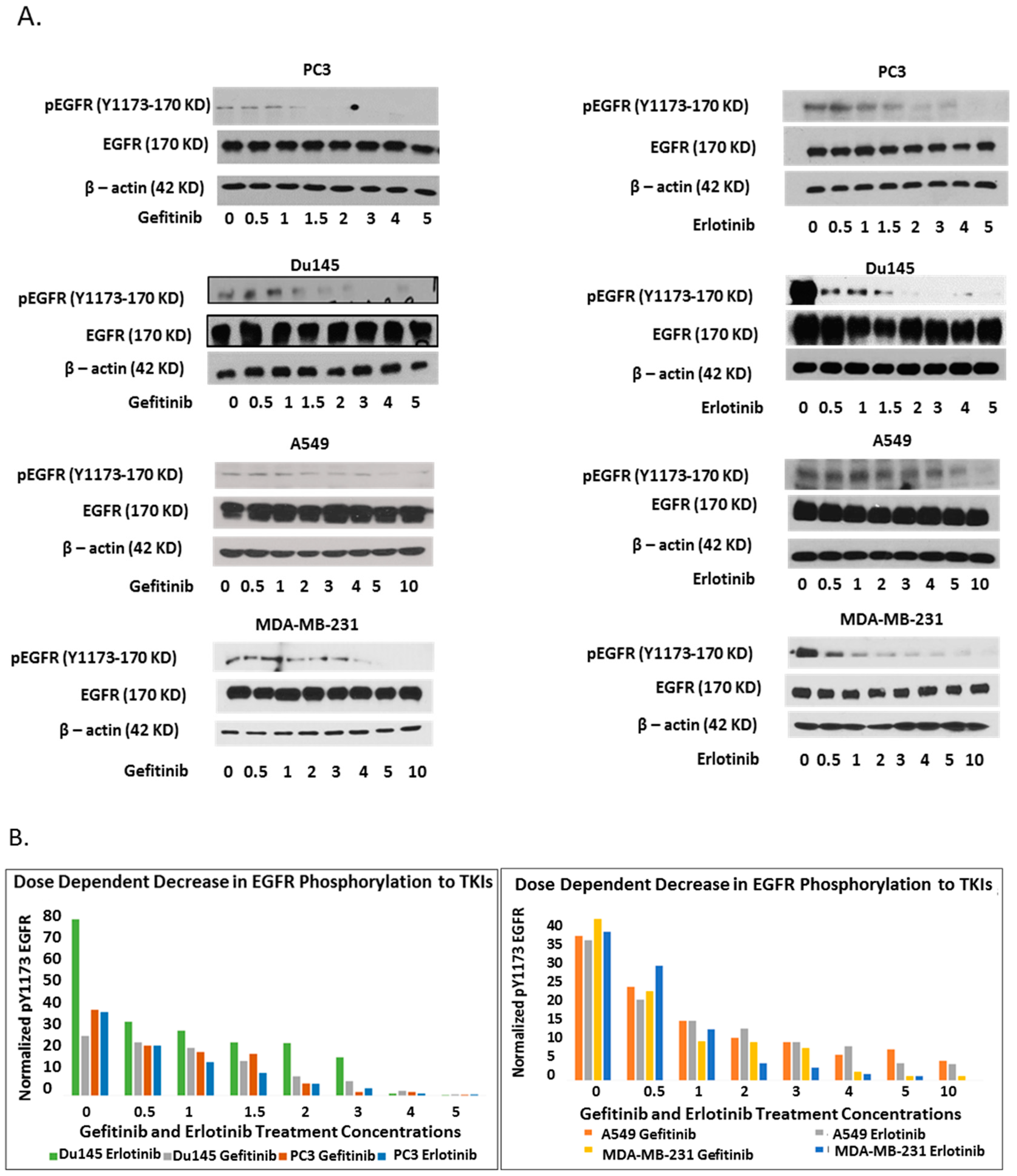
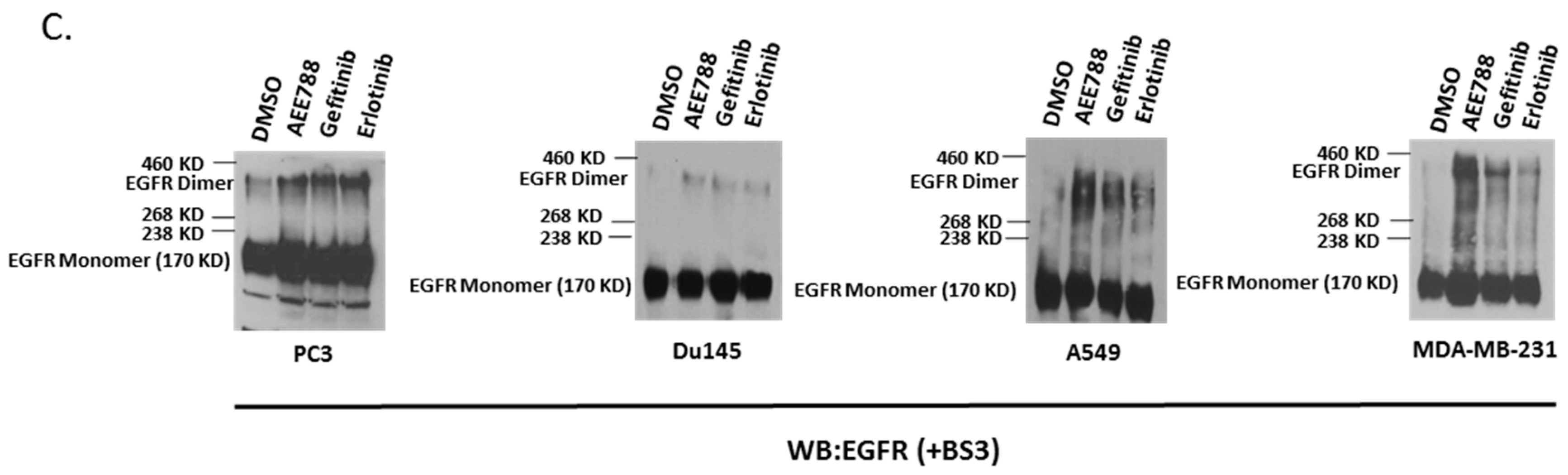
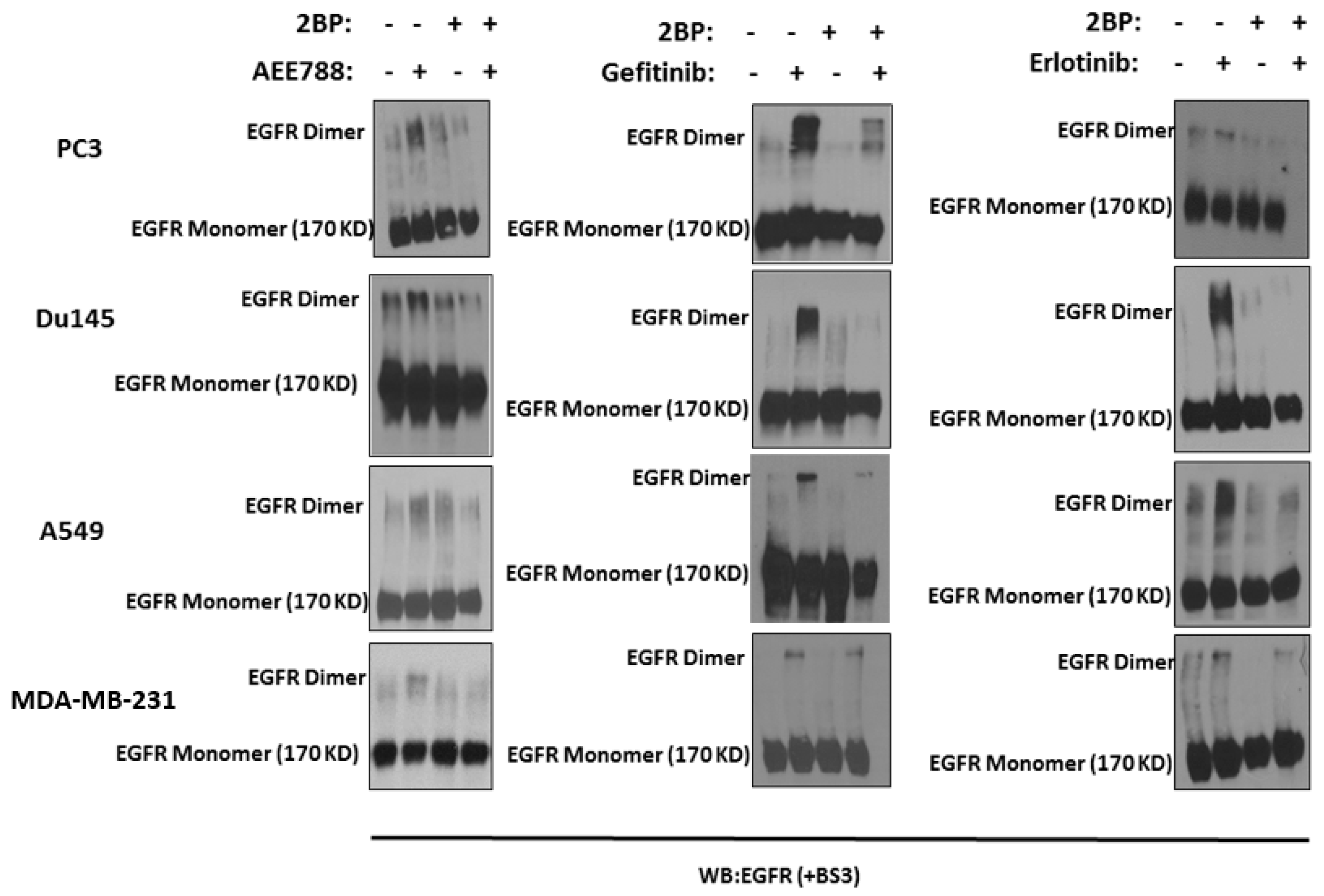
2.1. Epidermal Growth Factor Receptor (EGFR) Dimerization in Response to Acute Treatment of Tyrosine Kinase Inhibitors (TKIs)
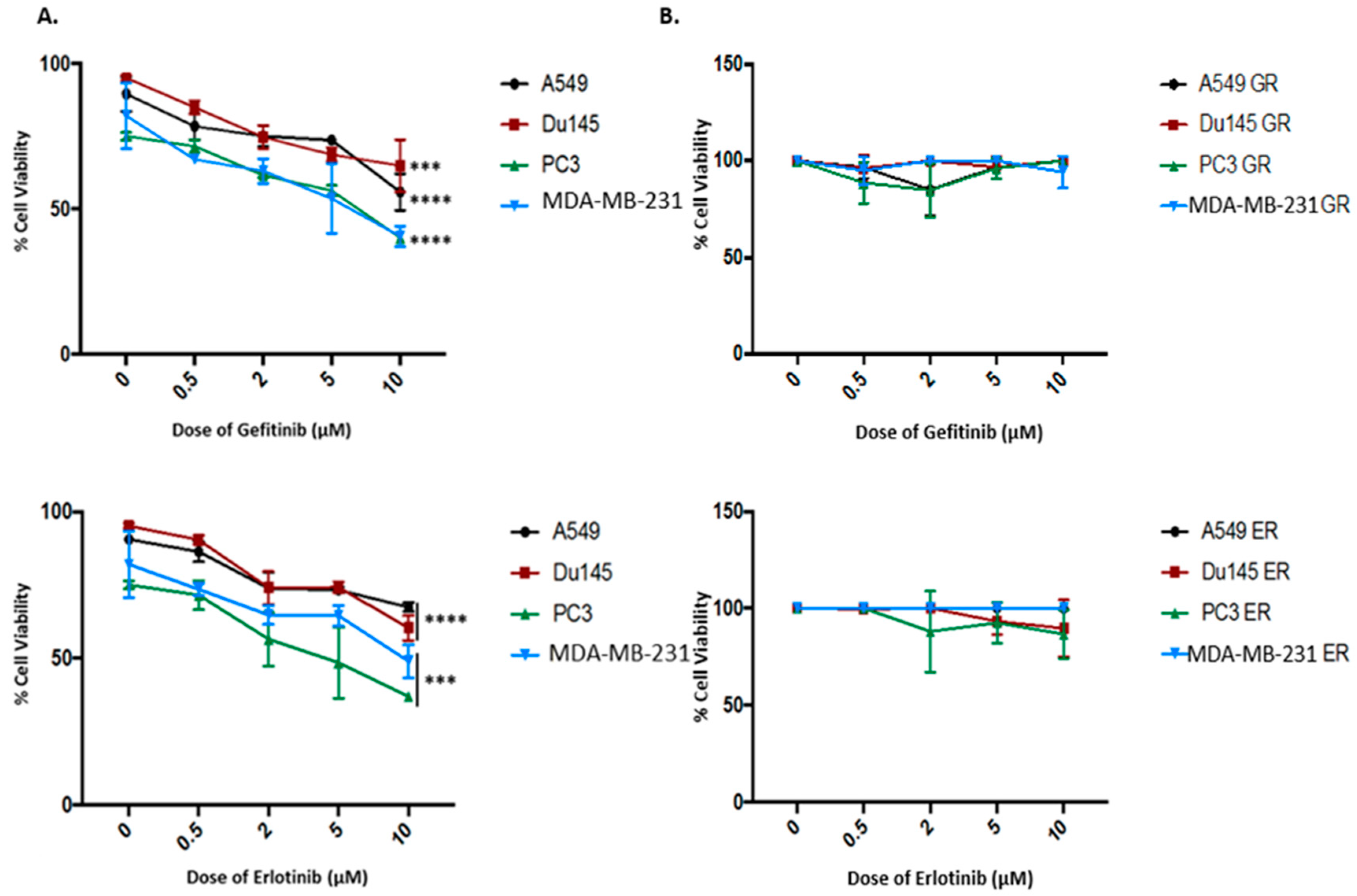
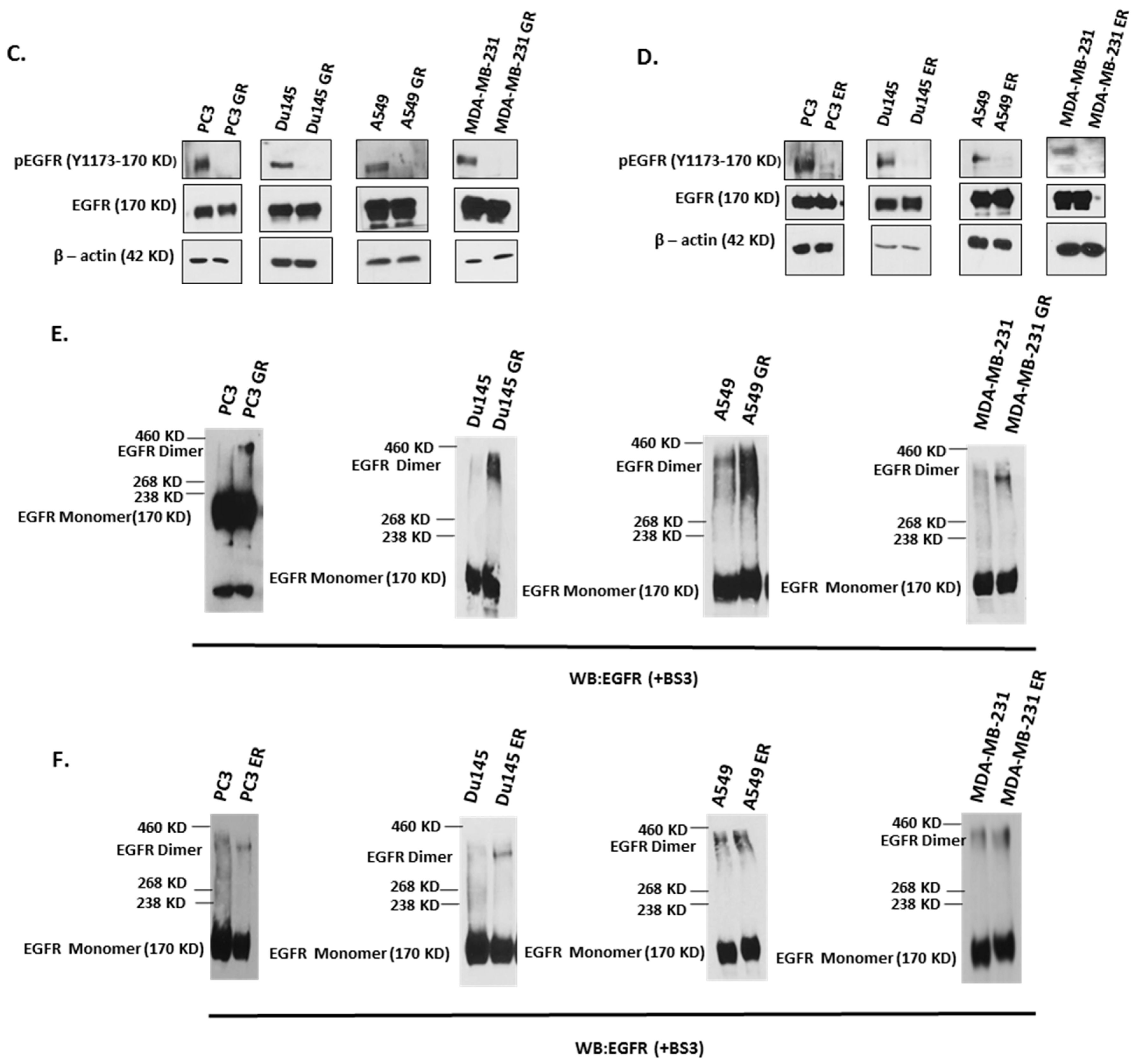
2.2. Kinase-Inactivated EGFR Dimers Increased in TKI Resistant Cells
2.3. Inhibition of Palmitoylation Abolishes TKI-Induced EGFR Dimer Formation
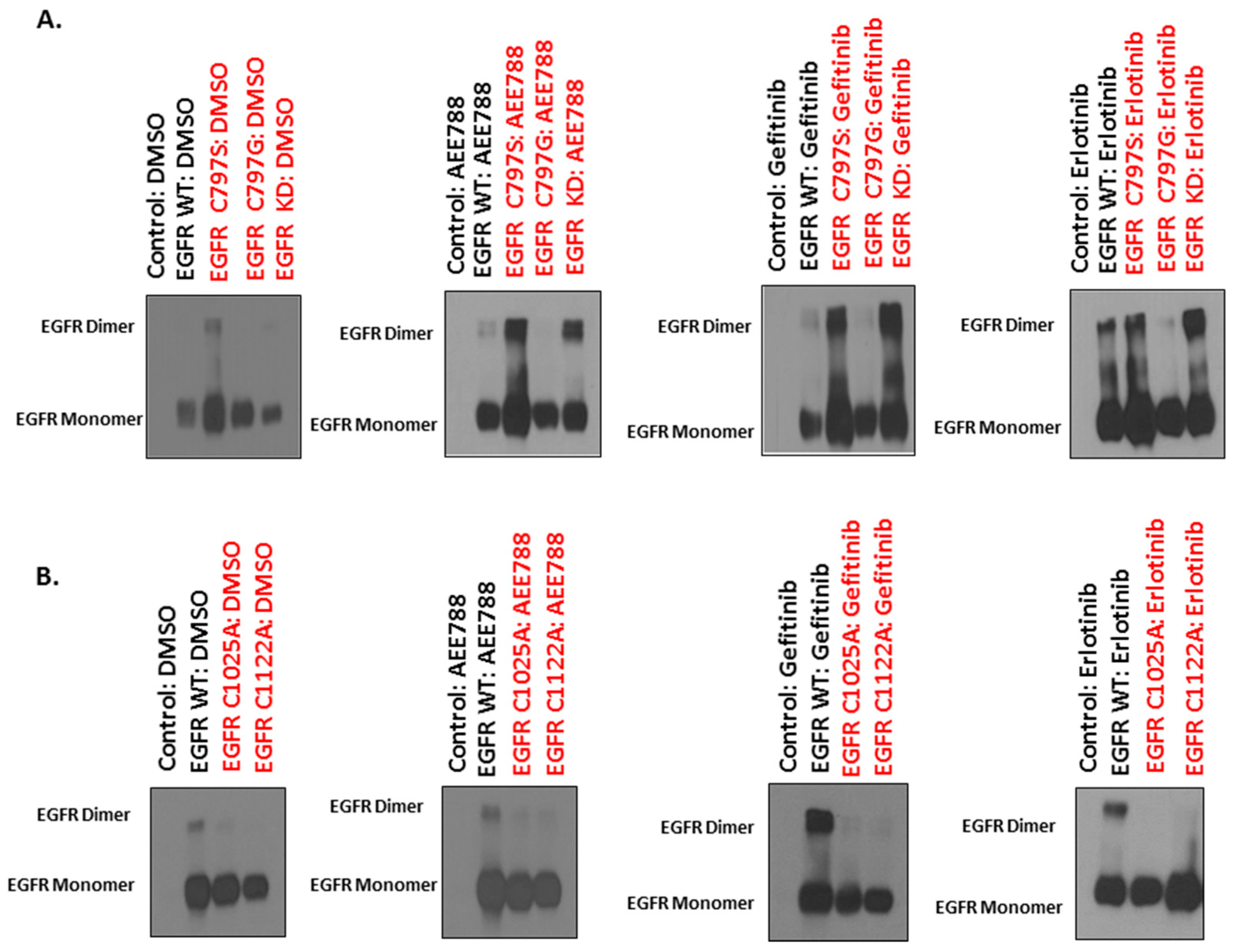
2.4. Mutations of Cysteine Residues Critical for EGFR Palmitoylation Abolished TKI-Induced Dimerization, and the Kinase Activity of EGFR Is Not Required for TKI-Induced Dimerization of EGFR
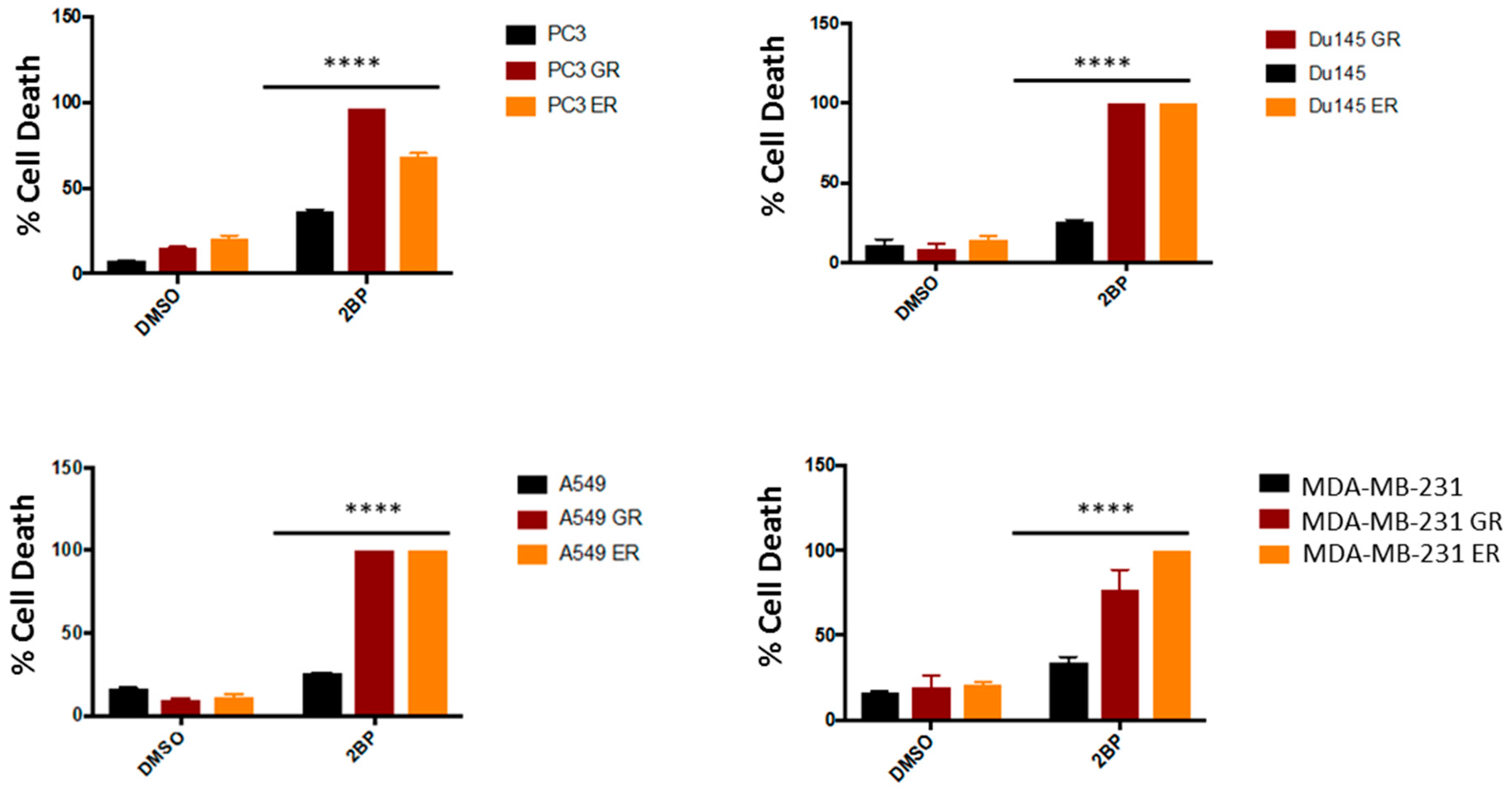
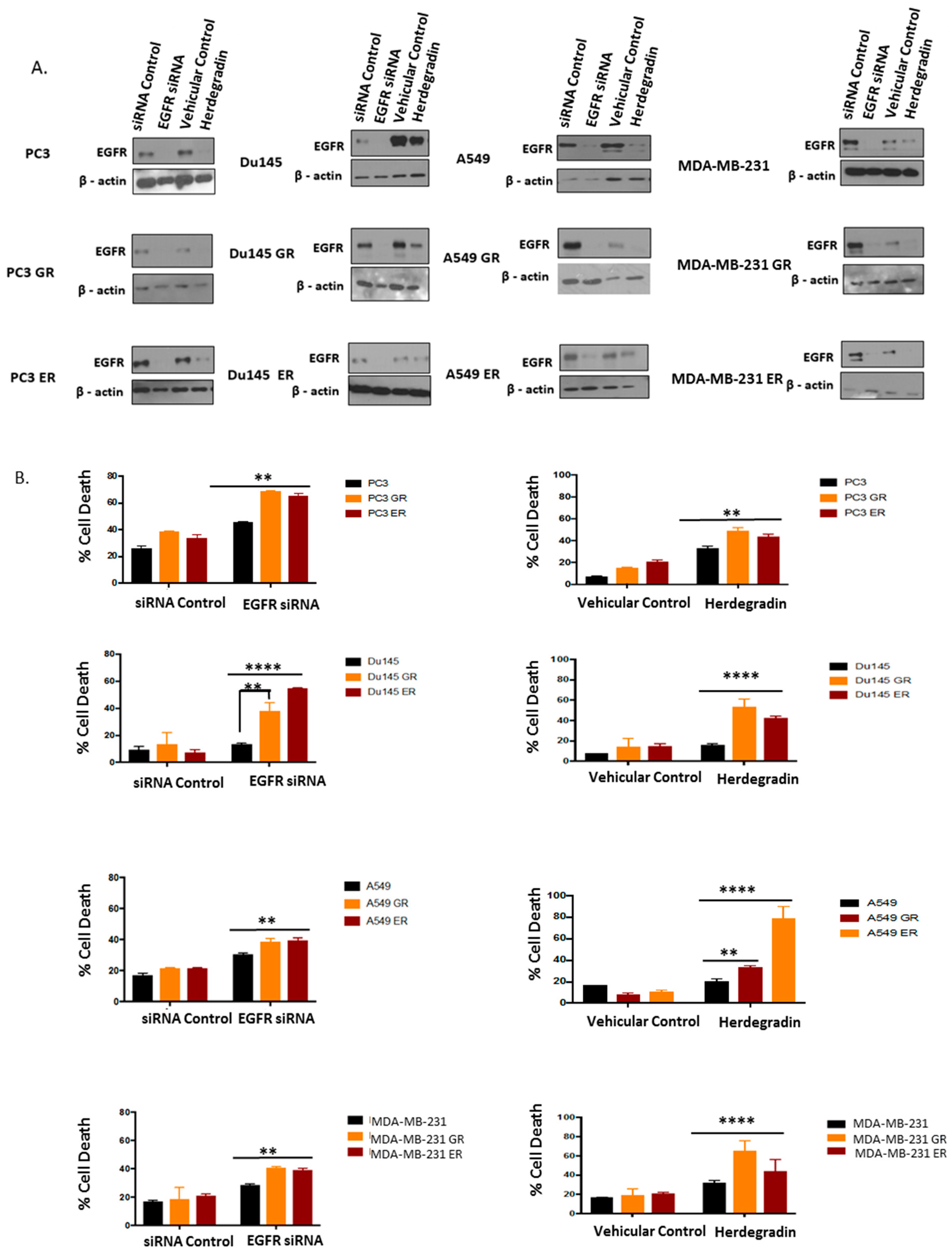
2.5. Both Inhibition of Palmitoylation and Targeted Reduction of EGFR Are Lethal to TKI-Resistant Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Reagents
4.2. Establishment of Gefitinib- and Erlotinib-Resistant Cells
4.3. Plasmids, siRNAs, and Peptide
4.4. Transfections and Western Blotting
4.5. EGFR Dimerization Assay
4.6. MTT Cell Proliferation/Cell Viability Assay
4.7. Trypan Blue Exclusion Assay
4.8. Statistics
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Roskoski, R.J. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharm. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Jin, H. EGFR-TKI resistance in NSCLC patients, mechanisms and strategies. Am. J. Cancer Res. 2014, 4, 411–435. [Google Scholar]
- Weihua, Z.; Tsan, R.; Huang, W.C.; Wu, Q.; Chiu, C.H.; Fidler, I.J.; Hung, M.C. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell 2008, 13, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J. The epidermal growth factor receptor as a target for cancer therapy. Endocr. Relat. Cancer 2001, 8, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.R.; Cepero, V.; Giordano, S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol. Cancer 2010, 9, 1–13. [Google Scholar] [CrossRef]
- Nan, X.; Xie, C.; Yu, X.; Liu, J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget 2017, 8, 75712–75726. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors, from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Mueller, K.L.; Bohin, N.; Ge, Y.; Boerner, J.L. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J. Cell Physiol. 2011, 226, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Cossu-Rocca, P.; Muroni, M.R.; Sanges, F.; Sotgiu, G.; Asunis, A.; Tanca, L.; Onnis, D.; Pira, G.; Manca, A.; Dore, S.; et al. EGFR kinase-dependent and kinase-independent roles in clear cell renal cell carcinoma. Am. J. Cancer Res. 2016, 6, 71–83. [Google Scholar]
- Riely, G.J.; Yu, H.A. EGFR: The paradigm of an oncogene-driven lung cancer. Clin. Cancer Res. 2015, 21, 2221–2226. [Google Scholar] [CrossRef]
- Ricordel, C.; Friboulet, L.; Facchinetti, F.; Soria, J.C. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann. Oncol. 2018, 29, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Syn, N.L.; Cho, B.C.; Soo, R.A. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer, mechanisms and therapeutic strategies. Cancer Treat. Rev. 2018, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Pao, W.; Sequist, L.V. Acquired resistance to TKIs in solid tumors: learning from lung cancer. Nat. Rev. Clin. Oncol. 2014, 11, 473–481. [Google Scholar] [CrossRef]
- Husain, H.; Scur, M.; Murtuza, A.; Bui, N.; Woodward, B.; Kurzrock, R. Strategies to overcome bypass mediating clinical resistance to EGFR tyrosine kinase inhibition in lung cancer. Mol. Cancer Ther. 2017, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Cortot, A.B.; Janne, P.A. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung cancer adenocarcinoma. Eur. Respir. Rev. 2014, 23, 356–366. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of tumor specimen at the time of acquired resistance to EGFR-TKI therapy in 155 Patients with EGFR mutant lung cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef]
- Canil, C.M.; Moore, M.J.; Winquist, E.; Baetz, T.; Pollack, M.; Chi, K.N.; Berry, S.; Ernst, D.S.; Douglas, L.; Brundage, M.; et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer, a trial of the national cancer institute of canada-clinical trials group. J. Clin. Oncol. 2005, 23, 455–460. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, S.; Zhao, W.; Qin, S.; Chu, Q.; Wu, K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol. Cancer 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Lisberg, A.; Garon, E.B. Epidermal growth factor tyrosine kinase inhibitor therapy inferior to second-line chemotherapy in EGFR wild-type non-small cell lung cancer patients: results of French nationwide observational study. Transl. Lung Cancer Res. 2017, 6, S39–S40. [Google Scholar] [CrossRef]
- Ojemuyiwa, M.A.; Madan, R.A.; Dahut, W.L. Tyrosine kinase inhibitors in the treatment of prostate cancer: taking the next step in clinical development. Expert Opin. Emerg. Drugs 2014, 19, 459–470. [Google Scholar] [CrossRef]
- Mahipal, A.; Kothan, N.; Gupta, S. Epidermal growth factor receptor inhibitors: coming of age. Cancer Control 2014, 21, 74–79. [Google Scholar] [CrossRef]
- Matsuda, N.; Lim, B.; Wang, X.; Ueno, N.T. Early clinical development of epidermal growth factor receptor targeted therapy in breast cancer. Expert Opin. Investig. Drugs 2017, 26, 463–479. [Google Scholar] [CrossRef]
- Ilaria, B.; Bracarda, S.; Caserta, C.; Minelli, A. Targeting of EGFR tyrosine kinase by ZD1839 (“Iressa”) in androgen-responsive prostate cancer in vitro. Mol. Genet. Metab. 2006, 88, 114–122. [Google Scholar] [CrossRef]
- Festuccia, C.; Gravia, G.L.; Biordi, L.; D’Ascenzo, S.; Dolo, V.; Corrado, F.; Ricevuto, E.; Tombolini, V. Effects of EGFR tyrosine kinase inhibitor erlotinib in prostate cancer cells in vitro. Prostate 2009, 69, 1529–1537. [Google Scholar] [CrossRef]
- Anderson, N.G.; Ahmad, T.; Chan, K.; Dobson, R.; Bundred, N.J. ZD1839 (Iressa), a novel epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, potently inhibits the growth of EGFR-positive cancer cell lines with or without erbB2 overexpression. Int. J. Cancer 2001, 94, 774–782. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, X.; Ali-Osman, F.; Keir, S.; Lo, H.W. EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial translocation of PUMA and PUMA-mediated apoptosis independent of EGFR kinase. Cancer Lett. 2015, 294, 101–110. [Google Scholar] [CrossRef][Green Version]
- Tan, X.; Thapa, N.; Sun, Y.; Anderson, R.A. A kinase-independent role for EGF receptor in autophagy initiation. Cell 2015, 160, 145–160. [Google Scholar] [CrossRef]
- Bollu, L.R.; Ren, J.; Blessing, A.M.; Katreddy, R.R.; Gao, G.; Xu, L.; Wang, J.; Su, F.; Weihua, Z. Involvement of de novo synthesized palmitate and mitochondrial EGFR in EGF induced mitochondrial fusion of cancer cells. Cell Cycle 2014, 13, 2415–2430. [Google Scholar] [CrossRef][Green Version]
- Tsuchihashi, K.; Okazaki, S.; Ohmura, M.; Ishikawa, M.; Sampetrean, O.; Onishi, H.; Wakimoto, H.; Yoshikawa, M.; Seishima, R.; Iwasaki, Y.; et al. The Egf receptor promotes the malignant potential of glioma by regulating amino acid transport system Xc(-). Cancer Res. 2016, 76, 2954–2963. [Google Scholar] [CrossRef]
- Katreddy, R.R.; Bollu, L.R.; Su, F.; Xian, N.; Srivastava, S.; Thomas, R.; Dai, Y.; Wu, B.; Xu, Y.; Rea, M.A.; et al. Targeted reduction of the EGFR protein, but not inhibition of its kinase activity, induces mitophagy and death of cancer cells through activation of mTORC2 and Akt. Oncogenesis 2018, 7, 1–13. [Google Scholar] [CrossRef]
- Cho, J.; Kim, S.; Du, J.; Meyerson, M. Autophosphorylation of the carboxy-terminal domain is not required for oncogenic transformation by lung cancer derived mutants. Int. J. Cancer 2018, 143, 679–685. [Google Scholar] [CrossRef]
- Munoz, L. Non-kinase targets of protein kinase inhibitors. Nat. Rev. 2017, 16, 424–440. [Google Scholar] [CrossRef]
- Bjorkelund, H.; Gedda, L.; Barta, P.; Malmqvist, M.; Anderson, K. Gefitinib induces epidermal growth factor receptor dimers which alters the interaction characteristics with 25I-EGF. PLoS ONE 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Lu, C.; Mi, L.Z.; Schurpf, T.; Walz, T.; Springer, T.A. Mechanisms for kinase-mediated dimerization of the epidermal growth factor receptor. J. Biol. Chem. 2012, 45, 38244–38253. [Google Scholar] [CrossRef]
- Bublil, E.M.; Pines, G.; Patel, G.; Fruhwirth, G.; Ng, T.; Yarden, Y. Kinase-mediated quasi-dimers of EGFR. FASEB J. 2010, 24, 4744–4755. [Google Scholar] [CrossRef]
- Traxler, P.; Allegrini, P.B.; Brandt, R.; Brueggen, J.; Cozens, R.; Fabbro, D.; Grosios, K.; Lane, H.A.; McSheehy, P.; Mestan, J.; et al. AEE788: A dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004, 64, 4931–4941. [Google Scholar] [CrossRef]
- Wong, S.S. Chemistry of Protein Conjugation and Crosslinking; CRC Press: Boca Raton, FL, USA, 1993; pp. 75–103. [Google Scholar]
- Fukata, Y.; Murakami, T.; Yokoi, N.; Fukata, M. Local palmitoylation cycles and specialized membrane domain organization. Curr. Top. Membr. 2016, 77, 97–141. [Google Scholar] [CrossRef]
- Anderson, A.M.; Ragan, M.A. Palmitoylation, a protein S-acylation with implications for breast cancer. NPJ Breast Cancer 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Bollu, L.R.; Katreddy, R.R.; Blessing, A.M.; Nguyen, P.; Zheng, B.; Wu, X.; Weihua, Z. Intracellular activation of EGFR by fatty acid synthase dependent palmitoylation. Oncotarget 2015, 6, 34992–35003. [Google Scholar] [CrossRef]
- Runkle, K.B.; Kharbanda, A.; Stypulkowski, E.; Cao, X.J.; Wang, W.; Garcia, B.A.; Witze, E.S. Inhibition of DHHC20mediated EGFR palmitoylation creates a dependence on EGFR signaling. Mol. Cell 2016, 62, 385–396. [Google Scholar] [CrossRef]
- Jennings, B.C.; Nadolski, M.J.; Ling, Y.; Baker, M.B.; Harrison, M.L.; Deschenes, R.J.; Linder, M.E. 2-Bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[b]thiophen-3-one inhibit DHHC-mediated palmitoylation in vitro. J. Lipid Res. 2009, 50, 233–242. [Google Scholar] [CrossRef]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Lado, M. Fatty acid synthase as a potential therapeutic target in cancer 2010. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Chu, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 560–562. [Google Scholar] [CrossRef]
- Chan, C.; Gill, G.N. Mutational analysis of the nucleotide binding site of the epidermal growth factor receptor and v-Src protein-tyrosine kinases. J. Biol. Chem. 1996, 271, 22619–22623. [Google Scholar] [CrossRef]
- Morgillo, F.; Corte, C.M.D.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR targeted therapies: lung cancer. ESMO Open 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017, 9, 52. [Google Scholar]
- Macdonald-Obermann, J.L.; Piwnica-Worms, D.; Pike, L.J. Mechanics of EGFR receptors/ErbB2 kinase activation revealed by luciferase fragment complementation imaging. Proc. Natl. Acad. Sci. USA 2012, 109, 137–142. [Google Scholar] [CrossRef]
- Coban, O.; Zanetti-Dominguez, L.C.; Matthews, D.R.; Rolfe, D.J.; Weitsman, G.; Barber, P.R.; Barbeau, J.; Devauges, V.; Kampmeier, F.; Winn, M.; et al. Effect of phosphorylation on EGFR dimer stability probed by single molecule dynamics and FRET/FLIM. Biophys. J. 2016, 108, 1013–1026. [Google Scholar] [CrossRef]
- Wang, S.; Tsui, S.T.; Liu, C.; Song, Y.; Liu, D. EGFR C797S mutation mediates resistance to third- generation inhibitors in T790M-positive non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Chu, H.; Yao, Y. Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non-small cell lung cancer with EGFR activating mutations. OncoTargets Ther. 2016, 9, 3711–3726. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Tortora, G.; D’Armiento, F.P.; de Rossa, G.; Staibano, S.; Autorino, R.; D’Armiento, M.; de Placido, S.; Catalano, G.; Bianco, A.R.; et al. Expression of epidermal growth factor receptor correlates with disease relaspse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 2002, 8, 3438–3444. [Google Scholar]
- Scher, H.I.; Sarkis, A.; Reuters, V.; Cohen, D.; Netto, G.; Petrylak, D.; Lianes, P.; Fuks, Z.; Mendelsohn, J.; Cordon-Cardo, C. Changing pattern of expression of the epidermal growth factor receptor and transforming growth factor alpha in the progression of prostatic neoplasms. Clin. Cancer Res. 1995, 1, 545–550. [Google Scholar]
- Gross, M.; Higano, C.; Pantuck, A.; Castellanos, O.; Green, E.; Nguyen, K.; Agus, D.B. A phase II trial of docetaxel and erlotinib as first line therapy for elderly patients with androgen-independent prostate cancer. BMC Cancer 2007, 7, 142. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Jiang, Y.; Liu, J.; Yang, X.; Quan, S. Synergistic interaction between MEK inhibitor and gefitinib in EGFR-TKI resistant human lung cancer cells. Oncol. Lett. 2015, 10, 2652–2656. [Google Scholar] [CrossRef]
- Lee, P.C.; Fang, Y.F.; Yamaguchi, H.; Wang, W.J.; Chen, T.S.; Hong, X.; Ke, B.; Xia, W.; Wei, Y.; Zha, Z.; et al. Targeting PKCδ as a therapeutic strategy against heterogeneous mechanisms of EGFR inhibitor resistance in EGFR-mutant lung cancer. Cancer Cell 2018, 34, 954–969. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, H.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- Chong, C.R.; Janne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2014, 19, 1389–1400. [Google Scholar] [CrossRef]
- Li, H.; You, L.; Xie, J.; Pan, H.; Han, W. The roles of subcellularly located EGFR in Autophagy. Cell Signal. Technol. 2017, 35, 223–230. [Google Scholar] [CrossRef]
- Ren, J.; Bollu, L.R.; Su, F.; Gao, G.; Xu, L.; Huang, W.C.; Hung, M.C.; Weihua, Z. EGFR-SGLT1 interaction does not respond to EGFR modulators, but inhibition of SGLT1 sensitizes prostate cancer cells to EGFR tyrosine kinase inhibitors. Prostate 2013, 73, 1453–1461. [Google Scholar] [CrossRef]
- Fung, C.; Chen, X.; Grandis, J.R.; Duvvui, U. EGFR Tyrosine Kinase Inhibition Induces Autophagy in Cancer Cells. Cancer Biol. Ther. 2012, 13, 1417–1424. [Google Scholar] [CrossRef]
- Lambert, S.; Kezunovic, D.V.; Karvinen, S.; Gniadecki, R. Ligand independent activation of EGFR by lipid raft disruption. J. Investig. Dermatol. 2006, 954–962. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Lupu, R. Targeting fatty acid synthase-driven lipid rafts, a novel strategy to overcome trastuzumab resistance in breast cancer cells. Med. Hypotheses 2005, 64, 997–1001. [Google Scholar] [CrossRef]
- Scaltriti, M.; Baselga, J. The epidermal growth factor receptor pathway, A Model for Targeted Therapy. Clin. Cancer Res. 2006, 12, 5268–5272. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, R.; Srivastava, S.; Katreddy, R.R.; Sobieski, J.; Weihua, Z. Kinase-Inactivated EGFR Is Required for the Survival of Wild-Type EGFR-Expressing Cancer Cells Treated with Tyrosine Kinase Inhibitors. Int. J. Mol. Sci. 2019, 20, 2515. https://doi.org/10.3390/ijms20102515
Thomas R, Srivastava S, Katreddy RR, Sobieski J, Weihua Z. Kinase-Inactivated EGFR Is Required for the Survival of Wild-Type EGFR-Expressing Cancer Cells Treated with Tyrosine Kinase Inhibitors. International Journal of Molecular Sciences. 2019; 20(10):2515. https://doi.org/10.3390/ijms20102515
Chicago/Turabian StyleThomas, Rintu, Shivangi Srivastava, Rajasekhara Reddy Katreddy, Jason Sobieski, and Zhang Weihua. 2019. "Kinase-Inactivated EGFR Is Required for the Survival of Wild-Type EGFR-Expressing Cancer Cells Treated with Tyrosine Kinase Inhibitors" International Journal of Molecular Sciences 20, no. 10: 2515. https://doi.org/10.3390/ijms20102515
APA StyleThomas, R., Srivastava, S., Katreddy, R. R., Sobieski, J., & Weihua, Z. (2019). Kinase-Inactivated EGFR Is Required for the Survival of Wild-Type EGFR-Expressing Cancer Cells Treated with Tyrosine Kinase Inhibitors. International Journal of Molecular Sciences, 20(10), 2515. https://doi.org/10.3390/ijms20102515




