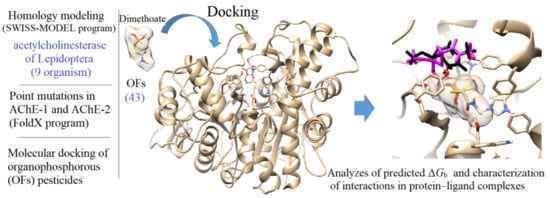
In Silico Study of the Resistance to Organophosphorus Pesticides Associated with Point Mutations in Acetylcholinesterase of Lepidoptera: B. mandarina, B. mori, C. auricilius, C. suppressalis, C. pomonella, H. armígera, P. xylostella, S. frugiperda, and S. litura
Abstract
1. Introduction
2. Results
2.1. Computational Protocol
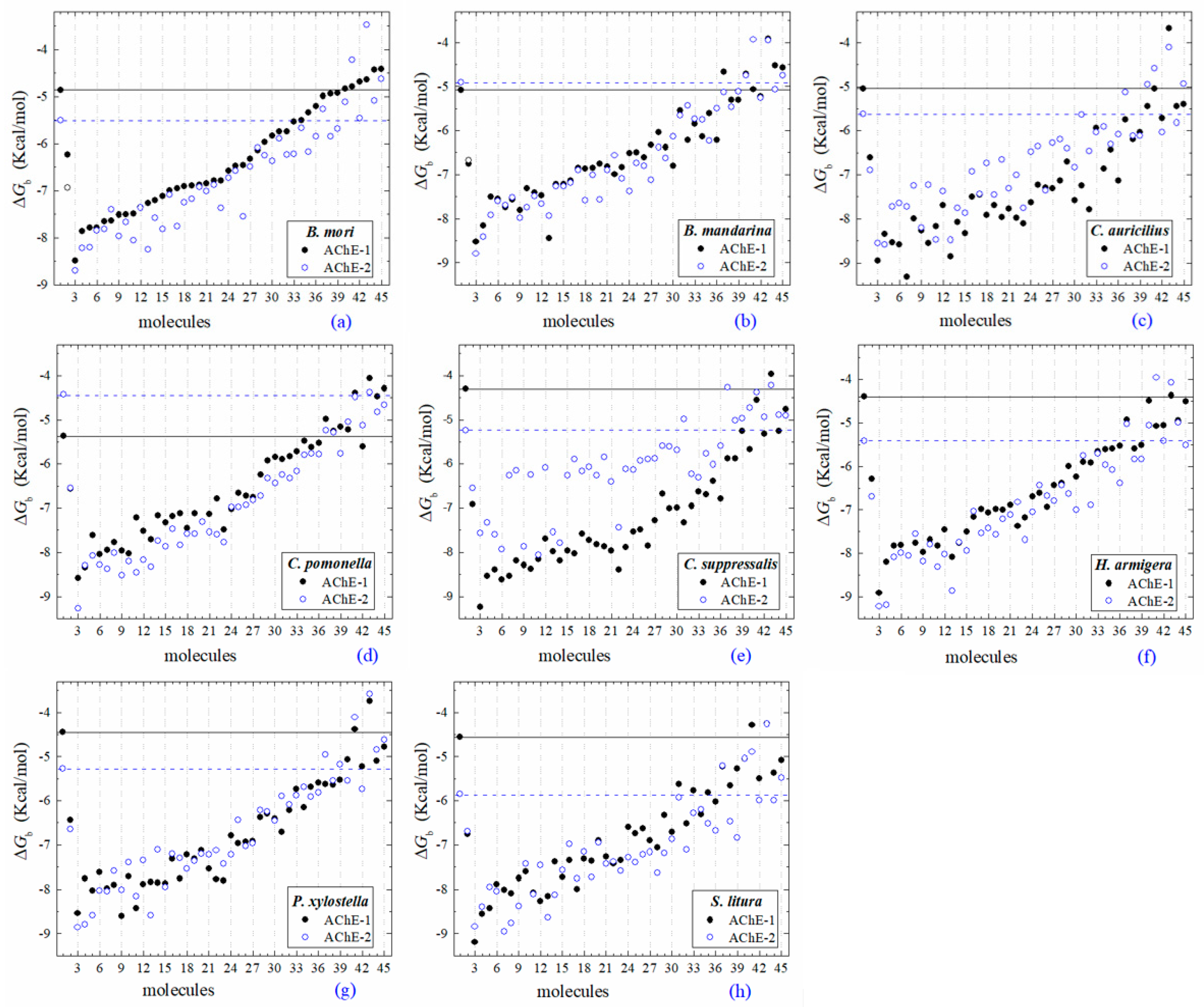
2.2. Molecular Docking of OPs on AChE Enzymes
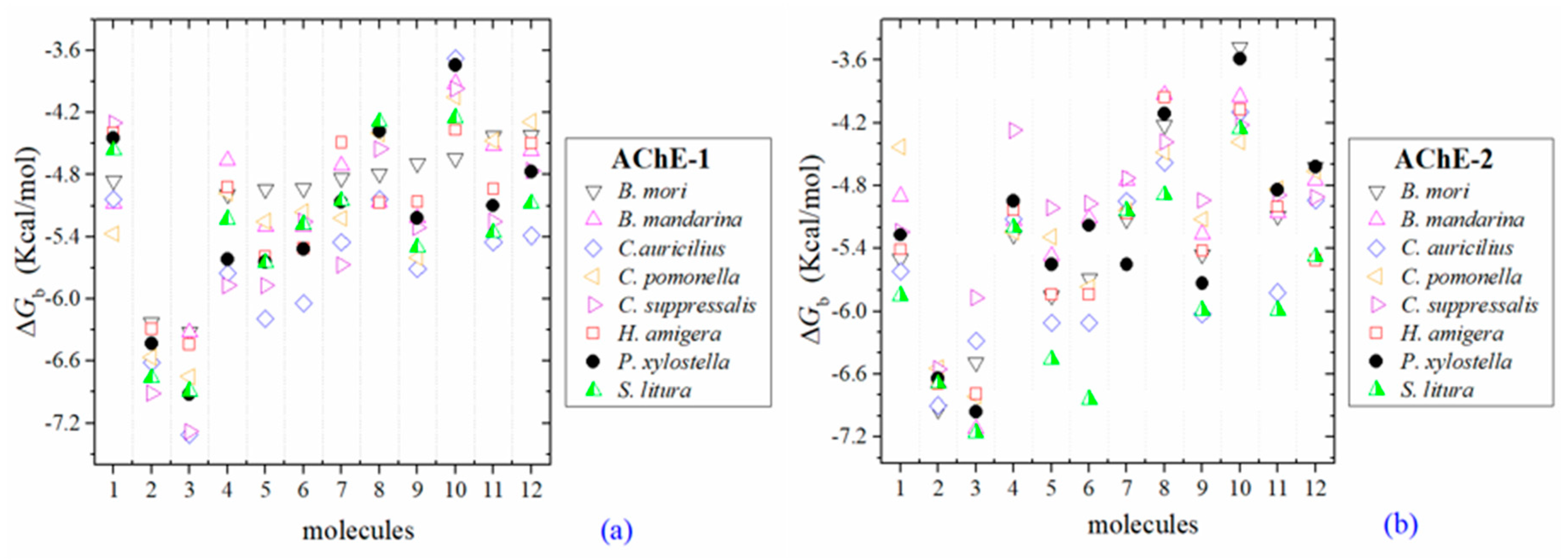
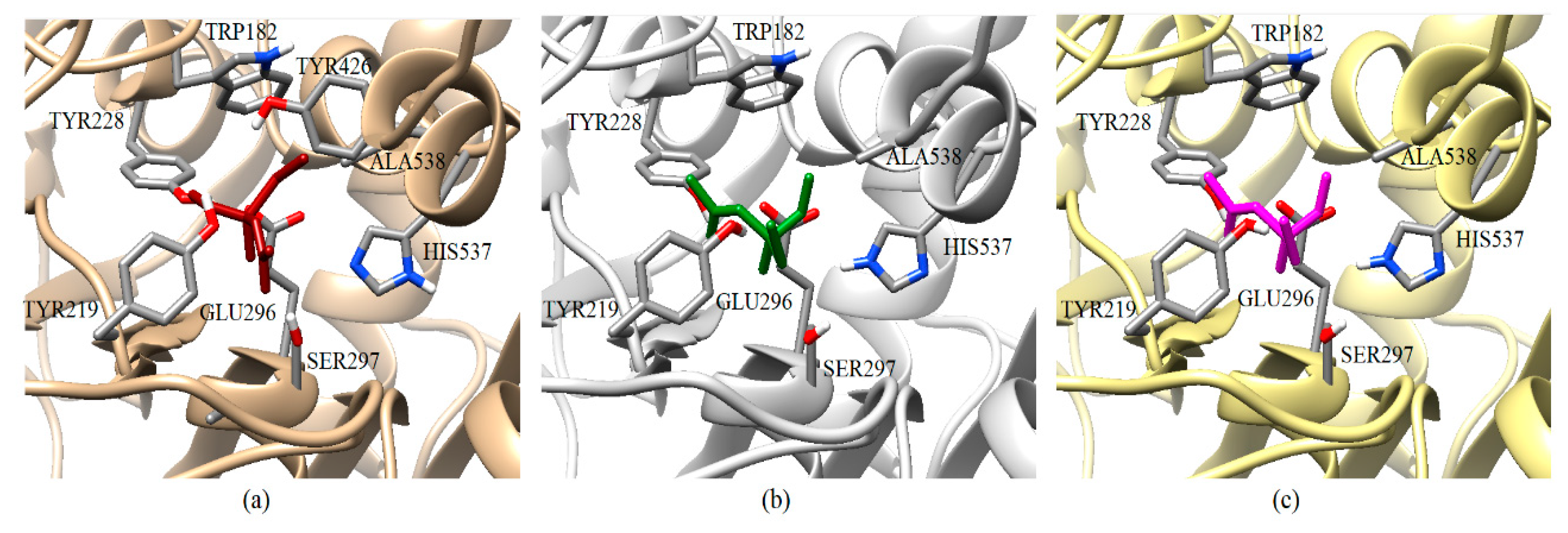
2.3. Docking of OPs Molecules on AChE-1 and AChE-2 (Wild Type)
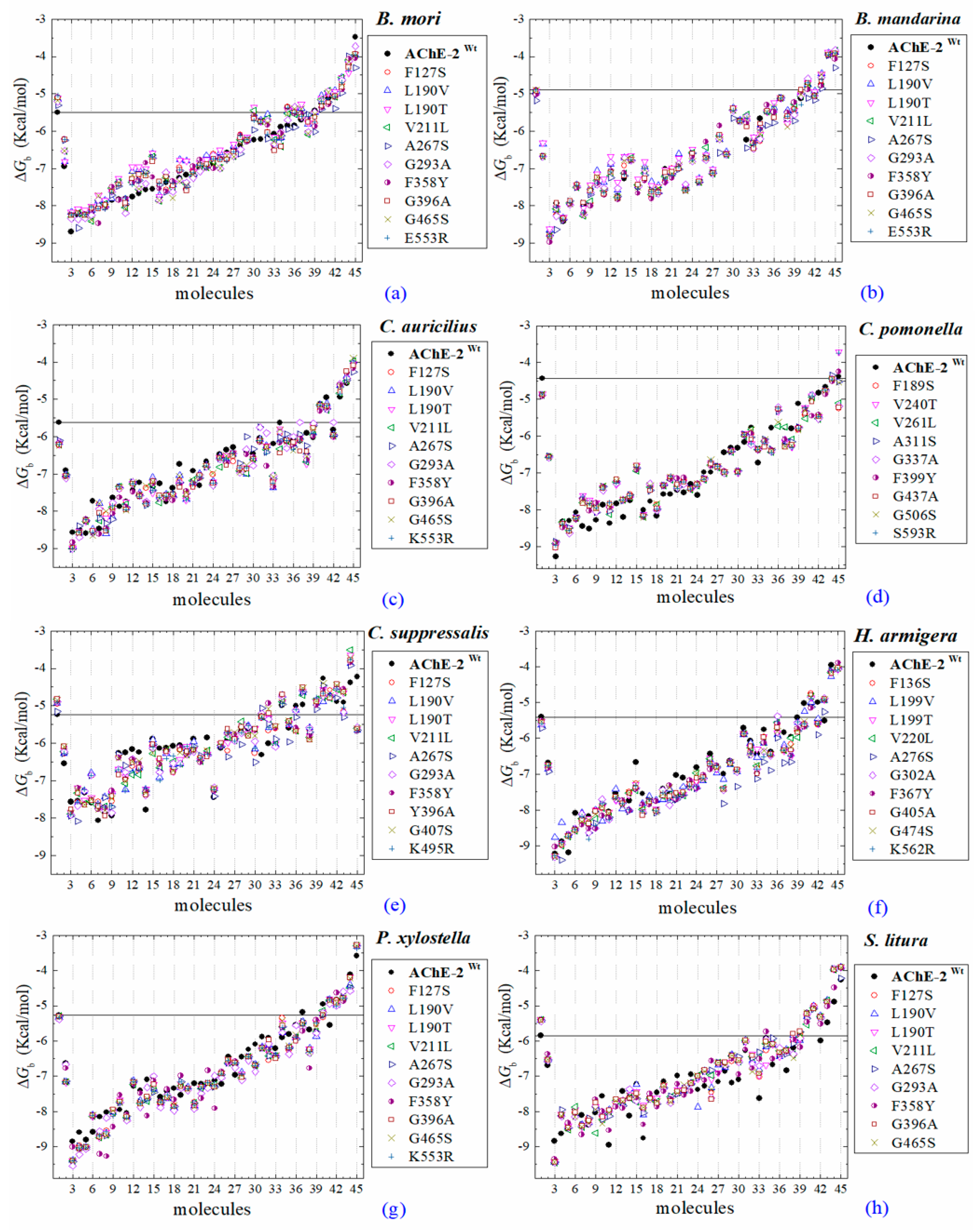
2.4. Docking of OPs Molecules on Modified AChE-1
2.5. Docking of OPs Molecules on Modified AChE-2
3. Discussion
3.1. Construction of Molecular Models
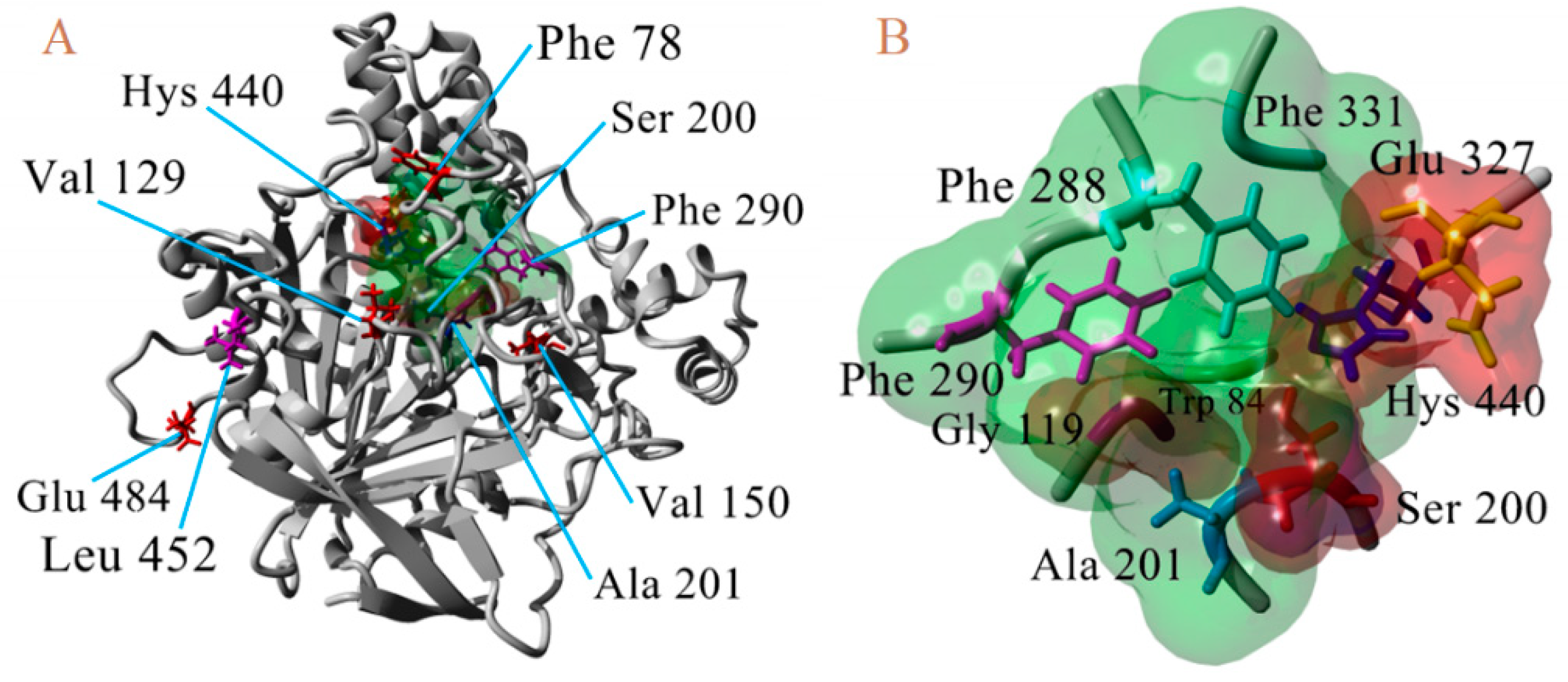
3.2. Docking Results of Acetylcholine Evaluations on AChE
3.3. Docking Results of Psoralen Evaluations on AChE
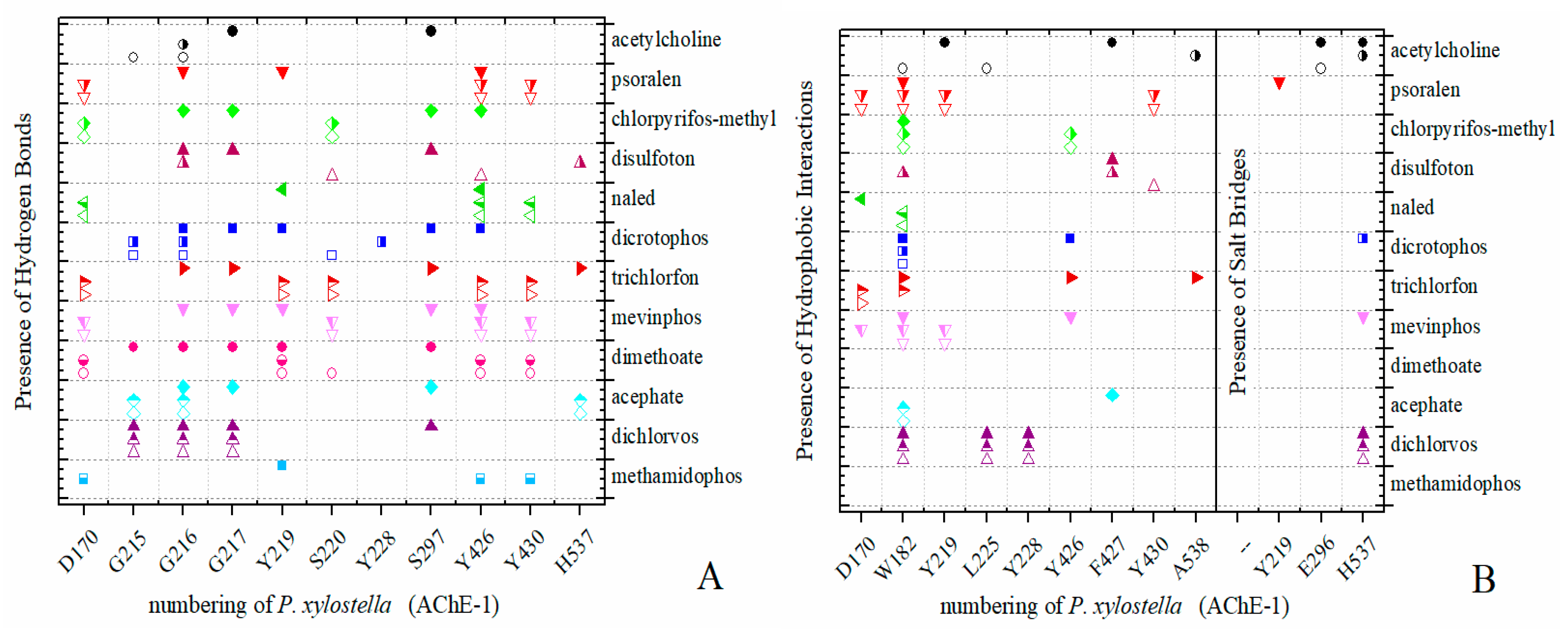
3.4. Resistance-Associate Mutation on AChE
4. Materials and Methods
4.1. Homology Modeling
4.2. Point Mutations on the AChE Structures
4.3. Ligands Used in Docking
4.4. Verification of Computational Methodology
4.5. Molecular Docking of Ligands Targeting AChE Enzymes
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| AChE-1wild type | form 1 acetylcholinesterase, wild type, without mutations |
| AChE-1modified | form 1 acetylcholinesterase, modified, with puntual mutations |
| AChE-2 wild type | form 2 acetylcholinesterase, wild type, without mutations |
| AChE-2 modified | form 2 acetylcholinesterase, modified, with puntual mutations |
| OP | organophosphorus |
| ΔGb | binding energy |
| RMSD | root-mean-square deviation |
| Qscore | quality of alignment |
Appendix A
Results for the Homology Modeling and Multiple Alignment of AChE Enzymes
| AChE-1 Model | Template | SeqI | GMQE | QMEANZs | SeqS | Range/Covererage |
|---|---|---|---|---|---|---|
| B. mandarina | 5YDJ | 68.77 | 0.73 | −1.64 | 0.52 | 103-642/0.83 |
| B. mori | 5YDJ | 70.62 | 0.74 | −1.60 | 0.53 | 103-642/0.80 |
| C. auricilius | 5YDJ | 66.39 | 0.72 | −1.70 | 0.51 | 114-653/0.88 |
| C. suppressalis | 5YDJ | 67.46 | 0.71 | −2.27 | 0.51 | 87-653/0.85 |
| C. pomonella | 5YDJ | 66.99 | 0.72 | −1.43 | 0.51 | 111-650/0.88 |
| H. armígera | 5YDJ | 67.27 | 0.72 | −1.45 | 0.51 | 115-654/0.87 |
| P. xylostella | 5YDJ | 67.49 | 0.74 | −1.47 | 0.51 | 98-638/0.90 |
| S. litura | 5YDJ | 67.27 | 0.73 | −1.42 | 0.51 | 114-653/0.88 |
| AChE-2 Model | Template | SeqI | GMQE | QMEAN | SeqS | Range/Covererage |
| B. mandarina | 1DX4 | 59.10 | 0.72 | −2.38 | 0.49 | 53-603/0.87 |
| B. mori | 1DX4 | 60.18 | 0.74 | −2.52 | 0.49 | 53-603/0.87 |
| C. auricilius | 1DX4 | 60.81 | 0.74 | −2.51 | 0.50 | 53-600/0.86 |
| C. suppressalis | 1DX4 | 58.08 | 0.71 | −3.00 | 0.48 | 53-542/0.84 |
| C. pomonella | 1DX4 | 60.72 | 0.74 | −2.46 | 0.49 | 53-603/0.87 |
| H. armígera | 1DX4 | 60.36 | 0.73 | −2.90 | 0.49 | 62-612/0.86 |
| P. xylostella | 1DX4 | 60.54 | 0.74 | −2.67 | 0.49 | 53-603/0.87 |
| S. litura | 1DX4 | 60.54 | 0.74 | −2.23 | 0.49 | 53-603/0.87 |
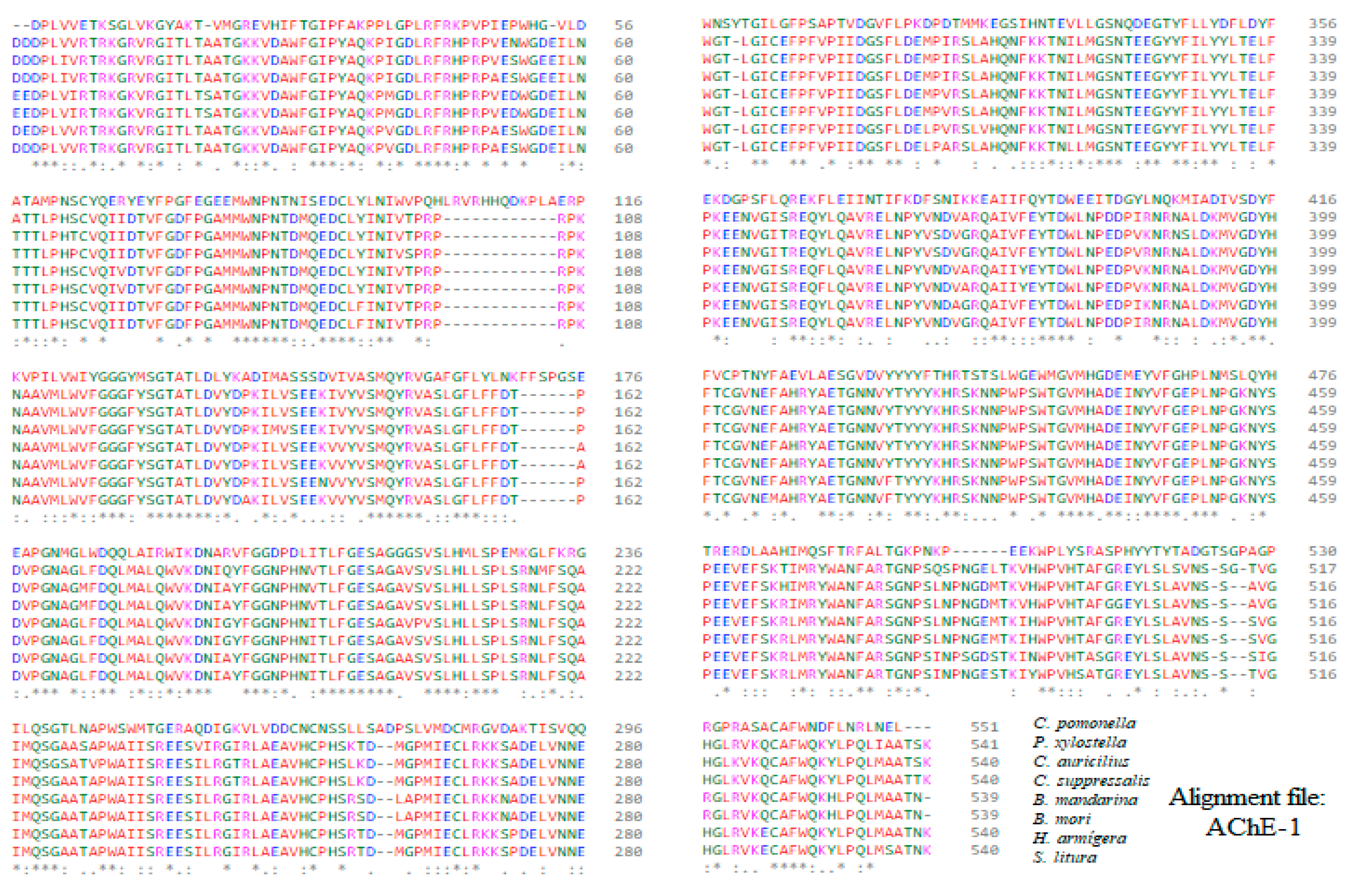
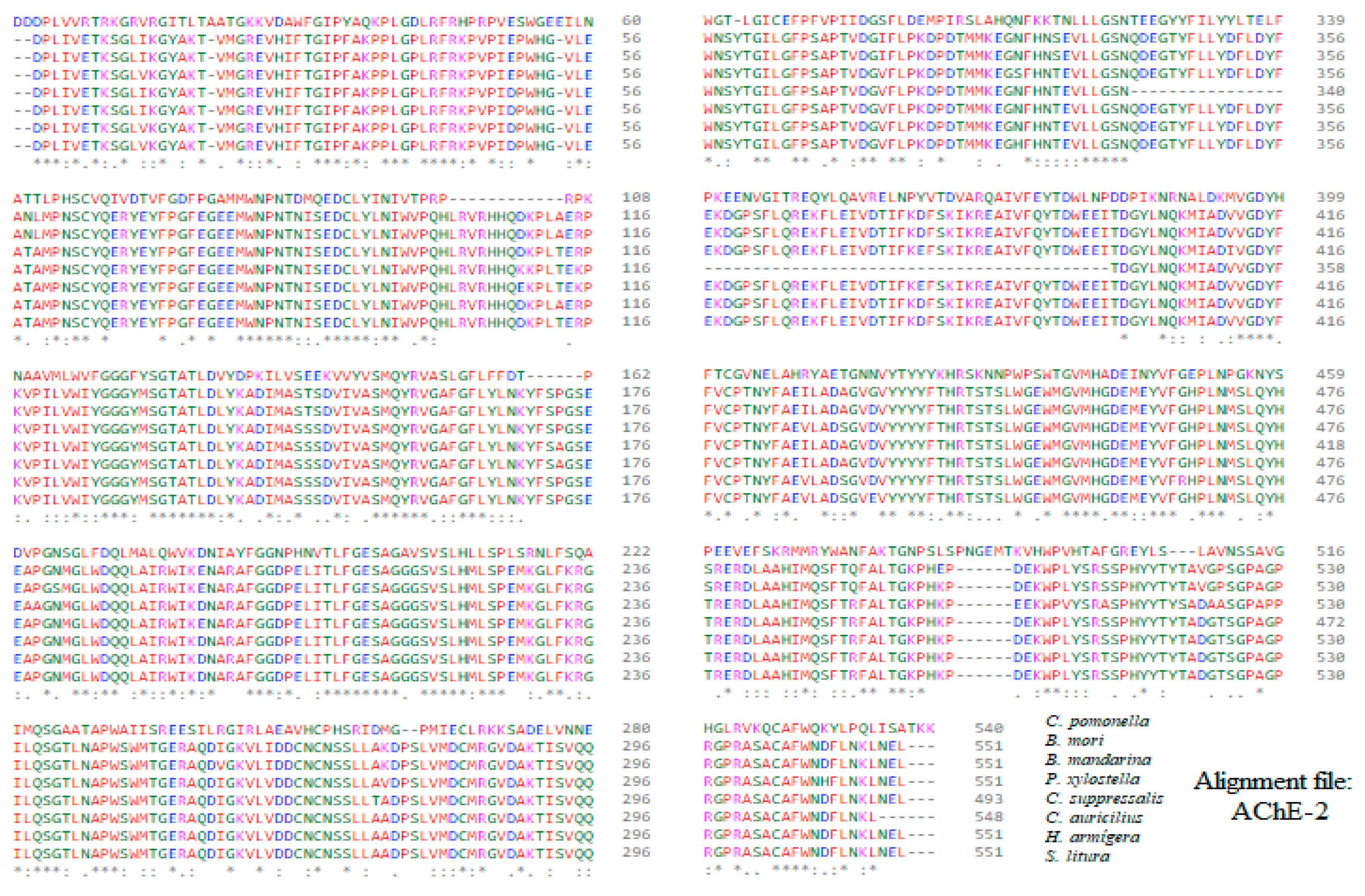
Appendix B
Verification of Computational Methodology
| Ligands | ΔGb * | ΔGb | ΔΔGb |
|---|---|---|---|
| Psoralen | −6.84 a | −6.97 | 0.13 |
| Dimefox | −4.14 b | −4.39 | 0.25 |
| Dichlorvos | −4.47 b | −5.52 | 1.05 |
| Phoxim ethyl phosphonate | −6.25 b | −7.33 | 1.08 |
| Heptenophos | −5.66 b | −6.83 | 1.17 |
| Methamidophos | −5.87 b | −4.45 | −1.42 |
References
- Hanfu, H.; O’Brochta, D.A. Advanced technologies for genetically manipulating the silkworm Bombyx mori, a model Lepidopteran insect. Proc. Biol. Sci. 2015, 282, 20150487. [Google Scholar]
- Roychoudhury, N.; Mishra, R. Bombyx mandarina Moore (Lepidoptera: Bombycidae): An Endangered Wild Indian Mulberry Silkworm Species. Tropical Forest Research Institute, Jabalpur, MP, India. Van Sangyan 2015, 2, 48–50. [Google Scholar]
- Invasive Species Compendium, CAB International. C. suppressalis (Striped Rice Stem Borer). 2018. Available online: https://www.cabi.org/ISC/datasheet/12855 (accessed on 1 June 2018).
- Invasive Species Compendium, CAB International. H. armigera (Cotton Bollworm). 2018. Available online: https://www.cabi.org/ISC/datasheet/26757 (accessed on 1 June 2018).
- Forrester, N.; Cahill, M.; Bird, L.; Layland, J. Management of pyrethroid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Pyrethroid resistance: Field resistance mechanisms. Bull. Entomol. Res. 1993, 1, 132. [Google Scholar]
- Invasive Species Compendium, CAB International. P. xylostella (Diamondback Moth). 2018. Available online: https://www.cabi.org/ISC/datasheet/42318 (accessed on 1 June 2018).
- Invasive Species Compendium, CAB International. S. litura (Taro Caterpillar). 2018. Available online: https://www.cabi.org/ISC/datasheet/44520 (accessed on 1 June 2018).
- Abbas, N.; Shad, S.; Razaq, M.; Waheed, A.; Aslam, M. Resistance of Spodoptera litura (Lepidoptera: Noctuidae) to profenofos: Relative fitness and cross-resistance. Crop Prot. 2014, 58, 49–54. [Google Scholar] [CrossRef]
- Guo, D.; Luo, J.; Zhou, Y.; Xiao, H.; He, K.; Yin, C.; Xu, J.; Li, F. ACE: An efficient and sensitive tool to detect insecticide resistance-associated mutations in insect acetylcholinesterase from RNA-Seq data. BMC Bioinform. 2017, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brevik, K.; Schoville, S.; Mota-Sanchez, D.; Chena, Y. Pesticide durability and the evolution of resistance: A novel application of survival analysis. Pest Manag. Sci. 2018, 74, 1953–1963. [Google Scholar] [CrossRef]
- Sakine, U. Insecticides and Advances in Integrated Pest Management. In Insecticide Resistance; Perveen, F., Ed.; InTech Press: London, UK, 2012; pp. 469–478. [Google Scholar]
- IRAC Insecticide Resistance Action Committee Mode of Action Classification Scheme; Prepared by: IRAC International MoA Working Group; Approved by: IRAC Executive Issued; IRAC: Baghdad, Iraq, 2018; pp. 1–26.
- Sparks, T.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; O’Brien, R. Inhibition of acetylcholinesterases by anionic organophosphorous compounds. Biochemistry 1968, 7, 1538–1545. [Google Scholar] [CrossRef]
- Aldridge, W. The nature of the reaction of organophosphorous compounds and carbamates with esterases. Bull. World Health Organ. 1971, 44, 25–30. [Google Scholar] [PubMed]
- Langel, U.; Jarv, J. Leaving group effects in butyrylcholinesterase reaction with organophosphorous inhibitors. Biochim. Biophys. Acta 1978, 525, 122–133. [Google Scholar] [CrossRef]
- Lushchekina, S.; Patrick, M. Catalytic bioscavengers against organophosphorus agents: Mechanistic issues of self-reactivating cholinesterases. Toxicology 2018, 409, 91–102. [Google Scholar] [CrossRef]
- Lushchekina, S.; Schopfer, L.; Grigorenko, B.; Nemukhin, A.; Varfolomeev, S.; Lockridge, O.; Masson, P. Optimization of cholinesterase-based catalytic bioscavengers against organophosphorus agents. Front. Pharmacol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Ordentlich, A.; Kronman, C.; Barak, D.; Stein, D.; Ariel, N.; Marcus, D.; Velan, B.; Shafferman, A. Engineering resistance to ‘aging’ of phosphorylated human acetylcholinesterase. Role of hydrogen bond network in the active center. FEBS Lett. 1993, 344, 215–220. [Google Scholar]
- Michigan State University Board of Trustees. Arthropod Pesticide Resistance Database (APRD). 2000. Available online: https://www.pesticideresistance.org (accessed on 1 June 2018).
- Insecticide Resistance Action Committee (IRAC) Resistance Management for Sustainable Agriculture and Improved Public Health. Available online: http://www.irac-online.org (accessed on 1 June 2018).
- Somani, G.; Kulkarni, C.; Shinde, P.; Shelke, R.; Laddha, K.; Sathaye, S. In vitro acetylcholinesterase inhibition by psoralen using molecular docking and enzymatic studies. J. Pharm. Bioallied. Sci. 2015, 7, 32–36. [Google Scholar] [CrossRef]
- Sadia-Chishty, M. A review on medicinal importance of babchi (Psoralea corylifolia). Int. J. Recent Scien. Res. 2016, 7, 11504–11512. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Protein Structure Homology-Modelling Server. Available online: https://swissmodel.expasy.org (accessed on 1 June 2018).
- Van Durme, J.; Delgado, J.; Stricher, F.; Serrano, L.; Schymkowitz, J.; Rousseau, F. A graphical interface for the FoldX forcefield. Bioinformatics 2011, 27, 1711–1712. [Google Scholar] [CrossRef]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Ny, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2014, 60, 2256–2268. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.; Adasme, M.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup, execution, and analysis of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef]
- The National Center for Biotechnology Information (NCBI). Available online: http://www.ncbi.nlm.nih.gov (accessed on 1 June 2018).
- Menozzi, P.; Shi, M.; Lougarre, A.; Tang, Z.; Fournier, D. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evol. Biol. 2004, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Omoto, C.; Field, L.; Williamson, M.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [PubMed]
- Cassanelli, S.; Reyes, M.; Rault, M.; Manicardi, G.; Sauphanor, B. Acetylcholinesterase mutation in an insecticide-resistant population of the codling moth Cydia pomonella (L.). Insect Biochem. Mol. Biol. 2006, 36, 642–653. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.; Thiessen, P.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 1 July 2018).
- Ranjan, A.; Chauhan, A.; Jindal, T. In silico and in vitro evaluation of human acetylcholinesterase inhibition by organophosphates. Environ. Toxicol. Pharmacol. 2018, 57, 131–140. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Prot. Bioinform. 2014, 48, 3.13.1–3.13.16. [Google Scholar] [CrossRef]
- Multiple Sequence Alignment. Clustal Omega. Available online: https://www.ebi.ac.uk/Tools/msa/clustalo/ (accessed on 1 July 2018).
- Chaudhry, M.; DASS, J.F.; Selvakumar, D.; Kumar, N. In-silico study of acetylcholinesterase inhibition by organophosphate pesticides. Int. J. Pharm. Bio Sci. 2013, 4, B788–B802. [Google Scholar]
- Sharma, A.K.; Gaur, K.; Tiwari, R.K.; Gaur, M.S. Computational interaction analysis of organophosphorus pesticides with different metabolic proteins in humans. J. Biomed. Res. 2011, 25, 335–347. [Google Scholar] [CrossRef]
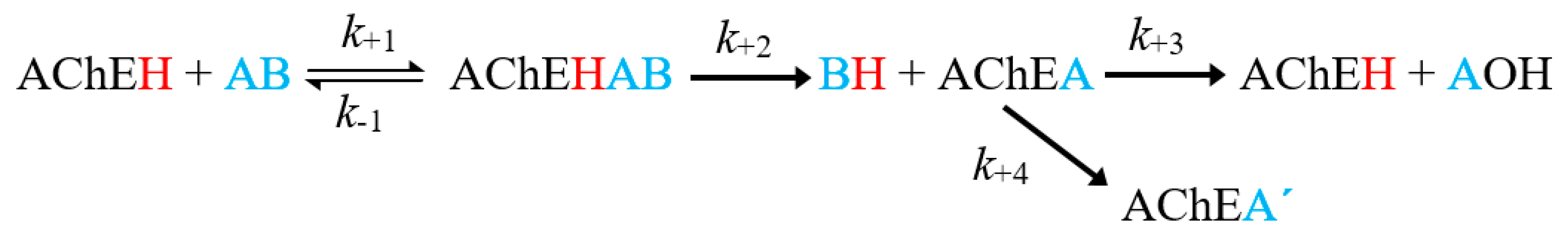

| Model | AChE-1 | AChE-2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Nres | %SI | RMSD | Qscore | Nres | %SI | RMSD | Qscore | |
| B. mandarina | 539 | 71.8 | 0.4837 | 0.935 | 551 | 62.9 | 0.3079 | 0.808 |
| B. mori | 539 | 72.0 | 0.4837 | 0.935 | 551 | 62.9 | 0.3079 | 0.808 |
| C. auricilius | 540 | 71.0 | 0.3956 | 0.941 | 548 | 63.1 | 0.3553 | 0.810 |
| C. suppressalis | 540 | 71.0 | 0.4593 | 0.936 | 493 | 62.7 | 0.5486 | 0.883 |
| C. pomonella | 551 | 44.1 | 1.3909 | 0.772 | 540 | 40.4 | 1.4337 | 0.678 |
| H. armígera | 540 | 72.0 | 0.3577 | 0.944 | 551 | 63.1 | 0.3514 | 0.806 |
| P. xylostella | 541 | 71.6 | 0.3695 | 0.942 | 551 | 63.3 | 0.3626 | 0.805 |
| S. litura | 540 | 72.0 | 0.3354 | 0.946 | 551 | 63.3 | 0.3784 | 0.804 |
| Genus Species | # Cases: | OPs Insecticide | |
|---|---|---|---|
| Plutella xylostella£ | 862 | 279 * | 1c, 2c*, 4c*, 5c, 6c, 7c, 10c, 11c, 12c, 13c, 15c, 18c, 19c, 21c, 22c, 24c, 26c, 28c, 28c, 29c, 30c, 31c, 32c, 33c, 34c, 35c, 36c, 37c, 40c*, and 43c |
| Helicoverpa armígera£ | 856 | 129 * | 3c, 4c*, 10c, 12c, 30c, 31c, 37c*, and 43c |
| Spodoptera litura£ | 644 | 251 * | 4c*, 5c, 6c, 10c, 15c, 30c, 35c, 37c*, 42c, and 43c* |
| Spodoptera exigua | 525 | 303 * | 4c*, 12c, 30c, and 37c* |
| Cydia pomonella£ | 193 | 46 * | 1c, 3c*, 4c*, 12c*, and 17c |
| Chilo suppressalis£ | 148 | 79 * | 1c, 4c, 5c, 11c, 15c, 21c, 33c, and 35c |
| Earias vittella | 128 | 32 * | 4c, 35c, and 37c |
| Spodoptera frugiperda£ | 125 | 57 * | 2c*, 4c, 5c, 6c, 10c, 12c,15c, and 38c |
| Heliothis virescens | 120 | --- | 1c, 12c, 16c, 17c, 27c, and 38c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Espinosa, F.; Méndez-Álvarez, D.; Pérez-Rodríguez, M.A.; Herrera-Mayorga, V.; Juárez-Saldivar, A.; Cruz-Hernández, M.A.; Rivera, G. In Silico Study of the Resistance to Organophosphorus Pesticides Associated with Point Mutations in Acetylcholinesterase of Lepidoptera: B. mandarina, B. mori, C. auricilius, C. suppressalis, C. pomonella, H. armígera, P. xylostella, S. frugiperda, and S. litura. Int. J. Mol. Sci. 2019, 20, 2404. https://doi.org/10.3390/ijms20102404
Reyes-Espinosa F, Méndez-Álvarez D, Pérez-Rodríguez MA, Herrera-Mayorga V, Juárez-Saldivar A, Cruz-Hernández MA, Rivera G. In Silico Study of the Resistance to Organophosphorus Pesticides Associated with Point Mutations in Acetylcholinesterase of Lepidoptera: B. mandarina, B. mori, C. auricilius, C. suppressalis, C. pomonella, H. armígera, P. xylostella, S. frugiperda, and S. litura. International Journal of Molecular Sciences. 2019; 20(10):2404. https://doi.org/10.3390/ijms20102404
Chicago/Turabian StyleReyes-Espinosa, Francisco, Domingo Méndez-Álvarez, Miguel A. Pérez-Rodríguez, Verónica Herrera-Mayorga, Alfredo Juárez-Saldivar, María A. Cruz-Hernández, and Gildardo Rivera. 2019. "In Silico Study of the Resistance to Organophosphorus Pesticides Associated with Point Mutations in Acetylcholinesterase of Lepidoptera: B. mandarina, B. mori, C. auricilius, C. suppressalis, C. pomonella, H. armígera, P. xylostella, S. frugiperda, and S. litura" International Journal of Molecular Sciences 20, no. 10: 2404. https://doi.org/10.3390/ijms20102404
APA StyleReyes-Espinosa, F., Méndez-Álvarez, D., Pérez-Rodríguez, M. A., Herrera-Mayorga, V., Juárez-Saldivar, A., Cruz-Hernández, M. A., & Rivera, G. (2019). In Silico Study of the Resistance to Organophosphorus Pesticides Associated with Point Mutations in Acetylcholinesterase of Lepidoptera: B. mandarina, B. mori, C. auricilius, C. suppressalis, C. pomonella, H. armígera, P. xylostella, S. frugiperda, and S. litura. International Journal of Molecular Sciences, 20(10), 2404. https://doi.org/10.3390/ijms20102404