Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells
Abstract
1. Introduction
2. Results and Discussion
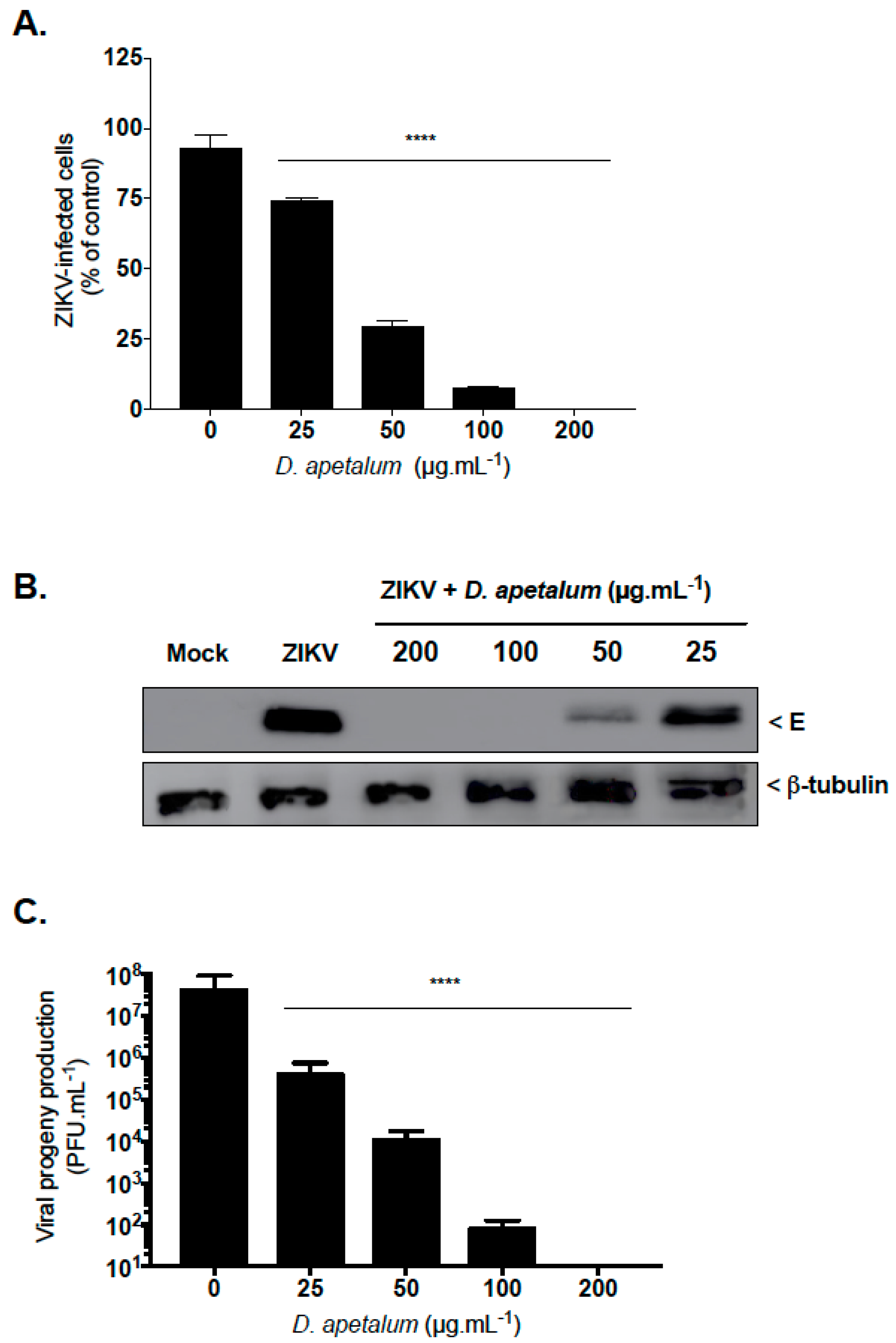
2.1. D. apetalum is an Effective Suppresor of ZIKV Infection at Non-Cytotoxic Concentration
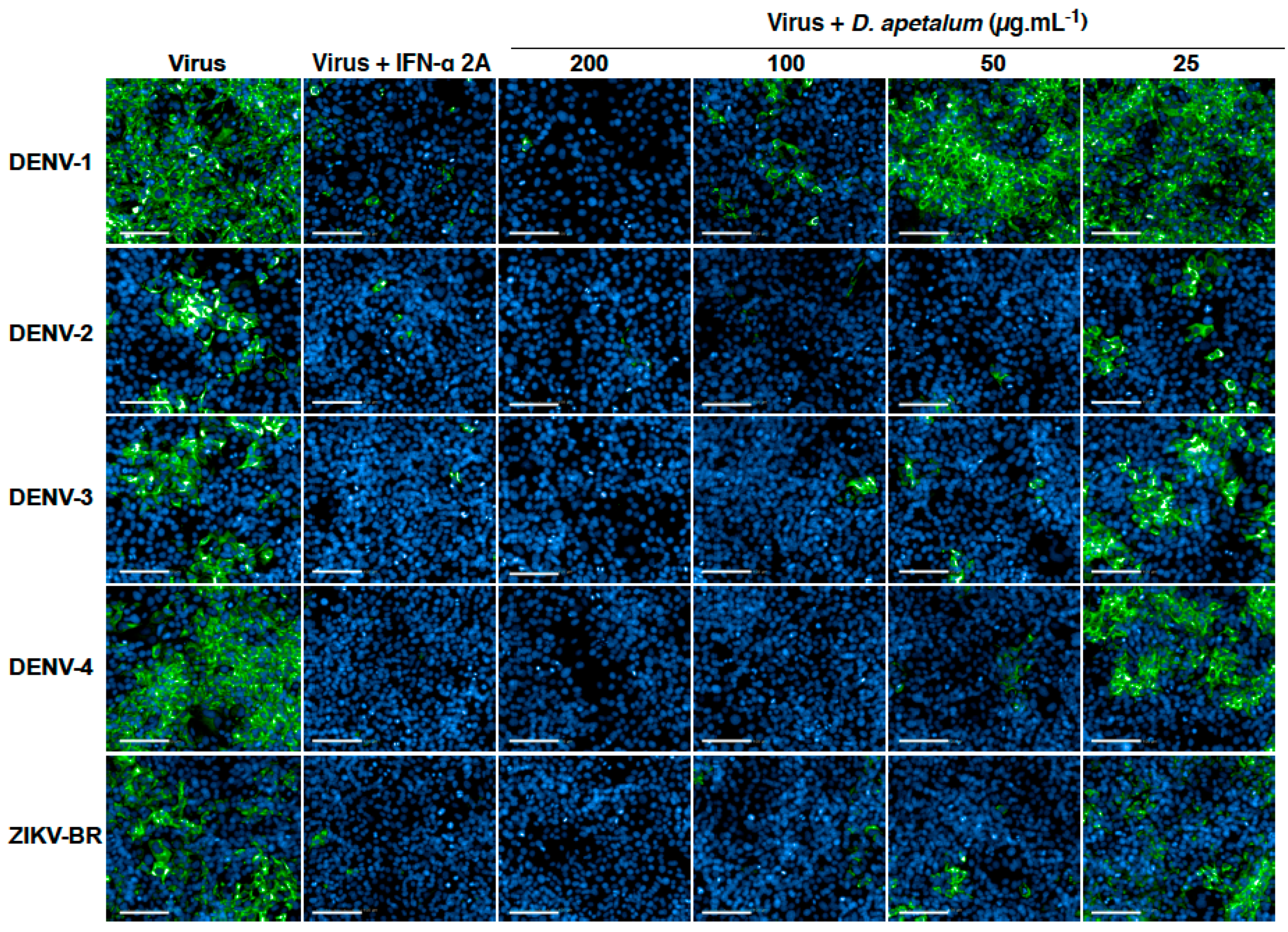
2.2. D. apetalum Extract Inhibits Infection by Four DENV Serotypes
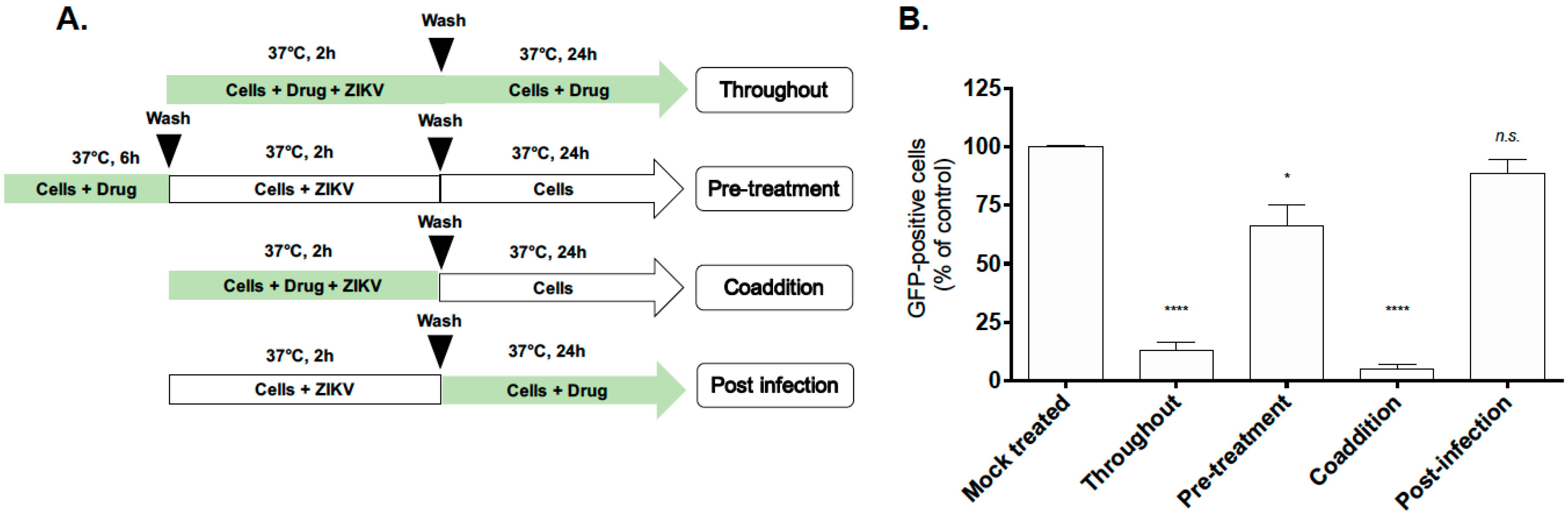
2.3. D. apetalum Extract-Mediated Inhibition of ZIKV Occurs at the Early Stage of the Viral Infetious Cycle
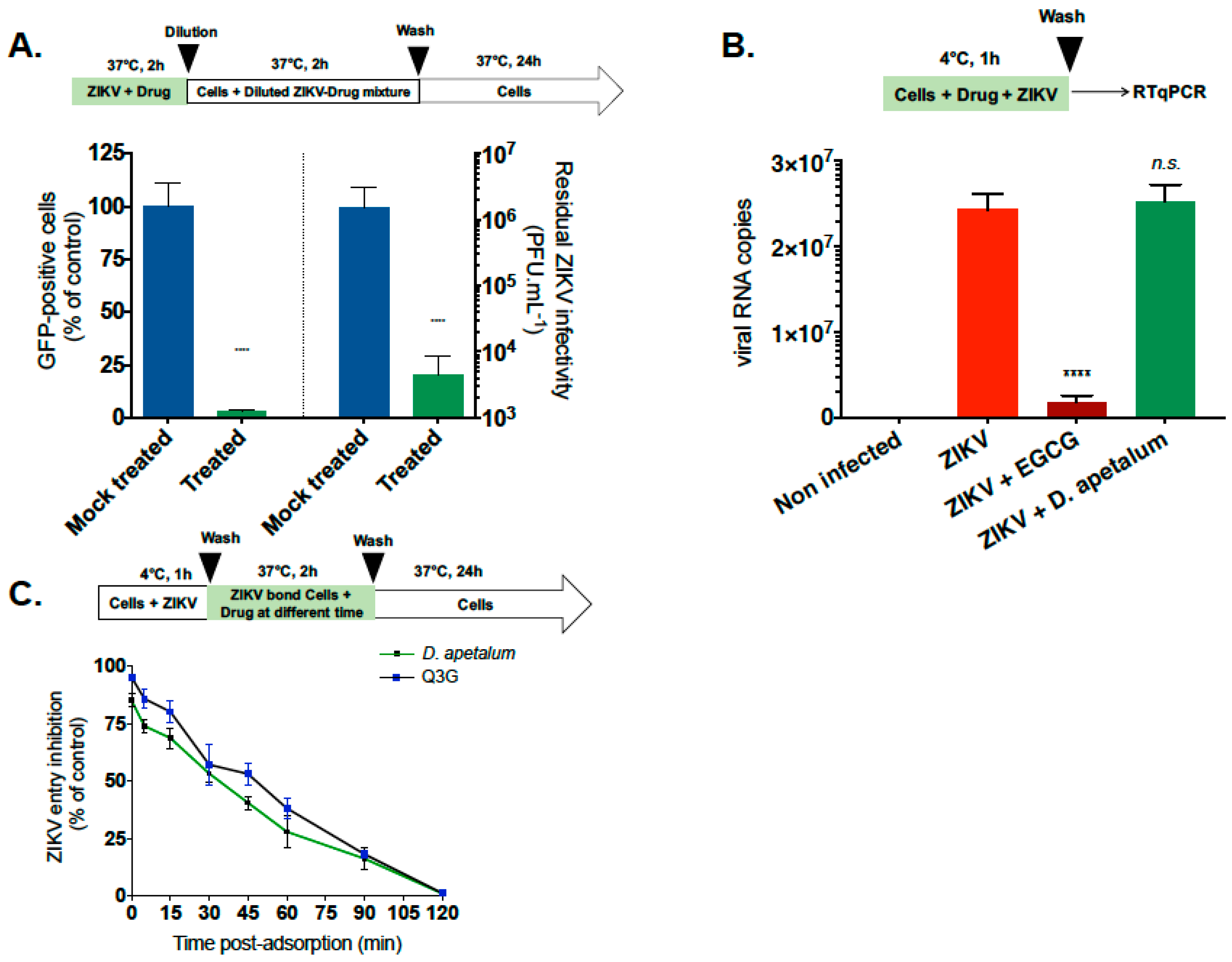
2.4. D. apetalum Extract Prevents ZIKV Entry by Inactivating Virus Particles
2.5. Concluding Remarks
3. Materials and Methods
3.1. Cells, Viruses and Reagents
3.2. Extraction of Plant Material
3.3. MTT Assay
3.4. Immunofluorescence and Flow Cytometry Assays
3.5. Virus Inactivation Assay
3.6. Western Blot
3.7. Plaque Forming Assay
3.8. RT-qPCR
3.9. Data analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A549 | Human lung epithelial cell line |
| DENV | Dengue virus |
| EGCG | Epigallocatechin gallate |
| h.pi | Hours post infection |
| Huh-7.5 | Human hepatoma cell line |
| MOI | Multiplicity of infection |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide |
| PFU | Plaque forming unit |
| Q3G | Isoquercitrin |
References
- Huber, R.G.; Lim, X.N.; Ng, W.C.; Sim, A.Y.L.; Poh, H.X.; Shen, Y.; Lim, S.Y.; Sundstrom, K.B.; Sun, X.; Aw, J.G.; et al. Structure mapping of dengue and Zika viruses reveals functional long-range interactions. Nat. Commun. 2019, 10, 1408. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Yi, P.; Xu, L.; Hawkins, H.K.; Rossi, S.L.; Soong, L.; Cai, J.; Menon, R.; Sun, J. Outcomes of Congenital Zika Disease Depend on Timing of Infection and Maternal-Fetal Interferon Action. Cell Rep. 2017, 21, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2017, 168, 542. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Gadea, G.; Despres, P. Dengue: A growing threat requiring vaccine development for disease prevention. Pathog. Glob. Health 2018, 112, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.-M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef]
- Mittal, R.; Nguyen, D.; Debs, L.H.; Patel, A.P.; Liu, G.; Jhaveri, V.M.; S Kay, S.-I.; Mittal, J.; Bandstra, E.S.; Younis, R.T.; et al. Zika Virus: An Emerging Global Health Threat. Front. Cell Infect. Microbiol. 2017, 7, 486. [Google Scholar] [CrossRef]
- Pascalis, H.; Turpin, J.; Roche, M.; Krejbich, P.; Gadea, G.; Nten, C.A.; Desprès, P.; Mavingui, P. The epidemic of Dengue virus type-2 Cosmopolitan genotype on Reunion Island relates to its active circulation in the Southwestern Indian Ocean neighboring islands. Heliyon 2019, 5, e01455. [Google Scholar] [CrossRef]
- Byler, K.G.; Ogungbe, I.V.; Setzer, W.N. In-silico screening for anti-Zika virus phytochemicals. J. Mol. Graph. Model 2016, 69, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Clain, E.; Sinigaglia, L.; Koishi, A.C.; Gorgette, O.; Gadea, G.; Viranaicken, W.; Krejbich-Trotot, P.; Mavingui, P.; Desprès, P.; Nunes Duarte Dos Santos, C.; et al. Extract from Aphloia theiformis, an edible indigenous plant from Reunion Island, impairs Zika virus attachment to the host cell surface. Sci. Rep. 2018, 8, 10856. [Google Scholar] [CrossRef] [PubMed]
- Norahmad, N.A.; Mohd Abd Razak, M.R.; Mohmad Misnan, N.; Md Jelas, N.H.; Sastu, U.R.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Zolkifli, N.A.; Thayan, R.; et al. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complement. Altern. Med. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Jayawardena, U.A.; Jayasinghe, C.D. Potential Use of Euphorbia hirta for Dengue: A Systematic Review of Scientific Evidence. J. Trop. Med. 2018, 2018, 2048530. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Tahir Ul Qamar, M.; Maryam, A.; Muneer, I.; Xing, F.; Ashfaq, U.A.; Khan, F.A.; Anwar, F.; Geesi, M.H.; Khalid, R.R.; Rauf, S.A.; et al. Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus. Sci. Rep. 2019, 9, 1433. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Lim, T.-H.; Rahim, N.-A.; Shu, M.-H.; Teoh, B.-T.; Sam, S.-S.; Danlami, M.-B.; Tan, K.-K.; Abubakar, S. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement. Altern. Med. 2013, 13, 91. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of dengue virus by curcuminoids. Antivir. Res. 2019, 162, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef]
- Clain, E.; Haddad, J.G.; Koishi, A.C.; Sinigaglia, L.; Rachidi, W.; Despres, P.; Duarte Dos Santos, C.N.; Guiraud, P.; Jouvenet, N.; El Kalamouni, C. The Polyphenol-Rich Extract from Psiloxylon mauritianum, an Endemic Medicinal Plant from Reunion Island, Inhibits the Early Stages of Dengue and Zika Virus Infection. Int. J. Mol. Sci. 2019, 20, 1860. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Duarte Dos Santos, C.N.; Bordignon, J. The citrus flavanone naringenin impairs dengue virus replication in human cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Despres, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef]
- Ledoux, A.; Cao, M.; Jansen, O.; Mamede, L.; Campos, P.-E.; Payet, B.; Clerc, P.; Grondin, I.; Girard-Valenciennes, E.; Hermann, T.; et al. Antiplasmodial, anti-chikungunya virus and antioxidant activities of 64 endemic plants from the Mascarene Islands. Int. J. Antimicrob. Agents 2018, 52, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Murali, A.; Singh, S.K.; Giri, R. Epigallocatechin gallate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. Int. J. Biol. Macromol. 2017, 104, 1046–1054. [Google Scholar] [CrossRef]
- Calland, N.; Sahuc, M.-E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C.; et al. Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action. J. Virol. 2015, 89, 10053–10063. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Shu, M.-H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; Zandi, K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016, 133, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Sliva, K.; Von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The green tea catechin, epigallocatechin gallate inhibits chikungunya virus infection. Antivir. Res. 2015, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Paemanee, A.; Hitakarun, A.; Roytrakul, S.; Smith, D.R. Screening of melatonin, alpha-tocopherol, folic acid, acetyl-L-carnitine and resveratrol for anti-dengue 2 virus activity. BMC Res. Notes 2018, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Calvo, Á.; Jiménez De Oya, N.; Martín-Acebes, M.A.; Garcia-Moruno, E.; Saiz, J.-C. Antiviral Properties of the Natural Polyphenols Delphinidin and Epigallocatechin Gallate against the Flaviviruses West Nile Virus, Zika Virus, and Dengue Virus. Front. Microbiol. 2017, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Zainal, N.; Chang, C.P.; Cheng, Y.L.; Wu, Y.W.; Anderson, R.; Wan, S.W.; Chen, C.L.; Ho, T.S.; AbuBakar, S.; Lin, Y.S. Resveratrol treatment reveals a novel role for HMGB1 in regulation of the type 1 interferon response in dengue virus infection. Sci. Rep. 2017, 7, 42998. [Google Scholar] [CrossRef]
- Dobi, A.; Bravo, S.B.; Veeren, B.; Paradela-Dobarro, B.; Alvarez, E.; Meilhac, O.; Viranaicken, W.; Baret, P.; Devin, A.; Rondeau, P. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: New insights into reactive oxygen species production. Free Radic. Res. 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Marimoutou, M.; Le Sage, F.; Smadja, J.; Lefebvre D’Hellencourt, C.; Gonthier, M.-P.; Robert-Da Silva, C. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismut. J. Inflamm. 2015, 12, 10. [Google Scholar] [CrossRef]
- Ralambondrainy, M.; Belarbi, E.; Viranaicken, W.; Baranauskiene, R.; Venskutonis, P.R.; Despres, P.; Roques, P.; El Kalamouni, C.; Selambarom, J. In vitro comparison of three common essential oils mosquito repellents as inhibitors of the Ross River virus. PLoS ONE 2018, 13, e0196757. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chen, T.Y.; Chung, C.Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.C.; Wang, G.H.; Lin, C.C.; Richardson, C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; Viranaicken, W.; Turpin, J.; El-Kalamouni, C.; Roche, M.; Krejbich-Trotot, P.; Desprès, P.; Gadea, G. The structural proteins of epidemic and historical strains of Zika virus differ in their ability to initiate viral infection in human host cells. Virology 2018, 516, 265–273. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Bai, L.-P.; Huang, W.-B.; Li, X.-Z.; Zhao, S.-S.; Zhong, N.-S.; Jiang, Z.-H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure–activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef]
- Johari, J.; Kianmehr, A.; Mustafa, M.; Abubakar, S.; Zandi, K. Antiviral Activity of Baicalein and Quercetin against the Japanese Encephalitis Virus. Int. J. Mol. Sci. 2012, 13, 16785–16795. [Google Scholar] [CrossRef]
- Li, F.; Lang, Y.; Ji, Z.; Xia, Z.; Han, Y.; Cheng, Y.; Liu, G.; Sun, F.; Zhao, Y.; Gao, M.; et al. A scorpion venom peptide Ev37 restricts viral late entry by alkalizing acidic organelles. J. Biol. Chem. 2019, 294, 182–194. [Google Scholar] [CrossRef]
- Lee, J.K.; Shin, O.S. Advances in Zika Virus–Host Cell Interaction: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 1101. [Google Scholar] [CrossRef]
- Moller-Tank, S.; Maury, W. Phosphatidylserine receptors: Enhancers of enveloped virus entry and infection. Virology 2014, 468–470, 565–580. [Google Scholar] [CrossRef]
- Elgner, F.; Sabino, C.; Basic, M.; Ploen, D.; Grunweller, A.; Hildt, E. Inhibition of Zika Virus Replication by Silvestrol. Viruses 2018, 10, 149. [Google Scholar] [CrossRef]
- Lee, J.L.; Loe, M.W.C.; Lee, R.C.H.; Chu, J.J.H. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Olagnier, D.; Hadj, S.B.; Han, X.; Tian, X.H.; Xu, H.T.; Yang, L.; Shi, Q.; Wang, P.; Wainberg, M.A.; et al. Sophoraflavenone G Restricts Dengue and Zika Virus Infection via RNA Polymerase Interference. Viruses 2017, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Frumence, E.; Roche, M.; Krejbich-Trotot, P.; El-Kalamouni, C.; Nativel, B.; Rondeau, P.; Missé, D.; Gadea, G.; Viranaicken, W.; Desprès, P. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-β production and apoptosis induction. Virology 2016, 493, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Gadea, G.; Bos, S.; Krejbich-Trotot, P.; Clain, E.; Viranaicken, W.; El-Kalamouni, C.; Mavingui, P.; Desprès, P. A robust method for the rapid generation of recombinant Zika virus expressing the GFP reporter gene. Virology 2016, 497, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.B.; Stella, V.; Bordignon, J.; Batista, W.C.; Borba, L.; Silva, L.H.; Hoffmann, F.G.; Probst, C.M.; Santos, C.N. Evidence for the co-circulation of dengue virus type 3 genotypes III and V in the Northern region of Brazil during the 2002–2004 epidemics. Mem. Inst. Oswaldo Cruz. 2008, 103, 483–488. [Google Scholar] [CrossRef]
- Kuczera, D.; Bavia, L.; Mosimann, A.L.; Koishi, A.C.; Mazzarotto, G.A.; Aoki, M.N.; Mansano, A.M.; Tomeleri, E.I.; Costa Junior, W.L.; Miranda, M.M.; et al. Isolation of dengue virus serotype 4 genotype II from a patient with high viral load and a mixed Th1/Th17 inflammatory cytokine profile in South Brazil. Virol. J. 2016, 13, 93. [Google Scholar] [CrossRef]

| Virus | CC50 (µg·mL−1) a | IC50 (µg·mL−1) b | SI d |
|---|---|---|---|
| DENV-1 | 263.5 | 96.35 | 2.7 |
| DENV-2 | 299.0 | 16.75 | 17.8 |
| DENV-3 | 293.0 | 25.90 | 11.3 |
| DENV-4 | 303.0 | 23.30 | 13.0 |
| ZIKV | 295.5 | 17.50 | 16.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, J.G.; Koishi, A.C.; Gaudry, A.; Nunes Duarte dos Santos, C.; Viranaicken, W.; Desprès, P.; El Kalamouni, C. Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. Int. J. Mol. Sci. 2019, 20, 2382. https://doi.org/10.3390/ijms20102382
Haddad JG, Koishi AC, Gaudry A, Nunes Duarte dos Santos C, Viranaicken W, Desprès P, El Kalamouni C. Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. International Journal of Molecular Sciences. 2019; 20(10):2382. https://doi.org/10.3390/ijms20102382
Chicago/Turabian StyleHaddad, Juliano G., Andrea Cristine Koishi, Arnaud Gaudry, Claudia Nunes Duarte dos Santos, Wildriss Viranaicken, Philippe Desprès, and Chaker El Kalamouni. 2019. "Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells" International Journal of Molecular Sciences 20, no. 10: 2382. https://doi.org/10.3390/ijms20102382
APA StyleHaddad, J. G., Koishi, A. C., Gaudry, A., Nunes Duarte dos Santos, C., Viranaicken, W., Desprès, P., & El Kalamouni, C. (2019). Doratoxylon apetalum, an Indigenous Medicinal Plant from Mascarene Islands, Is a Potent Inhibitor of Zika and Dengue Virus Infection in Human Cells. International Journal of Molecular Sciences, 20(10), 2382. https://doi.org/10.3390/ijms20102382






