Structural and Functional Changes in the Na+/H+ Exchanger Isoform 1, Induced by Erk1/2 Phosphorylation
Abstract
1. Introduction
1.1. NHE Structure and Subtypes Distribution
1.2. NHE1 Physiological and Pathological Roles
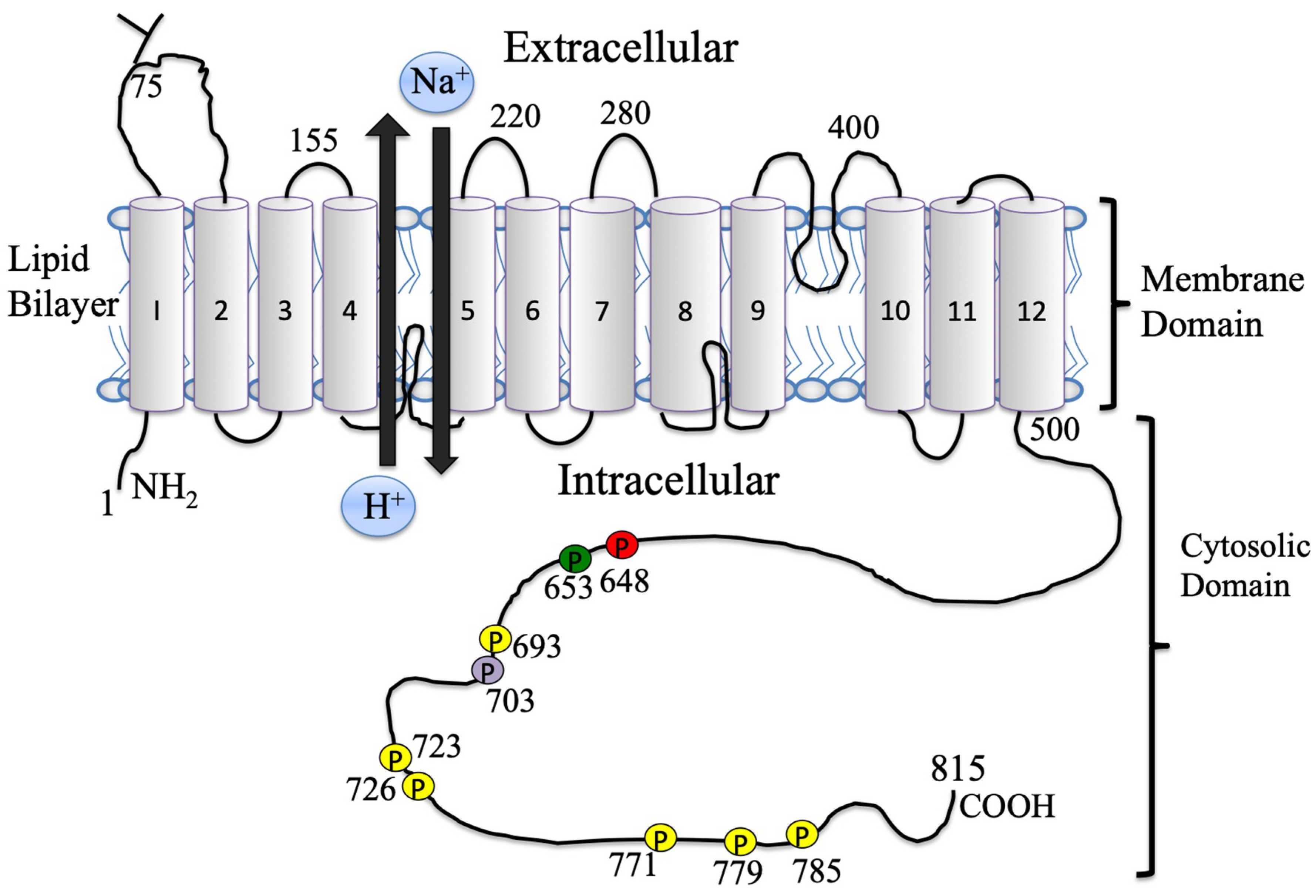
1.3. The Na+/H+ Exchanger Structural Aspects
2. Regulation of NHE1 Isoform of the Na+/H+ Exchanger, General
2.1. Rationale for Study of NHE1 Regulation
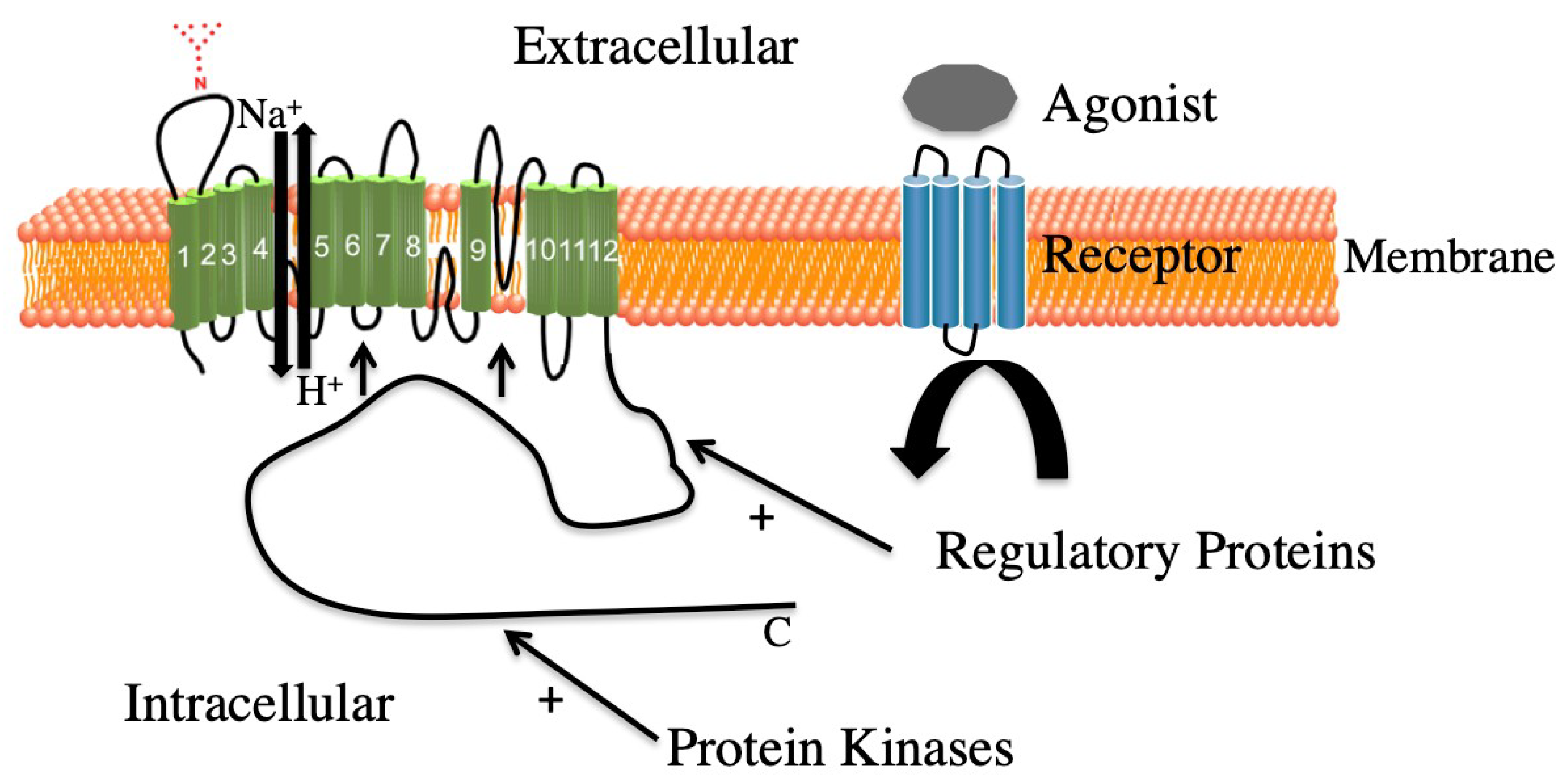
2.2. NHE1 Regulation
2.3. NHE1 Regulation, Phosphorylation
2.3.1. Phosphorylation, General
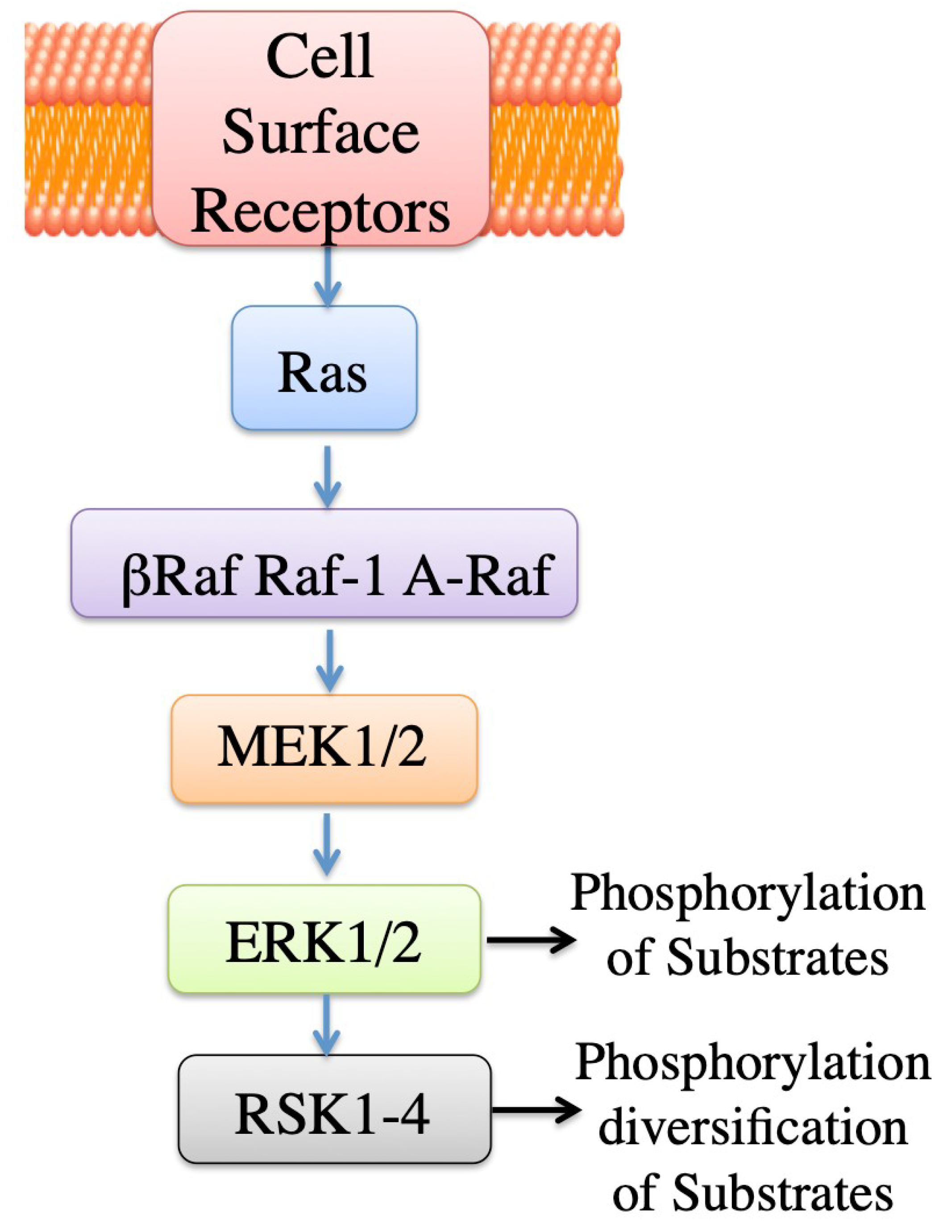
2.3.2. ERK Mediated Regulation, General
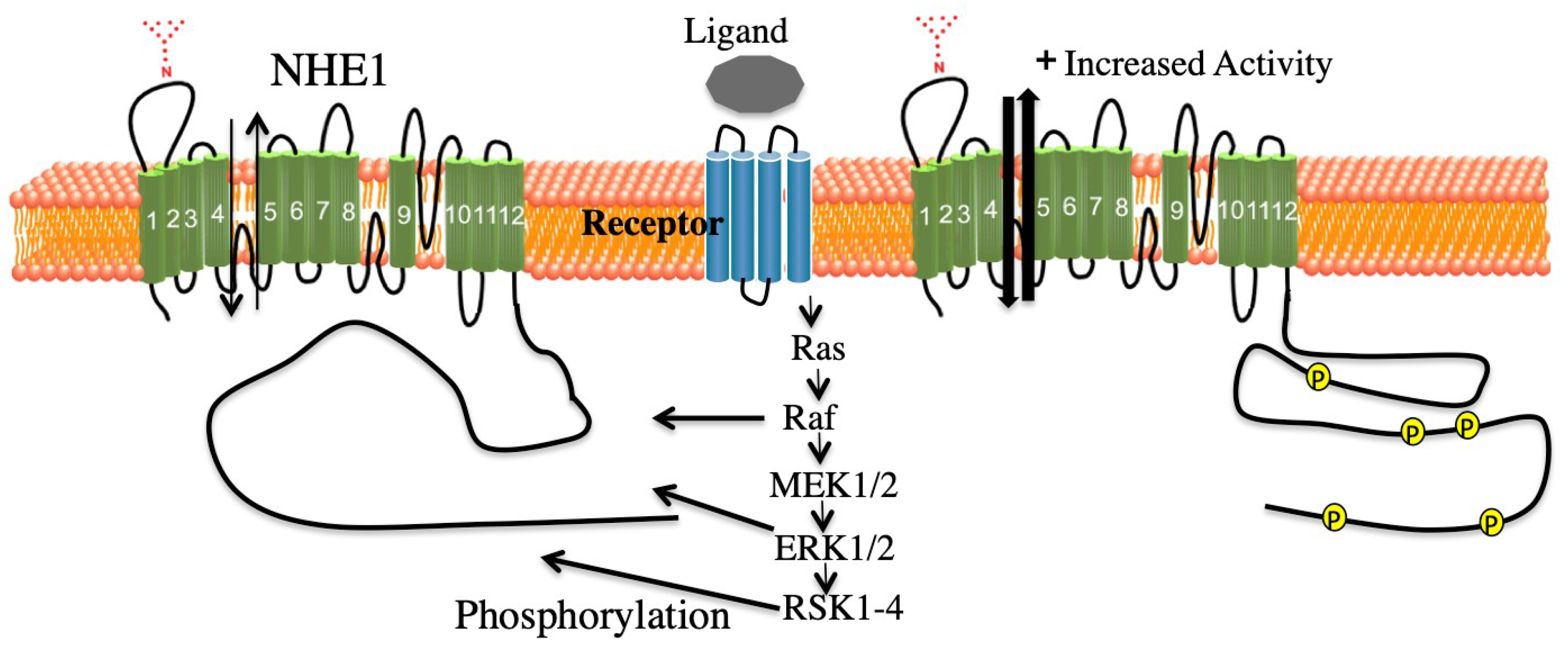
3. ERK Mediated Regulation of NHE1
3.1. ERK Pathway Regulation of NHE1 in the Myocardium
3.2. ERK Pathway Regulation of Breast Cancer Metastasis
4. NHE1 Scaffolding, ERK1/2, and Disordered Domains
4.1. Scaffolding and ERK1/2
4.2. ERK1/2 Scaffolding and the Disordered Domains of NHE1
4.3. ERK1/2 Phosphorylation and Binding and Conformational Changes in the NHE1 C-terminus
5. Conclusions
5.1. Summary of ERK Pathway Mediated Effects
5.2. Future Studies
Funding
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| CPA | Cation proton antiporter |
| ERK | Extracellular signal-regulated kinase |
| ERM | Ezrin, radixin, and moesin |
| MAPK | Mitogen activated protein kinase |
| NHE1 | Na+/H+ exchanger isoform one |
| NMR | Nuclear magnetic resonance |
| pHi | Intracellular pH |
References
- Fliegel, L. The Na+/H+ exchanger isoform 1. Int. J. Biochem. Cell Biol. 2005, 37, 33–37. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, T.; Humphreys, R.A.; Yoshida, H.; Kusumoto, K. The myocardial Na+-H+ exchange: Structure, regulation, and its role in heart disease. Circ. Res. 1999, 85, 777–786. [Google Scholar] [CrossRef]
- Fliegel, L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Expert Opin. Ther. Targets 2009, 13, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Malo, M.E.; Fliegel, L. Physiological role and regulation of the Na+/H+ exchanger. Can. J. Physiol. Pharmacol. 2006, 84, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Fliegel, L.; Dyck, J.R.B.; Wang, H.; Fong, C.; Haworth, R.S. Cloning and analysis of the human myocardial Na+/H+ exchanger. Mol. Cell. Biochem. 1993, 125, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Fliegel, L.; Sardet, C.; Pouysségur, J.; Barr, A. Identification of the protein and cdna of the cardiac Na+/H+ exchanger. FEBS Lett. 1991, 279, 25–29. [Google Scholar] [CrossRef]
- Fliegel, L. Functional and cellular regulation of the myocardial Na+/H+ exchanger. J. Thromb. Thrombolysis 1999, 8, 9–14. [Google Scholar] [CrossRef]
- Orlowski, J.; Kandasamy, R.A.; Shull, G.E. Molecular cloning of putative members of the Na+/H+ exchanger gene family. J. Biol. Chem. 1992, 267, 9331–9339. [Google Scholar] [PubMed]
- Lee, S.H.; Kim, T.; Park, E.S.; Yang, S.; Jeong, D.; Choi, Y.; Rho, J. Nhe10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival [corrected]. Biochem. Biophys. Res. Commun. 2008, 369, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Putney, L.K.; Denker, S.P.; Barber, D.L. The changing face of the Na+/H+ exchanger, Nhe1: Structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 527–552. [Google Scholar] [CrossRef]
- Sardet, C.; Franchi, A.; Pouysségur, J. Molecular cloning of the growth-factor-activatable human Na+/H+ antiporter. Cold Spring Harbor Symp. Quant. Biol. 1988, LIII, 1011–1018. [Google Scholar] [CrossRef]
- Petrecca, K.; Atanasiu, R.; Grinstein, S.; Orlowski, J.; Shrier, A. Subcellular localization of the Na+/H+ exchanger nhe1 in rat myocardium. Am. J. Physiol. 1999, 276, H709–H717. [Google Scholar] [CrossRef]
- Malakooti, J.; Dahdal, R.Y.; Schmidt, L.; Layden, T.J.; Dudeja, P.K.; Ramaswamy, K. Molecular cloning, tissue distribution, and functinal expression of the human Na+/H+ exchanger Nhe2. Am. J. Physiol. 1999, 277, G383–G390. [Google Scholar] [PubMed]
- Amemiya, M.; Loffing, J.; Lotscher, M.; Kaissling, B.; Alpern, R.J.; Moe, O.W. Expression of Nhe-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1995, 48, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Biemesderfer, D.; Pizzonia, J.; Abu-Alfa, A.; Exner, M.; Reilly, R.; Igarashi, P.; Aronson, P.S. Nhe3: A exchanger isoform of renal brush border. Am. J. Physiol. 1993, 265, F736–F742. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.R.; Orlowski, J.; Szabo, E.Z.; Zaun, H.C.; Schultheis, P.J.; Menon, A.G.; Shull, G.E. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (nhe5) from human brain. J. Biol. Chem. 1999, 274, 4377–4382. [Google Scholar] [CrossRef]
- Nakamura, N.; Tanaka, S.; Teko, Y.; Mitsui, K.; Kanazawa, H. Four Na+/H+ exchanger isoforms are distributed to golgi and post-golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 2005, 280, 1561–1572. [Google Scholar] [CrossRef]
- Numata, M.; Petrecca, K.; Lake, N.; Orlowski, J. Identification of a mitochondrial Na+/H+ exchanger. J. Biol. Chem. 1998, 273, 6951–6959. [Google Scholar] [CrossRef]
- Meima, M.E.; Mackley, J.R.; Barber, D.L. Beyond ion translocation: Structural functions of the sodium-hydrogen exchanger isoform-1. Curr. Opin. Nephrol. Hypertens. 2007, 16, 365–372. [Google Scholar] [CrossRef]
- Orlowski, J.; Grinstein, S. Diversity of the mammalian sodium/proton exchanger slc9 gene family. Pflugers Arch. 2004, 447, 549–565. [Google Scholar] [CrossRef]
- Brett, C.L.; Donowitz, M.; Rao, R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. 2005, 288, C223–C239. [Google Scholar] [CrossRef] [PubMed]
- Pouyssegur, J.; Sardet, C.; Franchi, A.; L‘Allemain, G.; Paris, S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic ph. Proc. Natl. Acad. Sci. USA 1984, 81, 4833–4837. [Google Scholar] [CrossRef]
- Guissart, C.; Li, X.; Leheup, B.; Drouot, N.; Montaut-Verient, B.; Raffo, E.; Jonveaux, P.; Roux, A.F.; Claustres, M.; Fliegel, L.; et al. Mutation of slc9a1, encoding the major Na+/H+ exchanger, causes ataxia-deafness lichtenstein-knorr syndrome. Hum. Mol. Genet. 2015, 24, 463–470. [Google Scholar] [CrossRef]
- Bell, S.M.; Schreiner, C.M.; Schultheis, P.J.; Miller, M.L.; Evans, R.L.; Vorhees, C.V.; Shull, G.E.; Scott, W.J. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am. J. Physiol. 1999, 276, C788–C795. [Google Scholar] [CrossRef]
- Cox, G.A.; Lutz, C.M.; Yang, C.-L.; Biemesderfer, D.; Bronson, R.T.; Fu, A.; Aronson, P.S.; Noebels, J.L.; Frankel, W.N. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mice. Cell 1997, 91, 139–148. [Google Scholar] [CrossRef]
- Putney, L.K.; Barber, D.L. Na-H exchange-dependent increase in intracellular ph times g2/m entry and transition. J. Biol. Chem. 2003, 278, 44645–44649. [Google Scholar] [CrossRef]
- Putney, L.K.; Barber, D.L. Expression profile of genes regulated by activity of the Na-H exchanger Nhe1. BMC Genom. 2004, 5, 46–59. [Google Scholar] [CrossRef]
- Wang, H.; Singh, D.; Fliegel, L. The Na+/H+ antiporter potentiates growth and retinoic- acid induced differentiation of p19 embryonal carcinoma cells. J. Biol. Chem. 1997, 272, 26545–26549. [Google Scholar] [CrossRef]
- Rao, G.N.; Sardet, C.; Pouyssegur, J.; Berk, B.C. Na+/H+ antiporter gene expression increases during retinoic acid-induced granulocytic differentiation of hl60 cells. J. Cell. Physiol. 1992, 151, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Barber, D.L. Ion transport proteins anchor and regulate the cytoskeleton. Curr. Opin. Cell Biol. 2002, 14, 214–220. [Google Scholar] [CrossRef]
- Denker, S.P.; Barber, D.L. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger nhe1. J. Cell. Biol. 2002, 159, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Denker, S.P.; Huang, D.C.; Orlowski, J.; Furthmayr, H.; Barber, D.L. Direct binding of the Na-H exchanger nhe1 to erm proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol. Cell 2000, 6, 1425–1436. [Google Scholar] [CrossRef]
- Tominaga, T.; Barber, D.L. Na-H exchange acts downstream of rhoa to regulate integrin-induced cell adhesion and spreading. Mol. Biol. Cell. 1998, 9, 2287–2303. [Google Scholar] [CrossRef]
- Hendus-Altenburger, R.; Kragelund, B.B.; Pedersen, S.F. Structural dynamics and regulation of the mammalian slc9a family of Na+/H+ exchangers. Curr. Top. Membr. 2014, 73, 69–148. [Google Scholar] [PubMed]
- Khaled, A.R.; Moor, A.N.; Li, A.; Kim, K.; Ferris, D.K.; Muegge, K.; Fisher, R.J.; Fliegel, L.; Durum, S.K. Trophic factor withdrawal: P38 mitogen-activated protein kinase activates Nhe1, which induces intracellular alkalinization. Mol. Cell. Biol. 2001, 21, 7545–7557. [Google Scholar] [CrossRef]
- Karki, P.; Fliegel, L. Overexpression of the Nhe1 isoform of the Na+/H+ exchanger causes elevated apoptosis in isolated cardiomyocytes after hypoxia/reoxygenation challenge. Mol. Cell. Biochem. 2010, 338, 47–57. [Google Scholar] [CrossRef]
- Harguindey, S.; Orive, G.; Luis Pedraz, J.; Paradiso, A.; Reshkin, S.J. The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin--one single nature. Biochim. Biophys. Acta 2005, 1756, 1–24. [Google Scholar] [CrossRef]
- Reshkin, S.J.; Bellizzi, A.; Caldeira, S.; Albarani, V.; Malanchi, I.; Poignee, M.; Alunni-Fabbroni, M.; Casavola, V.; Tommasino, M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 2000, 14, 2185–2197. [Google Scholar] [CrossRef]
- McLean, L.A.; Roscoe, J.; Jorgensen, N.K.; Gorin, F.A.; Cala, P.M. Malignant gliomas display altered pH regulation by Nhe1 compared with nontransformed astrocytes. Am. J. Phyiol. 2000, 278, C676–C688. [Google Scholar] [CrossRef]
- Rich, I.N.; Worthington-White, D.; Garden, O.A.; Musk, P. Apoptosis of leukemic cells accompanies reduction in intracellular ph after targeted inhibition of the Na+/H+ exchanger. Blood 2000, 95, 1427–1434. [Google Scholar]
- Reshkin, S.J.; Bellizzi, A.; Albarani, V.; Guerra, L.; Tommasino, M.; Paradiso, A.; Casavola, V. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na+/H+ exchange, motility, and invasion induced by serum deprivation. J. Biol. Chem. 2000, 275, 5361–5369. [Google Scholar] [CrossRef]
- Cardone, R.A.; Bagorda, A.; Bellizzi, A.; Busco, G.; Guerra, L.; Paradiso, A.; Casavola, V.; Zaccolo, M.; Reshkin, S.J. Protein kinase a gating of a pseudopodial-located rhoa/rock/p38/Nhe1 signal module regulates invasion in breast cancer cell lines. Mol. Biol. Cell 2005, 16, 3117–3127. [Google Scholar] [CrossRef]
- Paradiso, A.; Cardone, R.A.; Bellizzi, A.; Bagorda, A.; Guerra, L.; Tommasino, M.; Casavola, V.; Reshkin, S.J. The Na+-H+ exchanger-1 induces cytoskeletal changes involving reciprocal rhoa and rac1 signaling, resulting in motility and invasion in mda-mb-435 cells. Breast Cancer Res. 2004, 6, R616–R628. [Google Scholar] [CrossRef]
- Amith, S.R.; Fliegel, L. Regulation of the V exchanger (Nhe1) in breast cancer metastasis. Cancer Res. 2013, 73, 1259–1264. [Google Scholar] [CrossRef]
- Amith, S.R.; Fliegel, L. Na+/H+ exchanger-mediated hydrogen ion extrusion as a carcinogenic signal in triple-negative breast cancer etiopathogenesis and prospects for its inhibition in therapeutics. Semin. Cancer Biol. 2017, 43, 35–41. [Google Scholar] [CrossRef]
- Amith, S.R.; Fliegel, L. The Na+/H+ exchanger in metastasis. Aging (Albany, NY) 2016, 8, 1291. [Google Scholar] [CrossRef]
- Allen, D.G.; Xiao, X.H. Role of the cardiac Na+/H+ exchanger during ischemia and reperfusion. Cardiovasc. Res. 2003, 57, 934–941. [Google Scholar] [CrossRef]
- Lazdunski, M.; Frelin, C.; Vigne, P. The sodium/hydrogen exchange system in cardiac cells. Its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal ph. J. Mol. Cell. Cardiol. 1985, 17, 1029–1042. [Google Scholar] [CrossRef]
- Avkiran, M. Protection of the ischaemic myocardium by Na+/H+ exchange inhibitors: Potential mechanisms of action. Basic Res. Cardiol. 2001, 96, 306–311. [Google Scholar] [CrossRef]
- Karmazyn, M.; Sawyer, M.; Fliegel, L. The Na+/H+ exchanger: A target for cardiac therapeutic intervention. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 323–335. [Google Scholar] [CrossRef]
- Avkiran, M.; Marber, M.S. Na+/H+ exchange inhibitors for cardioprotective therapy: Progress, problems and prospects. J. Am. Coll. Cardiol. 2002, 39, 747–753. [Google Scholar] [CrossRef]
- Karmazyn, M. Amiloride enhances post ischemic recovery: Possible role of Na+/H+ exchange. Am. J. Physiol. 1988, 255, H608–H615. [Google Scholar]
- Scholz, W.; Albus, U.; Counillon, L.; Gogelein, H.; Lang, H.J.; Linz, W.; Weichert, A.; Scholkens, B.A. Protective effects of hoe642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc. Res. 1995, 29, 260–268. [Google Scholar] [CrossRef]
- Gumina, R.J.; Mizumura, T.; Beier, N.; Schelling, P.; Schultz, J.J.; Gross, G.J. A new sodium/hydrogen exchange inhibitor, emd 85131, limits infarct size in dogs when administered before or after coronary artery occlusion. J. Pharmacol. Exp. Ther. 1998, 286, 175–183. [Google Scholar]
- Moor, A.; Gan, X.T.; Karmazyn, M.; Fliegel, L. Activation of Na+/H+ exchanger-directed protein kinases in the ischemic and ischemic-reperfused rat myocardium. J. Biol. Chem. 2001, 276, 16113–16122. [Google Scholar] [CrossRef]
- Kusumoto, K.; Haist, J.V.; Karmazyn, M. Na+/H+ exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am. J. Physiol. 2001, 280, H738–H745. [Google Scholar] [CrossRef]
- Yoshida, H.; Karmazyn, M. Na+/H+ exchange inhibition attenuates hypertrophy and heart failure in 1-wk postinfarction rat myocardium. Am. J. Physiol. 2000, 278, H300–H304. [Google Scholar] [CrossRef]
- Kilic, A.; Velic, A.; De Windt, L.J.; Fabritz, L.; Voss, M.; Mitko, D.; Zwiener, M.; Baba, H.A.; van Eickels, M.; Schlatter, E.; et al. Enhanced activity of the myocardial Na+/H+ exchanger Nhe-1 contributes to cardiac remodeling in atrial natriuretic peptide receptor-deficient mice. Circulation 2005, 112, 2307–2317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mraiche, F.; Oka, T.; Gan, X.T.; Karmazyn, M.; Fliegel, L. Activated Nhe1 is required to induce early cardiac hypertrophy in mice. Basic Res. Cardiol. 2011, 106, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Karmazyn, M.; Liu, Q.; Gan, X.T.; Brix, B.J.; Fliegel, L. Aldosterone increases Nhe-1 expression and induces nhe-1-dependent hypertrophy in neonatal rat ventricular myocytes. Hypertension 2003, 42, 1171–1176. [Google Scholar] [CrossRef]
- Cingolani, H.E.; Alvarez, B.V.; Ennis, I.L.; Camilion de Hurtado, M.C. Stretch-induced alkalinization of feline papillary muscle: An autocrine-paracrine system. Circ. Res. 1998, 83, 775–780. [Google Scholar] [CrossRef]
- Camilion de Hurtado, M.C.; Portiansky, E.L.; Perez, N.G.; Rebolledo, O.R.; Cingolani, H.E. Regression of cardiomyocyte hypertrophy in shr following chronic inhibition of the Na+/H+ exchanger. Cardiovasc. Res. 2002, 53, 862–868. [Google Scholar] [CrossRef]
- Baartscheer, A.; Hardziyenka, M.; Schumacher, C.A.; Belterman, C.N.; van Borren, M.M.; Verkerk, A.O.; Coronel, R.; Fiolet, J.W. Chronic inhibition of the Na+/H+ Exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br. J. Pharmacol. 2008, 154, 1266–1275. [Google Scholar] [CrossRef]
- Cingolani, H.E.; Ennis, I.L. Sodium-hydrogen exchanger, cardiac overload, and myocardial hypertrophy. Circulation 2007, 115, 1090–1100. [Google Scholar] [CrossRef]
- Dulce, R.A.; Hurtado, C.; Ennis, I.L.; Garciarena, C.D.; Alvarez, M.C.; Caldiz, C.; Pierce, G.N.; Portiansky, E.L.; Chiappe de Cingolani, G.E.; Camilion de Hurtado, M.C. Endothelin-1 induced hypertrophic effect in neonatal rat cardiomyocytes: Involvement of Na+/H+ and Na+/Ca2+ exchangers. J. Mol. Cell. Cardiol. 2006, 41, 807–815. [Google Scholar] [CrossRef]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by ph. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.J.; von Ballmoos, C.; Newstead, S.; Uzdavinys, P.; Dotson, D.L.; Iwata, S.; Beckstein, O.; Cameron, A.D.; Drew, D. A two-domain elevator mechanism for sodium/proton antiport. Nature 2013, 501, 573–577. [Google Scholar] [CrossRef]
- Paulino, C.; Kuhlbrandt, W. Ph- and sodium-induced changes in a sodium/proton antiporter. eLife 2014, 3, e01412. [Google Scholar] [CrossRef]
- Wohlert, D.; Kuhlbrandt, W.; Yildiz, O. Structure and substrate ion binding in the sodium/proton antiporter panhap. eLife 2014, 3, e03579. [Google Scholar] [CrossRef]
- Dutta, D.; Fliegel, L. Structure and function of yeast and fungal Na+/H+ antiporters. IUBMB Life 2018, 70, 23–31. [Google Scholar] [CrossRef]
- Padan, E. Functional and structural dynamics of nhaa, a prototype for Na+ and H+ antiporters, which are responsible for Na+ and H+ homeostasis in cells. Biochim. Biophys. Acta 2014, 1837, 1047–1062. [Google Scholar] [CrossRef]
- Goswami, P.; Paulino, C.; Hizlan, D.; Vonck, J.; Yildiz, O.; Kuhlbrandt, W. Structure of the archaeal Na+/H+ antiporter nhap1 and functional role of transmembrane helix 1. EMBO J. 2011, 30, 439–449. [Google Scholar] [CrossRef]
- Padan, E.; Danieli, T.; Keren, Y.; Alkoby, D.; Masrati, G.; Haliloglu, T.; Ben-Tal, N.; Rimon, A. Nhaa antiporter functions using 10 helices, and an additional 2 contribute to assembly/stability. Proc. Natl. Acad. Sci. USA 2015, 112, E5575–E5582. [Google Scholar] [CrossRef]
- Ullah, A.; Dutta, D.; Fliegel, L. Expression and characterization of the sos1 arabidopsis salt tolerance protein. Mol. Cell. Biochem. 2016, 415, 133–143. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Pang, T.; Su, X.; Shigekawa, M. A novel topology model of the human Na+/H+ exchanger isoform 1. J. Biol. Chem. 2000, 275, 7942–7949. [Google Scholar] [CrossRef]
- Landau, M.; Herz, K.; Padan, E.; Ben-Tal, N. Model structure of the Na+/H+ exchanger 1 (Nhe1): Functional and clinical implications. J. Biol. Chem. 2007, 282, 37854–37863. [Google Scholar] [CrossRef]
- Liu, Y.; Basu, A.; Li, X.; Fliegel, L. Topological analysis of the Na+/H+ exchanger. Biochim. Biophys. Acta 2015, 1848, 2385–2393. [Google Scholar] [CrossRef]
- Jinadasa, T.; Josephson, C.B.; Boucher, A.; Orlowski, J. Determinants of cation permeation and drug sensitivity in predicted transmembrane helix 9 and adjoining exofacial re-entrant loop 5 of Na+/H+ exchanger Nhe1. J. Biol. Chem. 2015, 290, 18173–18186. [Google Scholar] [CrossRef]
- Dutta, D.; Fliegel, L. Molecular modeling and inhibitor docking analysis of the Na+/H+ exchanger isoform one. Biochem. Cell Biol. 2019, in press. [Google Scholar] [CrossRef]
- Orlowski, J.; Shull, G. Characteristics of the plasma membrane Na+/H+ exchanger gene family. In The Na+/H+ Exchanger; Fliegel, L., Ed.; R.G. Landes Company: Austin, TX, USA, 1996; pp. 123–148. [Google Scholar]
- Jaballah, M.; Mohamed, I.A.; Alemrayat, B.; Al-Sulaiti, F.; Mlih, M.; Mraiche, F. Na+/H+ exchanger isoform 1 induced cardiomyocyte hypertrophy involves activation of p90 ribosomal s6 kinase. PLoS ONE 2015, 10, e0122230. [Google Scholar] [CrossRef]
- Mohamed, I.A.; Gadeau, A.P.; Fliegel, L.; Lopaschuk, G.; Mlih, M.; Abdulrahman, N.; Fillmore, N.; Mraiche, F. Na+/H+ exchanger isoform 1-induced osteopontin expression facilitates cardiomyocyte hypertrophy. PLoS ONE 2015, 10, e0123318. [Google Scholar] [CrossRef]
- Mraiche, F.; Fliegel, L. Elevated expression of activated Na+/H+ exchanger protein induces hypertrophy in isolated rat neonatal ventricular cardiomyocytes. Mol. Cell. Biochem. 2011, 358, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Mraiche, F.; Zhou, D.; Karmazyn, M.; Oka, T.; Fliegel, L.; Haddad, G.G. Elevated myocardial Na+/H+ exchanger isoform 1 activity elicits gene expression that leads to cardiac hypertrophy. Physiol. Genom. 2010, 42, 374–383. [Google Scholar] [CrossRef]
- Amith, S.R.; Wilkinson, J.M.; Baksh, S.; Fliegel, L. Na+/H+ exchanger (Nhe1) as a novel co-adjuvant target in paclitaxel therapy of triple-negative breast cancer cells. Oncotarget 2015, 6, 1262–1275. [Google Scholar] [CrossRef]
- Amith, S.R.; Wilkinson, J.M.; Fliegel, L. Na+/H+ exchanger Nhe1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget 2016, 7, 21091–21113. [Google Scholar] [CrossRef]
- Avkiran, M.; Cook, A.R.; Cuello, F. Targeting Na+/H+ exchanger regulation for cardiac protection: A rsky approach? Curr. Opin. Pharmacol. 2008, 8, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Karmazyn, M. Nhe-1: Still a viable therapeutic target. J. Mol. Cell. Cardiol. 2013, 61, 77–82. [Google Scholar] [CrossRef]
- Karmazyn, M.; Moffat, M.P. Role of Na/H exchange in cardiac physiology and pathophysiology: Mediation of myocardial reperfusion injury by the ph paradox. Cardiovasc. Res. 1993, 27, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Haworth, R.S.; Avkiran, M. Receptor-mediated regulation of the cardiac sarcolemmal Na+/H+ exchanger. Mechanisms and (patho)physiological significance. In The Na+/H+ Exchanger, From Molecular to Its Role in Disease; Karmazyn, M., Avkiran, M., Fliegel, L., Eds.; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2003; pp. 191–209. [Google Scholar]
- Bianchini, L.; L‘Allemain, G.; Pouyssegur, J. The p42/44 mitogen-activated protein kinase cascade determinant in mediating activation of the Na+/H+ exchanger (Nhe1 isoform) in response to growth factors. J. Biol. Chem. 1997, 272, 271–279. [Google Scholar] [CrossRef]
- Phan, V.N.; Kusuhara, M.; Lucchesi, P.A.; Berk, B.C. A 90kd Na+/H+ exchanger kinase has increased activity in spontaneously hypertensive rat vascular smooth muscle cells. Circ. Res. 1997, 29, 1265–1272. [Google Scholar]
- Sardet, C.; Counillon, L.; Franchi, A.; Pouyssegur, J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kd. Science 1990, 247, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Snabaitis, A.K.; Yokoyama, H.; Avkiran, M. Roles of mitogen-activated protein kinases and protein kinase c in a1a-adrenoreceptor-mediated stimulation of the sarcolemmal Na+/H+ exchanger. Circ. Res. 2000, 86, 214–220. [Google Scholar] [CrossRef]
- Tominaga, T.; Ishizaki, T.; Narumiya, S.; Barber, D.L. P160rock mediates rhoa activation of Na/H exchange. EMBO J. 1998, 17, 4712–4722. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Abe, J.-i.; Berk, B.C. Angiotensin II stimulates p90rsk in vascular smooth muscle cells: A potential Na+/H+ exchanger kinase. Hypertension 1997, 29, 1265–1272. [Google Scholar]
- Fliegel, L.; Karmazyn, M. The cardiac Na-H exchanger: A key downstream mediator for the cellular hypertrophic effects of paracrine, autocrine and hormonal factors. Biochem. Cell Biol. 2004, 82, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, S.; Bertrand, B.; Shigekawa, M.; Fafournoux, P.; Pouyssegur, J. Growth factor activation and “H+-sensing” of the Na+/H+ exchanger isoform 1 (Nhe1). J. Biol. Chem. 1994, 269, 5583–5588. [Google Scholar]
- Bertrand, B.; Wakabayashi, S.; Ikeda, T.; Pouyssegur, J.; Shigekawa, M. The Na+/H+ exchanger isoform 1 (Nhe1) is a novel member of the calmodulin-binding proteins. J. Biol. Chem. 1994, 269, 13703–13709. [Google Scholar] [PubMed]
- Wakabayashi, S.; Ikeda, T.; Iwamoto, T.; Pouyssegur, J.; Shigekawa, M. Calmodulin-binding autoinhibitory domain controls “ph-sensing“ in the Na+/H+ exchanger Nhe1 through sequence specific interaction. Biochemistry 1997, 36, 12854–12861. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Bertrand, B.; Ikeda, T.; Pouyssegur, J.; Shigekawa, M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (Na+/H+ Nhe1) highly H+-sensitive and Ca2+ regulation-defective. J. Biol. Chem. 1994, 269, 13710–13715. [Google Scholar]
- Snabaitis, A.K.; Cuello, F.; Avkiran, M. Protein kinase b/akt phosphorylates and inhibits the cardiac Na+/H+ exchanger nhe1. Circ. Res. 2008, 103, 881–890. [Google Scholar] [CrossRef]
- Lin, X.; Barber, D.L. A calcineurin homologous protein inhibits gtpase-stimulated Na/H exchange. J. Biol. Chem. 1996, 93, 12631–12636. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Wakabayashi, S.; Shigekawa, M. Expression of calcineurin b homologous protein 2 protects serum deprivation-induced cell death by serum-independent activation of Na+/H+ exchanger. J. Biol. Chem. 2002, 277, 43771–43777. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Kay, C.M.; Muller-Esterl, W.; Fliegel, L. The Na+/H+ exchanger cytoplasmic tail: Structure, function, and interactions with tescalcin. Biochemistry 2003, 42, 7448–7456. [Google Scholar] [CrossRef] [PubMed]
- Mailander, J.; Muller-Esterl, W.; Dedio, J. Human homolog of mouse tescalcin associates with Na+/H+ exchanger type-1. FEBS Lett. 2001, 507, 331–335. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Hisamitsu, T.; Nakamura, T.Y. Regulation of the cardiac Na+/H+ exchanger in health and disease. J. Mol. Cell. Cardiol. 2013, 61, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Alvarez, B.; Casey, J.R.; Reithmeier, R.A.; Fliegel, L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. J. Biol. Chem. 2002, 277, 36085–36091. [Google Scholar] [CrossRef]
- Vaheri, A.; Carpen, O.; Heiska, L.; Helander, T.S.; Jaaskelainen, J.; Majander-Nordenswan, P.; Sainio, M.; Timonen, T.; Turunen, O. The ezrin protein family: Membrane-cytoskeleton interactions and disease associations. Curr. Opin. Cell. Biol. 1997, 9, 659–666. [Google Scholar] [CrossRef]
- Silva, N.L.C.L.; Haworth, R.S.; Singh, D.; Fliegel, L. The carboxyl-terminal region of the Na+/H+ exchanger interacts with mammalian heat shock protein. Biochemistry 1995, 34, 10412–10420. [Google Scholar] [CrossRef]
- Odunewu-Aderibigbe, A.; Fliegel, L. Protein mediated regulation of the Nhe1 isoform of the Na+/H+ exchanger in renal cells. A regulatory role of hsp90 and akt kinase. Cell Signal. 2017, 36, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Odunewu-Aderibigbe, A.; Fliegel, L. Heat shock proteins and the Na+/H+ exchanger. Channels (Austin) 2017, 11, 380–382. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, D.; Yao, H.; Gavrialov, O.; McConnell, M.J.; Gelb, B.D.; Haddad, G.G. Novel functional interaction between Na+/H+ exchanger 1 and tyrosine phosphatase shp-2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2406–R2416. [Google Scholar] [CrossRef][Green Version]
- Aharonovitz, O.; Zaun, H.C.; Balla, T.; York, J.D.; Orlowski, J.; Grinstein, S. Intracellular ph regulation by Na+/H+ exchange requires phosphatidylinositol 4,5-bisphosphate. J. Cell Biol. 2000, 150, 213–224. [Google Scholar] [CrossRef]
- Aharonovitz, O.; Demaurex, N.; Woodside, M.; Grinstein, S. Atp dependence is not an intrinsic property of Na+/H+ exchanger Nhe1: Requirement for an ancillary factor. Am. J. Physiol. 1999, 276, C1303–C1311. [Google Scholar] [CrossRef]
- Shimada-Shimizu, N.; Hisamitsu, T.; Nakamura, T.Y.; Hirayama, N.; Wakabayashi, S. Na+/H+ exchanger 1 is regulated via its lipid-interacting domain, which functions as a molecular switch: A pharmacological approach using indolocarbazole compounds. Mol. Pharmacol. 2014, 85, 18–28. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Nakamura, T.Y.; Kobayashi, S.; Hisamitsu, T. Novel phorbol ester-binding motif mediates hormonal activation of Na+/H+ exchanger. J. Biol. Chem. 2010, 285, 26652–26661. [Google Scholar] [CrossRef]
- Fliegel, L. Molecular biology of the myocardial Na+/H+ exchanger. J. Mol. Cell. Cardiol. 2008, 44, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Odunewu-Aderibigbe, A.; Fliegel, L. The Na+/H+ exchanger and ph regulation in the heart. IUBMB Life 2014, 66, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Li, X.; Schrama, D.; Fliegel, L. B-Raf is associated with and activates the Nhe1 isoform of the Na+/H+ exchanger. J. Biol. Chem. 2011, 286, 13096–13105. [Google Scholar] [CrossRef] [PubMed]
- Meima, M.E.; Webb, B.A.; Witkowska, H.E.; Barber, D.L. The sodium-hydrogen exchanger Nhe1 is an akt substrate necessary for actin filament reorganization by growth factors. J. Biol. Chem. 2009, 284, 26666–26675. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Seger, R. The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 2006, 24, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Kolch, W. Coordinating erk/mapk signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005, 6, 827–837. [Google Scholar] [CrossRef]
- Amith, S.R.; Fong, S.; Baksh, S.; Fliegel, L. Na +/H +exchange in the tumour microenvironment: Does nhe1 drive breast cancer carcinogenesis? Int. J. Dev. Biol. 2015, 59, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Snider, J.L.; Skalli, O.; Welbourne, T.; Cardelli, J.A. Na+/H+ exchangers and rhoa regulate acidic extracellular ph-induced lysosome trafficking in prostate cancer cells. Traffic 2009, 10, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Araos, J.; Naranjo, L.; Toledo, F.; Beltran, A.R.; Ramirez, M.A.; Gutierrez, J.; Pardo, F.; Leiva, A.; Sobrevia, L. Sodium/proton exchanger isoform 1 regulates intracellular pH and cell proliferation in human ovarian cancer. Biochim. Biophys. Acta 2017, 1863, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Zhang, S.; Treskov, I.; Kovacs, A.; Weinheimer, C.; Muslin, A.J. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation 2004, 110, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; O’Neill, M.M.; MacDonnell, S.; Brookes, P.S.; Yan, C.; Berk, B.C. The rsk inhibitor bix02565 limits cardiac ischemia/reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 177–186. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, G.; Wang, J.; Jin, W.; Wang, L.; Li, H.; Ma, L.; Li, Q.; Pang, T. Nhe1 mediates mda-mb-231 cells invasion through the regulation of mt1-mmp. Exp. Cell Res. 2011, 317, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Silva, N.L.C.L.; Lucchesi, P.A.; Haworth, R.; Wang, K.; Michalak, M.; Pelech, S.; Fliegel, L. Phosphorylation and regulation of the Na+/H+ exchanger through mitogen-activated protein kinase. Biochemistry 1997, 36, 9151–9158. [Google Scholar] [CrossRef] [PubMed]
- Haworth, R.S.; Dashnyam, S.; Avkiran, M. Ras triggers acidosis-induced activation of the extracellular-signal-regulated kinase pathway in cardiac myocytes. Biochem. J. 2006, 399, 493–501. [Google Scholar] [CrossRef]
- Sabri, A.; Byron, K.L.; Samarel, A.M.; Bell, J.; Lucchesi, P.A. Hydrogen peroxide activates mitogen-activated protein kinases and Na+/H+ exchange in neonatal rat cardiac myocytes. Circ. Res. 1998, 82, 1053–1062. [Google Scholar] [CrossRef]
- Wei, S.; Rothstein, E.C.; Fliegel, L.; Dell’Italia, L.J.; Lucchesi, P.A. Differential map kinase activation and Na+/H+ exchanger phosphorylation by H(2)O(2) in rat cardiac myocytes. Am. J. Physiol. 2001, 281, C1542–C1550. [Google Scholar] [CrossRef]
- Rothstein, E.C.; Byron, K.L.; Reed, R.E.; Fliegel, L.; Lucchesi, P.A. H2O2-induced Ca2+ overload in nrvm involves erk1/2 map kinases: Role for an nhe-1-dependent pathway. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H598–H605. [Google Scholar] [CrossRef]
- Lehoux, S.; Abe, J.; Florian, J.A.; Berk, B.C. 14-3-3 binding to Na+/H+ exchanger isoform-1 is associated with serum-dependent activation of Na+/H+ exchange. J. Biol. Chem. 2001, 276, 15794–15800. [Google Scholar] [CrossRef]
- Maekawa, N.; Abe, J.; Shishido, T.; Itoh, S.; Ding, B.; Sharma, V.K.; Sheu, S.S.; Blaxall, B.C.; Berk, B.C. Inhibiting p90 ribosomal s6 kinase prevents (Na+)-H+ exchanger-mediated cardiac ischemia-reperfusion injury. Circulation 2006, 113, 2516–2523. [Google Scholar] [CrossRef]
- Coccaro, E.; Karki, P.; Cojocaru, C.; Fliegel, L. Phenylephrine and sustained acidosis activate the neonatal rat cardiomyocyte Na+/H+ exchanger through phosphorylation of amino acids ser770 and ser771. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H846–H858. [Google Scholar] [CrossRef]
- Stupak, J.; Liu, H.; Wang, Z.; Brix, B.J.; Fliegel, L.; Li, L. Nanoliter sample handling combined with microspot maldi-ms for detection of gel-separated phosphoproteins. J. Proteome Res. 2005, 4, 515–522. [Google Scholar] [CrossRef]
- Liu, H.; Stupak, J.; Zheng, J.; Keller, B.O.; Brix, B.J.; Fliegel, L.; Li, L. Open tubular immobilized metal–ion affinity chromatography combined with maldi ms and ms/ms for identification of protein phosphorylation sites. Anal. Chem. 2004, 76, 4223–4232. [Google Scholar] [CrossRef]
- Malo, M.E.; Li, L.; Fliegel, L. Mitogen-activated protein kinase-dependent activation of the Na+/H+ exchanger is mediated through phosphorylation of amino acids ser770 and ser771. J. Biol. Chem. 2007, 282, 6292–6299. [Google Scholar] [CrossRef]
- Karki, P.; Coccaro, E.; Fliegel, L. Sustained intracellular acidosis activates the myocardial Na+/H+ exchanger independent of amino acid ser703 and p90rsk. Biochim. Biophys. Acta 2010, 1798, 1565–1576. [Google Scholar] [CrossRef]
- Hendus-Altenburger, R.; Pedraz-Cuesta, E.; Olesen, C.W.; Papaleo, E.; Schnell, J.A.; Hopper, J.T.; Robinson, C.V.; Pedersen, S.F.; Kragelund, B.B. The human Na+/H+ exchanger 1 is a membrane scaffold protein for extracellular signal-regulated kinase 2. BMC Biol. 2016, 14, 31. [Google Scholar] [CrossRef]
- Li, X.; Khan, M.F.; Schriemer, D.C.; Fliegel, L. Structural changes in the c-terminal regulatory region of the Na+/H+ exchanger mediate phosphorylation induced regulation. J. Mol. Cell. Cardiol. 2013, 61, 153–163. [Google Scholar] [CrossRef]
- Fliegel, L. Beta-raf activation of the myocardial Na+/H+ exchanger. Channels (Austin) 2017, 11, 181–182. [Google Scholar] [CrossRef]
- Hendus-Altenburger, R.; Lambrughi, M.; Terkelsen, T.; Pedersen, S.F.; Papaleo, E.; Lindorff-Larsen, K.; Kragelund, B.B. A phosphorylation-motif for tuneable helix stabilisation in intrinsically disordered proteins - lessons from the sodium proton exchanger 1 (nhe1). Cell Signal. 2017, 37, 40–51. [Google Scholar] [CrossRef]
- Chang, G.; Wang, J.; Zhang, H.; Zhang, Y.; Wang, C.; Xu, H.; Zhang, H.; Lin, Y.; Ma, L.; Li, Q.; et al. Cd44 targets Na/H exchanger 1 to mediate mda-mb-231 cells’ metastasis via the regulation of erk1/2. Br. J. Cancer 2014, 110, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Cardone, R.A.; Bellizzi, A.; Busco, G.; Weinman, E.J.; Dell’aquila, M.E.; Casavola, V.; Azzariti, A.; Mangia, A.; Paradiso, A.; Reshkin, S.J. The nherf1 pdz2 domain regulates pka-rhoa-p38-mediated Nhe1 activation and invasion in breast tumor cells. Mol. Biol. Cell 2007, 18, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Reshkin, S.J.; Greco, M.R.; Cardone, R.A. Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130100. [Google Scholar] [CrossRef] [PubMed]
- Spugnini, E.P.; Sonveaux, P.; Stock, C.; Perez-Sayans, M.; De Milito, A.; Avnet, S.; Garcia, A.G.; Harguindey, S.; Fais, S. Proton channels and exchangers in cancer. Biochim. Biophys. Acta 2015, 1848, 2715–2726. [Google Scholar] [CrossRef]
- Reshkin, S.J.; Cardone, R.A.; Harguindey, S. Na+-H+ exchanger, pH regulation and cancer. Recent Pat. Anticancer Drug Discov. 2013, 8, 85–99. [Google Scholar] [CrossRef]
- Brisson, L.; Driffort, V.; Benoist, L.; Poet, M.; Counillon, L.; Antelmi, E.; Rubino, R.; Besson, P.; Labbal, F.; Chevalier, S.; et al. Nav1.5 Na+ channels allosterically regulate the Nhe-1 exchanger and promote the activity of breast cancer cell invadopodia. J. Cell Sci. 2013, 126, 4835–4842. [Google Scholar] [CrossRef]
- Daniel, C.; Bell, C.; Burton, C.; Harguindey, S.; Reshkin, S.J.; Rauch, C. The role of proton dynamics in the development and maintenance of multidrug resistance in cancer. Biochim. Biophys. Acta 2013, 1832, 606–617. [Google Scholar] [CrossRef]
- Greco, M.R.; Antelmi, E.; Busco, G.; Guerra, L.; Rubino, R.; Casavola, V.; Reshkin, S.J.; Cardone, R.A. Protease activity at invadopodial focal digestive areas is dependent on nhe1-driven acidic pHe. Oncol. Rep. 2014, 31, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Busco, G.; Cardone, R.A.; Greco, M.R.; Bellizzi, A.; Colella, M.; Antelmi, E.; Mancini, M.T.; Dell’Aquila, M.E.; Casavola, V.; Paradiso, A.; et al. Nhe1 promotes invadopodial ecm proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010, 24, 3903–3915. [Google Scholar] [CrossRef]
- Stock, C.; Cardone, R.A.; Busco, G.; Krahling, H.; Schwab, A.; Reshkin, S.J. Protons extruded by Nhe1: Digestive or glue? Eur. J. Cell Biol. 2008, 87, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Amith, S.R.; Vincent, K.M.; Wilkinson, J.M.; Postovit, L.M.; Fliegel, L. Defining the Na+/H+ exchanger nhe1 interactome in triple-negative breast cancer cells. Cell Signal. 2017, 29, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, G.; Stock, C.M.; Lemaire, J.; Lund, S.F.; Jensen, M.F.; Damsgaard, B.; Petersen, K.S.; Wiwel, M.; Ronnov-Jessen, L.; Schwab, A.; et al. The Na+/H+ exchanger nhe1, but not the Na+, HCO3(-) cotransporter nbcn1, regulates motility of mcf7 breast cancer cells expressing constitutively active erbb2. Cancer Lett. 2012, 317, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Chiang, C.Y.; Srivastava, J.; Gersten, M.; White, S.; Bell, R.; Kurschner, C.; Martin, C.H.; Smoot, M.; Sahasrabudhe, S.; et al. A human map kinase interactome. Nat. Methods 2010, 7, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, M.; Patel, H.; Barber, D.L. Na+/H+ exchanger Nhe1 as plasma membrane scaffold in the assembly of signaling complexes. Am. J. Physiol. 2004, 287, C844–C850. [Google Scholar] [CrossRef]
- Tompa, P.; Fuxreiter, M. Fuzzy complexes: Polymorphism and structural disorder in protein-protein interactions. Trends Biochem. Sci. 2008, 33, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Fuxreiter, M.; Tompa, P.; Simon, I. Local structural disorder imparts plasticity on linear motifs. Bioinformatics (Oxford) 2007, 23, 950–956. [Google Scholar] [CrossRef]

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fliegel, L. Structural and Functional Changes in the Na+/H+ Exchanger Isoform 1, Induced by Erk1/2 Phosphorylation. Int. J. Mol. Sci. 2019, 20, 2378. https://doi.org/10.3390/ijms20102378
Fliegel L. Structural and Functional Changes in the Na+/H+ Exchanger Isoform 1, Induced by Erk1/2 Phosphorylation. International Journal of Molecular Sciences. 2019; 20(10):2378. https://doi.org/10.3390/ijms20102378
Chicago/Turabian StyleFliegel, Larry. 2019. "Structural and Functional Changes in the Na+/H+ Exchanger Isoform 1, Induced by Erk1/2 Phosphorylation" International Journal of Molecular Sciences 20, no. 10: 2378. https://doi.org/10.3390/ijms20102378
APA StyleFliegel, L. (2019). Structural and Functional Changes in the Na+/H+ Exchanger Isoform 1, Induced by Erk1/2 Phosphorylation. International Journal of Molecular Sciences, 20(10), 2378. https://doi.org/10.3390/ijms20102378



