Identification of Fatty Acid Desaturase 6 in Golden Pompano Trachinotus Ovatus (Linnaeus 1758) and Its Regulation by the PPARαb Transcription Factor
Abstract
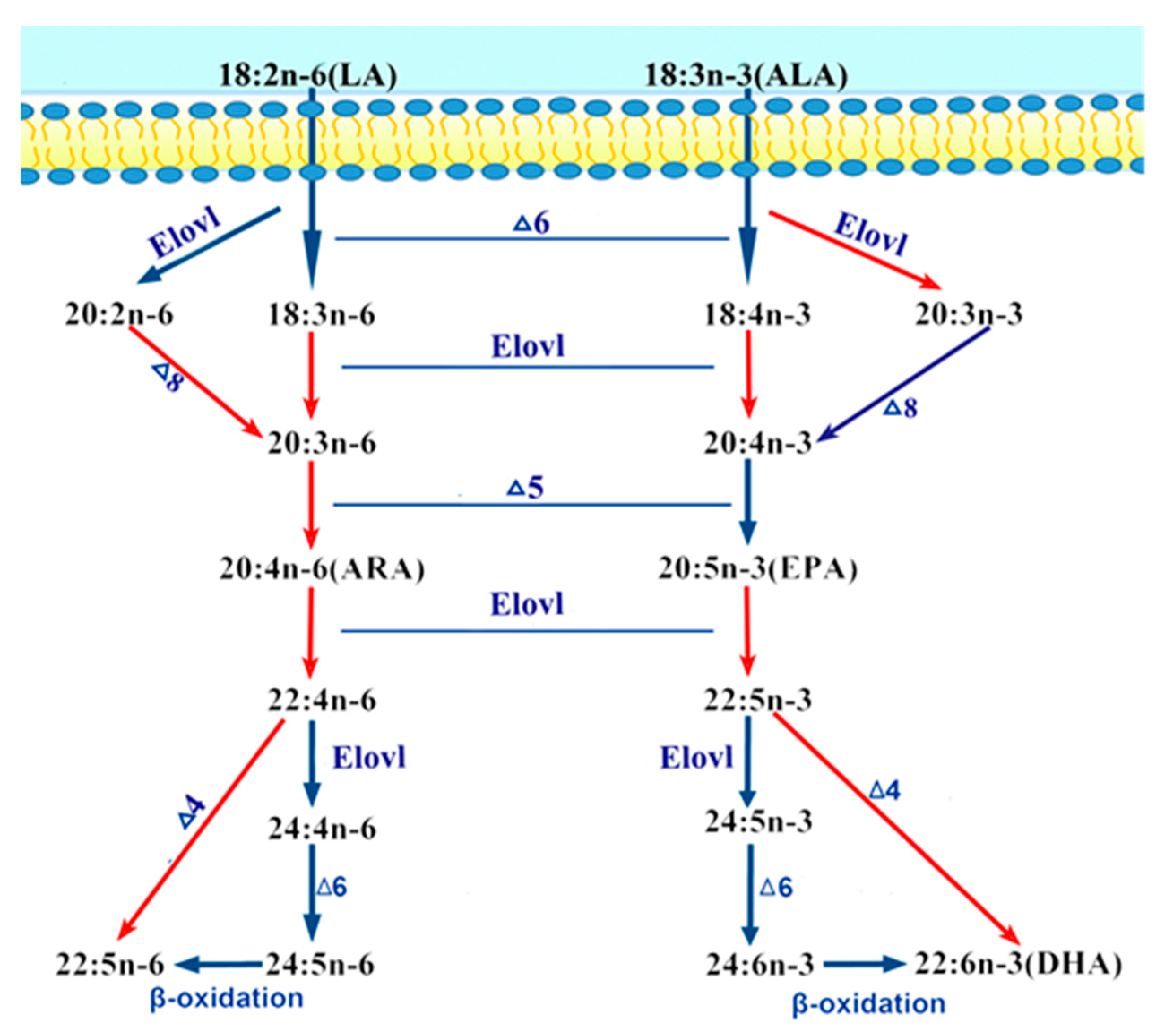
1. Introduction
2. Results
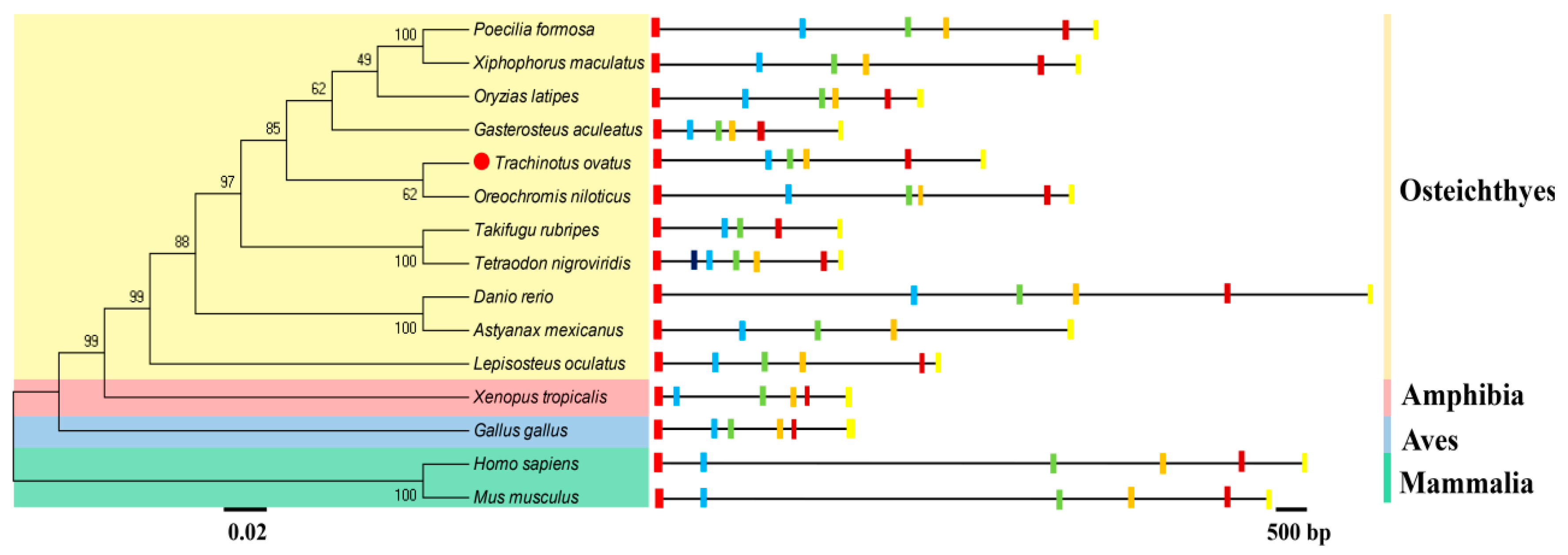
2.1. Molecular Cloning and Phylogenetics of T. ovatus Fads6
2.2. Heterologous Expression of the Desaturase ORF in Saccharomyces cerevisiae
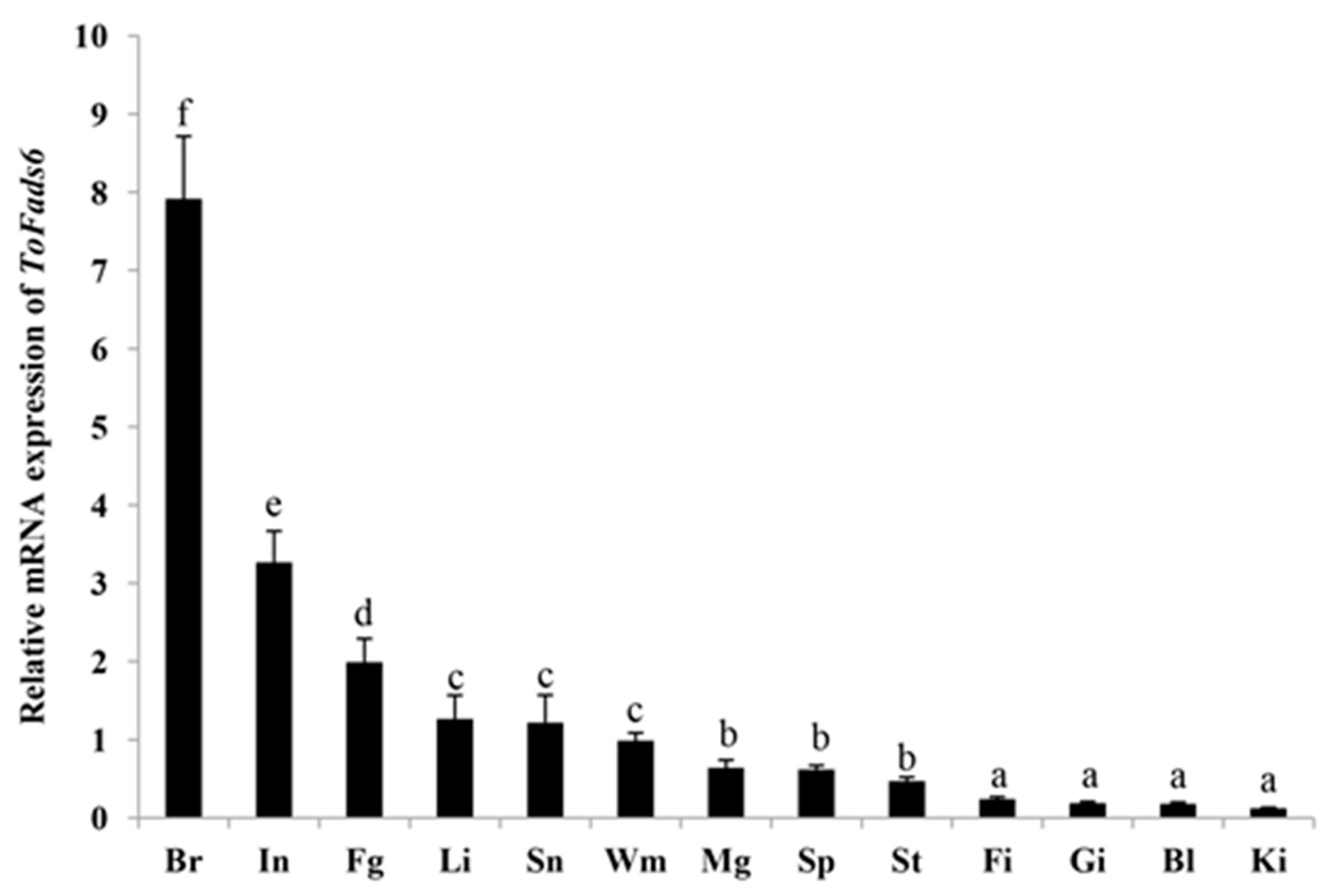
2.3. Tissue Distribution of ToFads6
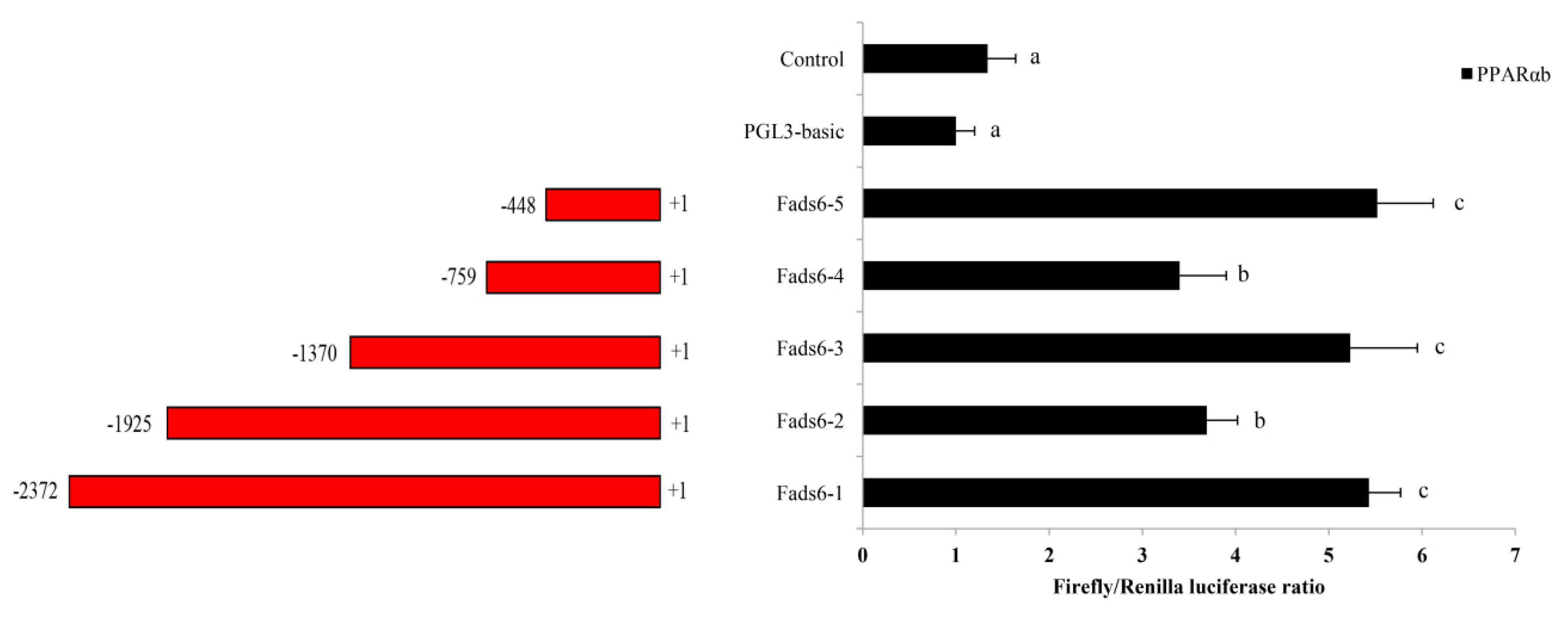
2.4. Promoter Analysis of PPARαb Regulation
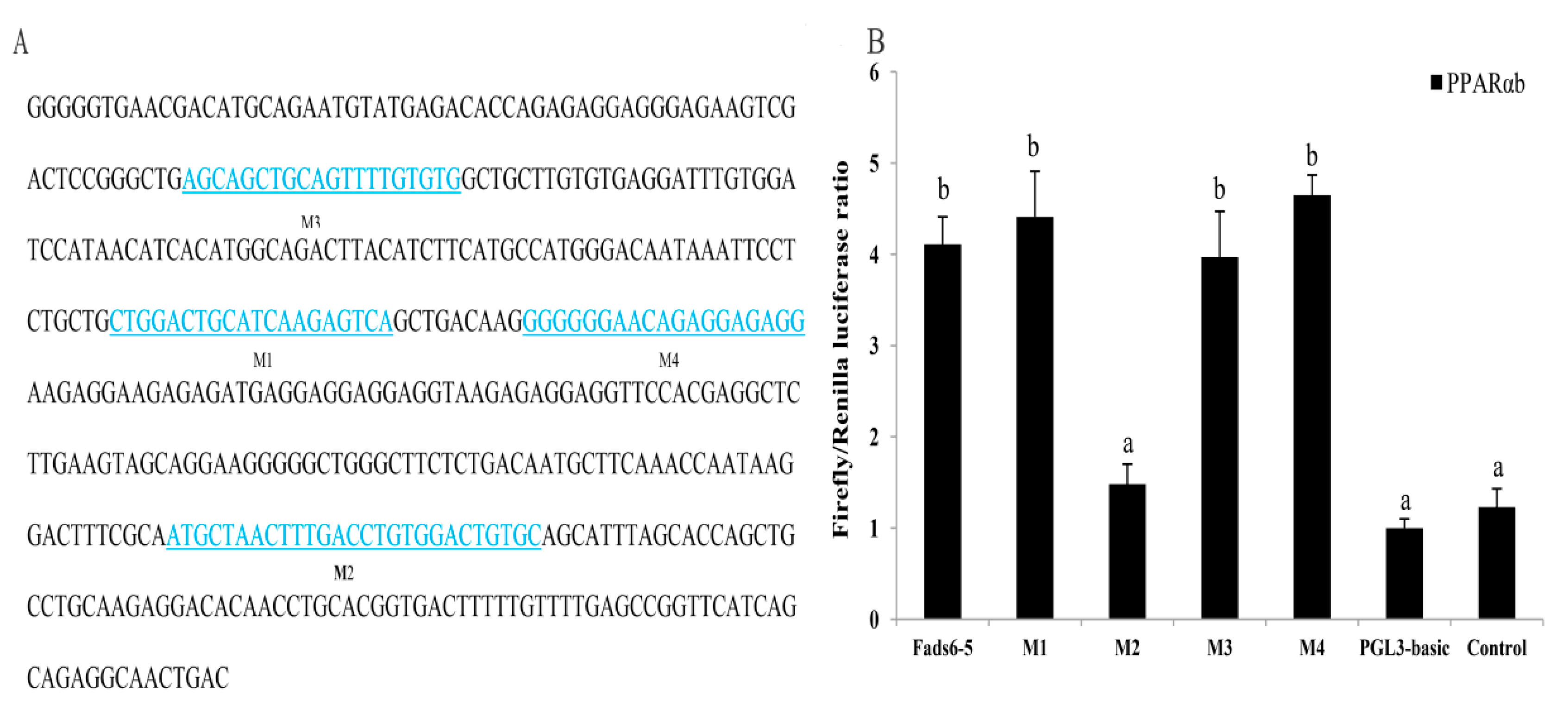
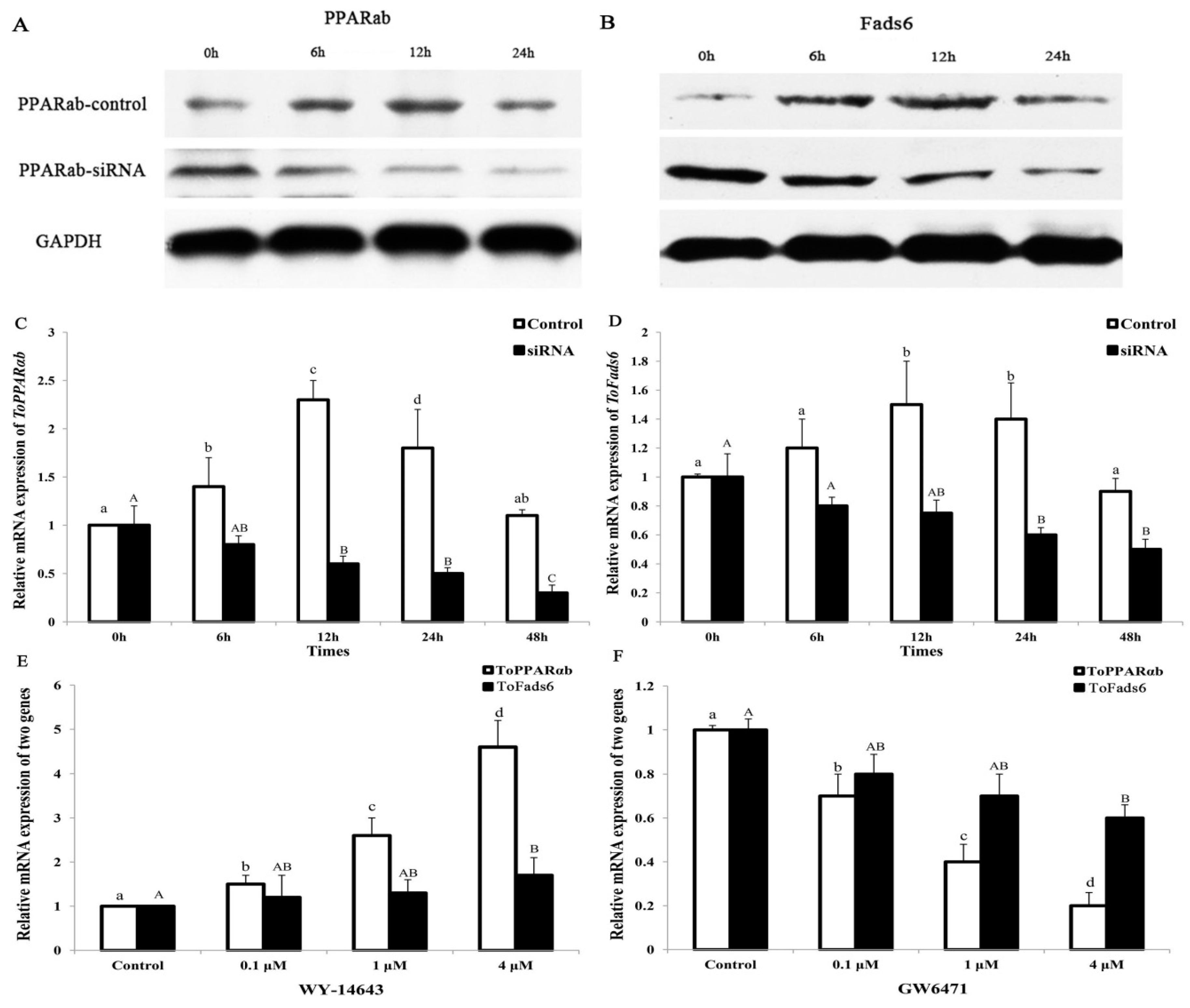
2.5. Transcriptional Regulation of ToFads6 by PPARαb
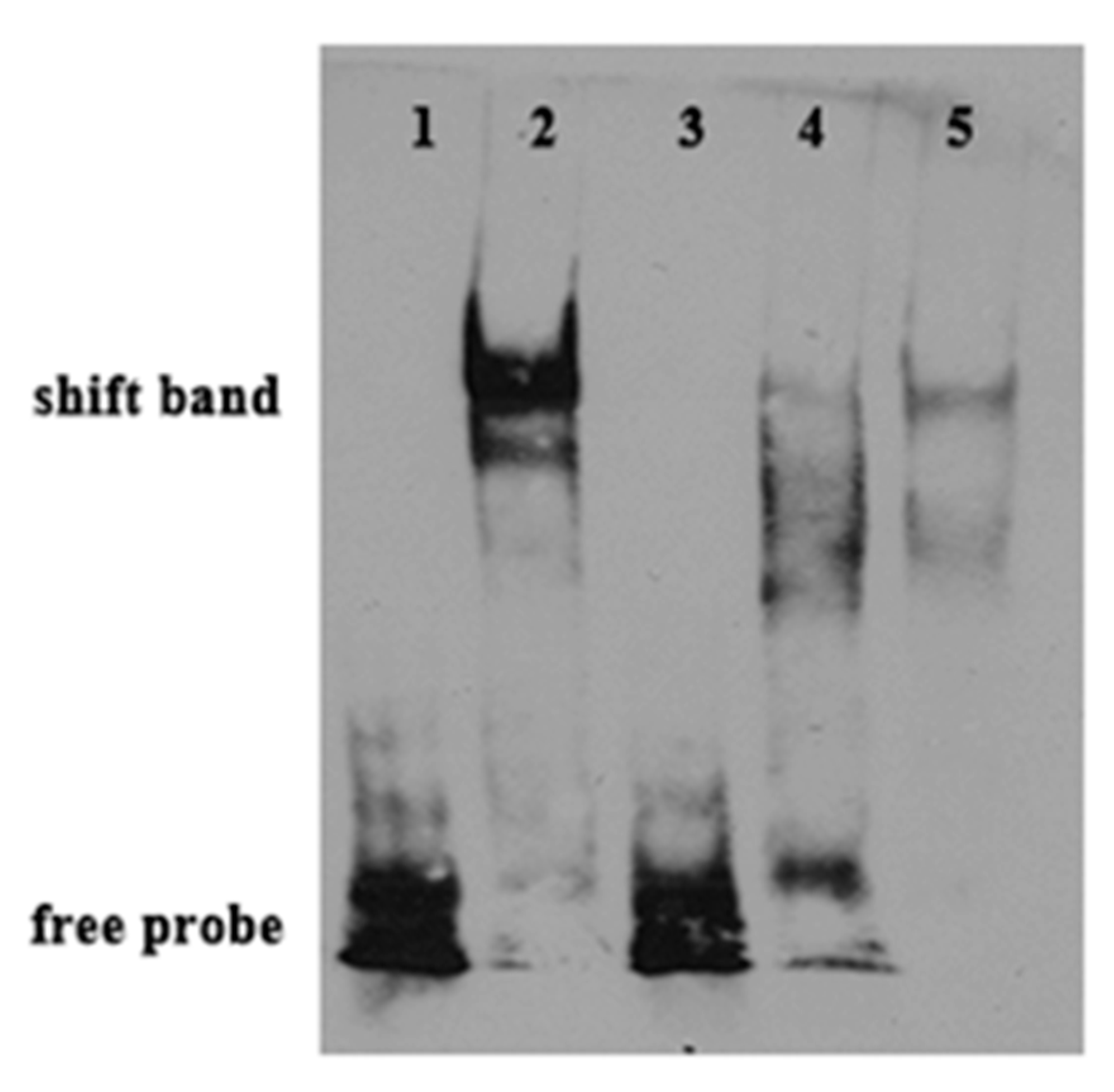
2.6. Binding of PPARαb to Fads6 Promoters
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Tissue Collection
4.3. Gene Cloning and Bioinformatics of T. ovatus Fads6
4.4. Functional Characterization of the ToFads6 Desaturase
4.5. Real-Time Quantitative PCR Analysis
4.6. Preparation of a Fads6 Polyclonal Antibody and Western Blotting Analysis
4.7. Cloning of the Fads6 Promoter and Construction of Deletion Mutants
4.8. Construction of Truncated Mutants for the Identification of Predicted Transcription Factor (TF) Binding Sites in the Fads6 Promoter
4.9. Cell Culture, Transfection, and Luciferase Assay
4.10. Expression Analysis of ToFads6 with ToPPARαb
4.11. Electrophoretic Mobility Shift Assay (EMSA)
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations:
| Fads | Fatty acid desaturases |
| EMSA | Electrophoretic mobile shift assays |
| LC-PUFA | Long-chain polyunsaturated fatty acids |
| PPARαb | Transcription factor peroxisome proliferator-activated receptor alpha b |
| Elovl | Elongation of very long-chain fatty acids |
| HNF4 | Hepatocyte nuclear factor-4 |
| NR1H3 | Nuclear receptor subfamily 1 group H member 3 |
| SREBP | Sterol-regulatory element binding protein |
| NF-κB | Nuclear factor-kappa B |
| PPREs | PPAR response elements |
| FAMEs | Fatty acid methyl esters |
| TLC | Thin-layer chromatography |
| TOCF | T. ovatus caudal fin |
References
- Castro, C.L.F.; Tocher, D.R.; Monroig, Ó. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Park, W.J.; Kothapalli, K.S.; Lawrence, P.; Tyburczy, C.; Brenna, J.T. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J. Lipid Res. 2009, 50, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Monroig, Ó.; Li, Y.Y.; Tocher, D.R. Delta-8 desaturation activity varies among fatty acyl desaturases of teleost fish: High activity in delta-6 desaturases of marine species. Comp. Biochem. Physiol. B 2011, 159, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Vagner, M.; Santigosa, E. Characterization and modulation of gene expression and enzymatic activity of delta-6 desaturase in teleosts: A review. Aquaculture 2011, 315, 131–143. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ntambi, J.M. Fatty acid desaturation and chain elongation in mammals. In Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Vance, D.E., Vance, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 191–212. [Google Scholar]
- Gregory, M.K.; See, V.H.L.; Gibson, R.A.; Schuller, K.A. Cloning and functional characterization of a fatty acyl elongase from southern bluefin tuna (Thunnus maccoyii). Comp. Biochem. Physiol. B 2010, 155, 178–185. [Google Scholar] [CrossRef]
- Li, Y.Y.; Monroig, Ó.; Zhang, L.; Wang, S.Q.; Zheng, X. Vertebrate fatty acyl desaturase with D4 activity. Proc. Natl. Acad. Sci. USA 2010, 107, 16840–16845. [Google Scholar] [CrossRef]
- Morais, S.; Mourente, G.; Ortega, A.; Tocher, J.A.; Tocher, D.R. Expression of fatty acyl desaturase and elongase genes, and evolution of DHA: EPA ratio during development of unfed larvae of Atlantic bluefin tuna (Thunnus thynnus L.). Aquaculture 2011, 313, 129–139. [Google Scholar] [CrossRef]
- Wang, S.Q.; Monroig, Ó.; Tang, G.X.; Zhang, L.; You, C.H.; Tocher, D.R.; Li, Y.Y. Investigating long-chain polyunsaturated fatty acid biosynthesis in teleost fish: Functional characterization of fatty acyl desaturase (Fads2) and Elovl5 elongase in the catadromous species, Japanese eel Anguilla japonica. Aquaculture 2014, 434, 57–65. [Google Scholar] [CrossRef]
- Xie, D.Z.; Chen, F.; Lin, S.Y.; Wang, S.Q.; You, C.H.; Monroig, Ó.; Tocher, D.R.; Li, Y.Y. Cloning, functional characterization and nutritional regulation of Δ6 fatty acyl desaturase in the herbivorous euryhaline teleost Scatophagus argus. PLoS ONE 2014, 9, e90200. [Google Scholar] [CrossRef]
- Ngoh, S.Y.; Tan, D.; Shen, X.; Kathiresan, P.; Jiang, J.; Liew, W.C.; Thevasagayam, N.M.; Kwan, H.Y.; Saju, J.M.; Prakki, S.R.; et al. Nutrigenomic and nutritional analyses reveal the effects of pelleted feeds on Asian seabass (Lates calcarifer). PLoS ONE 2015, 10, e0145456. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Suzuki, Y.; Nishikawa, T.; Otsuki, T.; Sugiyama, T.; Irie, R.; Wakamatsu, A.; Hayashi, K.; Sato, H.; Nagai, K.; et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat. Genet. 2004, 36, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huo, K.; Ma, L.; Tang, L.; Li, D.; Huang, X.; Yuan, Y.; Li, C.; Wang, W.; Guan, W.; et al. Toward an understanding of the protein interaction network of the human liver. Mol. Syst. Biol. 2011, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Giancaspero, T.A.; Galluccio, M.; Miccolis, A.; Leone, P.; Eberini, I.; Iametti, S.; Indiveri, C.; Barile, M. Human FAD synthase is a bi-functional enzyme with a FAD hydrolase activity in the molybdopterin binding domain. Biochem. Biophys. Res. Commun. 2015, 465, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kota, B.P.; Huang, T.H.; Roufogalis, B.D. An overview on biological mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M.V.; Lodhi, I.J.; Yin, L.; Malapaka, R.R.V.; Xu, H.E.; Turk, J.; Semenkovich, C. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 2009, 138, 476–488. [Google Scholar] [CrossRef]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the roles of PPARa in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- Tang, C.R.; Cho, H.P.; Nakamura, M.T.; Clarke, S.D. Regulation of human Delta-6 desaturase gene transcription: Identification of a functional direct repeat-1 element. J. Lipid Res. 2003, 44, 686–695. [Google Scholar] [CrossRef]
- Dong, X.J.; Tan, P.; Cai, Z.N.; Xu, H.L.; Li, J.Q.; Ren, W.; Xu, H.G.; Zuo, R.T.; Zhou, J.F.; Mai, K.S. Regulation of FADS2 transcription by SREBP-1 and PPAR-α influences LC-PUFA biosynthesis in fish. Sci. Rep. 2017, 7, 40024. [Google Scholar] [CrossRef]
- Navidshad, B.; Royan, M. Peroxisome Proliferator-Activated Receptor Alpha (PPAR alpha), a key regulator of lipid metabolism in avians. Crit. Rev. Eukaryot. Gene Expr. 2016, 26, 303–308. [Google Scholar] [CrossRef]
- Dong, Y.W.; Zhao, J.H.; Chen, J.L.; Wang, S.Q.; Liu, Y.; Zhang, Q.H.; You, C.H.; Monroig, O.; Tocher, D.R.; Li, Y.Y. Cloning and characterization of ∆6/∆5 fatty acyl desaturase (Fad) gene promoter in the marine teleost Siganus canaliculatus. Gene 2018, 647, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Guo, H.Y.; Zhu, C.Y.; Ma, Z.H.; Jiang, S.G.; Zhang, D.C. Genetic polymorphism of breeding populations of golden pompano (Trachinotus ovatus). South China Fish. Sci. 2014, 10, 67–71. [Google Scholar]
- Zhen, P.L.; Ma, Z.H.; Guo, H.Y.; Jiang, S.G.; Zhang, D.C. Ontogenetic development of caudal skeletons in Trachinotus ovatus larvae. South China Fish. Sci. 2014, 10, 45–50. [Google Scholar]
- Sun, X.X.; Guo, H.Y.; Zhu, K.C.; Zhang, N.; Yu, W.B.; Wu, N.; Jiang, S.G.; Zhang, D.C. Feed type regulates the fatty acid profiles of golden pompano Trachinotus ovatus (Linnaeus 1758). J Appl. Anim. Res. 2018, 46, 60–63. [Google Scholar] [CrossRef]
- Zhu, K.C.; Song, L.; Zhao, C.P.; Guo, H.Y.; Zhang, N.; Guo, L.; Liu, B.S.; Jiang, S.G.; Zhang, D.C. The Transcriptional factor PPARαb positively regulates Elovl5 elongase in golden pompano Trachinotus ovatus (Linnaeus 1758). Front. Physiol. 2018, 9, 1340. [Google Scholar] [CrossRef]
- Shanklin, J.; Whittle, E.; Fox, B.G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 1994, 33, 12787–12794. [Google Scholar] [CrossRef]
- Geay, F.; Tinti, E.; Mellery, J.; Michaux, C.; Larondelle, Y.; Perpète, E.; Kestemont, P. Cloning and functional characterization of Δ6 fatty acid desaturase (FADS2) in Eurasian perch (Perca fluviatilis). Comp. Biochem. Physiol. B 2016, 191, 112–125. [Google Scholar] [CrossRef]
- Zuo, R.T.; Mai, K.S.; Xu, W.; Dong, X.J.; Ai, Q.H. Molecular cloning, tissue distribution and nutritional regulation of a Δ6-fatty acyl desaturase-like enzyme in large yellow croaker (Larimichthys crocea). Aquac. Res. 2016, 47, 445–459. [Google Scholar] [CrossRef]
- Huang, Y.S.; Lin, Z.D.; Rong, H.; Hao, M.L.; Zou, W.G.; Li, S.K.; Wen, X.B. Cloning, tissue distribution, functional characterization and nutritional regulation of Δ6 fatty acyl desaturase in chu’s croaker Nibea coibor. Aquaculture 2017, 479, 208–216. [Google Scholar] [CrossRef]
- Xu, G.X.; Guo, C.C.; Shan, H.Y.; Kong, H.Z. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Kuah, M.K.; Jaya-Ram, A.; Shu-Chien, A.C. The capacity for long-chain polyunsaturated fatty acid synthesis in a carnivorous vertebrate: Functional characterisation and nutritional regulation of a Fads2 fatty acyl desaturase with Delta 4 activity and an Elovl5 elongase in striped snakehead (Channa striata). Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2014, 1851, 248–260. [Google Scholar]
- Morais, S.; Castanheira, F.; Martinez-Rubio, L.; Conceicao, L.E.C.; Tocher, D.R. Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Delta 4 activity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2012, 1821, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Oboh, A.; Kabeya, N.; Carmona-Antonanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two alternative pathways for docosahexaenoic acid (DHA, 22:6n-3) biosynthesis are widespread among teleost fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Mai, K.S.; Xu, W.; Yuan, Y.H.; Zhang, Y.J.; Ai, Q.H. Characterization, mRNA expression and regulation of Delta 6 fatty acyl desaturase (Fads2) by dietary n-3 long chain polyunsaturated fatty acid (LC-PUFA) levels in grouper larvae (Epinephelus coioides). Aquaculture 2014, 434, 212–219. [Google Scholar] [CrossRef]
- Lopes-Marques, M.; Ozorio, R.; Amaral, R.; Tocher, D.R.; Monroig, O.; Castro, L.F.C. Molecular and functional characterization of a fads2 orthologue in the Amazonian teleost, Arapaima gigas. Comp. Biochem. Physiol. B 2017, 203, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tanomman, S.; Ketudat-Cairns, M.; Jangprai, A.; Boonanuntanasarn, S. Characterization of fatty acid delta-6 desaturase gene in Nile tilapia and heterogenous expression in Saccharomyces cerevisiae. Comp. Biochem. Physiol. B 2013, 166, 148–156. [Google Scholar] [CrossRef]
- Bell, J.G.; McEvoy, J.; Tocher, D.R.; McGhee, F.; Campbell, P.J.; Sargent, J.R. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J. Nutr. 2001, 131, 1535–1543. [Google Scholar] [CrossRef]
- O’Malley, B.W.; Towle, H.C.; Schwartz, R.J. Regulation of gene expression in eucaryotes. Annu. Rev. Genet. 1977, 11, 239–275. [Google Scholar] [CrossRef]
- Zhu, K.C.; Chen, L.P.; Zhao, J.K.; Wang, H.J.; Wang, W.M.; Li, Z.; Wang, H.L. Molecular characterization and expression patterns of myogenin in compensatory growth of Megalobrama amblycephala. Comp. Biochem. Physiol. B 2014, 170, 10–17. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Higgins, D.G. ClustalW and ClustalX version 2. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. 2013, 3, 2725–2729. [Google Scholar] [CrossRef]
- Agaba, M.K.; Tocher, D.R.; Zheng, X.Z.; Dickson, C.A.; Dick, J.R.; Teale, A.J. Cloning and functional characterisation of polyunsaturated fatty acid elongases from marine and freshwater teleost fish. Comp. Biochem. Physiol. B 2005, 142, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mai, K.S.; He, G.; Ai, Q.H.; Zhang, W.B.; Xu, W.; Wang, J.; Liufu, Z.G.; Zhang, Y.J.; Zhou, H.H. Characterization of two Δ5 fatty acyl desaturases in abalone (Haliotis discus hannai Ino). Aquaculture 2013, 416, 48–56. [Google Scholar] [CrossRef]
- Hastings, N.; Agaba, M.K.; Tocher, D.R.; Leaver, M.J.; Dick, J.R.; Sargent, J.R.; Teale, A.J. A vertebrate fatty acid desaturase with Δ5 and Δ6 activities. Proc. Natl. Acad. Sci. USA 2001, 98, 14304–14309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.C.; Yu, W.B.; Guo, H.Y.; Zhang, N.; Guo, L.; Liu, B.S.; Jiang, S.G.; Zhang, D.C. Genomic structure, expression pattern and polymorphisms of GILT in golden pompano Trachinotus ovatus (Linnaeus 1758). Gene 2018, 665, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wan, X.L.; Huang, C.X.; Wang, W.M.; Liu, H.; Wang, H.L. Study on the immune response to recombinant Hsp70 protein from Megalobrama amblycephala. Immunobiology 2014, 219, 850–858. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 2. [Google Scholar]
- Wei, S.N.; Yu, W.P.; Qin, Q.W. Establishment of a new fish cell line from the caudal fin of golden pompano Trachinotus ovatus and its susceptibility to iridovirus. J. Fish. Biol. 2018, 92, 1675–1686. [Google Scholar] [CrossRef]
- Ding, Y.; Ao, J.Q.; Huang, X.H.; Chen, X.H. Identification of two subgroups of type I IFNs in perciforme fish large yellow croaker Larimichthys crocea provides novel insights into function and regulation of fish type I IFNs. Front. Immunol. 2016, 7, 343. [Google Scholar] [CrossRef]
- Sun, L.Y.; Zhang, D.C.; Jiang, S.G.; Guo, H.Y.; Zhu, C.Y. Isolation and characterization of 21 polymorphic microstatellites in golden pompano Trachinotus ovatus. Conserv. Genet. Resour. 2013, 5, 1107–1109. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, S.Q.; Chen, J.L.; Zhang, Q.H.; Liu, Y.; You, C.H.; Monroig, O.; Tocher, D.R.; Li, Y.Y. Hepatocyte Nuclear factor 4α (HNF4α) is a transcription factor of vertebrate fatty acyl desaturase gene as identified in marine teleost Siganus canaliculatus. PLoS ONE 2016, 11, e0160361. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Monroig, O.; Wang, T.J.; Yuan, Y.H.; Navarro, J.C.; Hontoria, F.; Liao, K.; Tocher, D.R.; Mai, K.S.; Xu, W. Functional characterization and differential nutritional regulation of putative Elovl5 and Elovl4 elongases in large yellow croaker (Larimichthys crocea). Sci. Rep. 2017, 7, 2303. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Mu, Y.; Ao, J.; Chen, X. Peroxiredoxin IV regulates pro-inflammatory responses in large yellow croaker (Pseudosciaena crocea) and protects against bacterial challenge. J. Proteome Res. 2010, 9, 1424–1436. [Google Scholar] [CrossRef]


| FA Substrate | Product | Conversion (%) | Activity |
|---|---|---|---|
| C18:2n-6 | C18:3n-6 | 0 | Δ6 |
| C18:3n-3 | C18:4n-3 | 0 | Δ6 |
| C20:2n-6 | C20:3n-6 | 0.17 ± 0.02 | Δ8 |
| C20:3n-6 | C20:4n-6 | 0.54 ± 0.06 | Δ5 |
| C22:5n-3 | C22:6n-3 | 39.29 ± 4.4 | Δ4 |
| C22:4n-6 | C22:5n-6 | 1.04 ± 0.11 | Δ4 |
| Putative Binding Sites | Nucleotide Sequence | Mutation |
|---|---|---|
| M1 | 5′ CTGGACTGCATCAAGAGTCA 3′ | deletion |
| M2 | 5′ ATGCTAACTTTGACCTGTGGACTGTGC 3′ | deletion |
| M3 | 5′ AGCAGCTGCAGTTTTGTGTG 3′ | deletion |
| M4 | 5′ GGGGGGAACAGAGGAGAGG 3′ | deletion |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.-C.; Song, L.; Guo, H.-Y.; Guo, L.; Zhang, N.; Liu, B.-S.; Jiang, S.-G.; Zhang, D.-C. Identification of Fatty Acid Desaturase 6 in Golden Pompano Trachinotus Ovatus (Linnaeus 1758) and Its Regulation by the PPARαb Transcription Factor. Int. J. Mol. Sci. 2019, 20, 23. https://doi.org/10.3390/ijms20010023
Zhu K-C, Song L, Guo H-Y, Guo L, Zhang N, Liu B-S, Jiang S-G, Zhang D-C. Identification of Fatty Acid Desaturase 6 in Golden Pompano Trachinotus Ovatus (Linnaeus 1758) and Its Regulation by the PPARαb Transcription Factor. International Journal of Molecular Sciences. 2019; 20(1):23. https://doi.org/10.3390/ijms20010023
Chicago/Turabian StyleZhu, Ke-Cheng, Ling Song, Hua-Yang Guo, Liang Guo, Nan Zhang, Bao-Suo Liu, Shi-Gui Jiang, and Dian-Chang Zhang. 2019. "Identification of Fatty Acid Desaturase 6 in Golden Pompano Trachinotus Ovatus (Linnaeus 1758) and Its Regulation by the PPARαb Transcription Factor" International Journal of Molecular Sciences 20, no. 1: 23. https://doi.org/10.3390/ijms20010023
APA StyleZhu, K.-C., Song, L., Guo, H.-Y., Guo, L., Zhang, N., Liu, B.-S., Jiang, S.-G., & Zhang, D.-C. (2019). Identification of Fatty Acid Desaturase 6 in Golden Pompano Trachinotus Ovatus (Linnaeus 1758) and Its Regulation by the PPARαb Transcription Factor. International Journal of Molecular Sciences, 20(1), 23. https://doi.org/10.3390/ijms20010023







