Biomolecular Clusters Distribution up to Mega Dalton Region Using MALDI-Quadrupole Ion Trap Mass Spectrometer
Abstract
1. Introduction
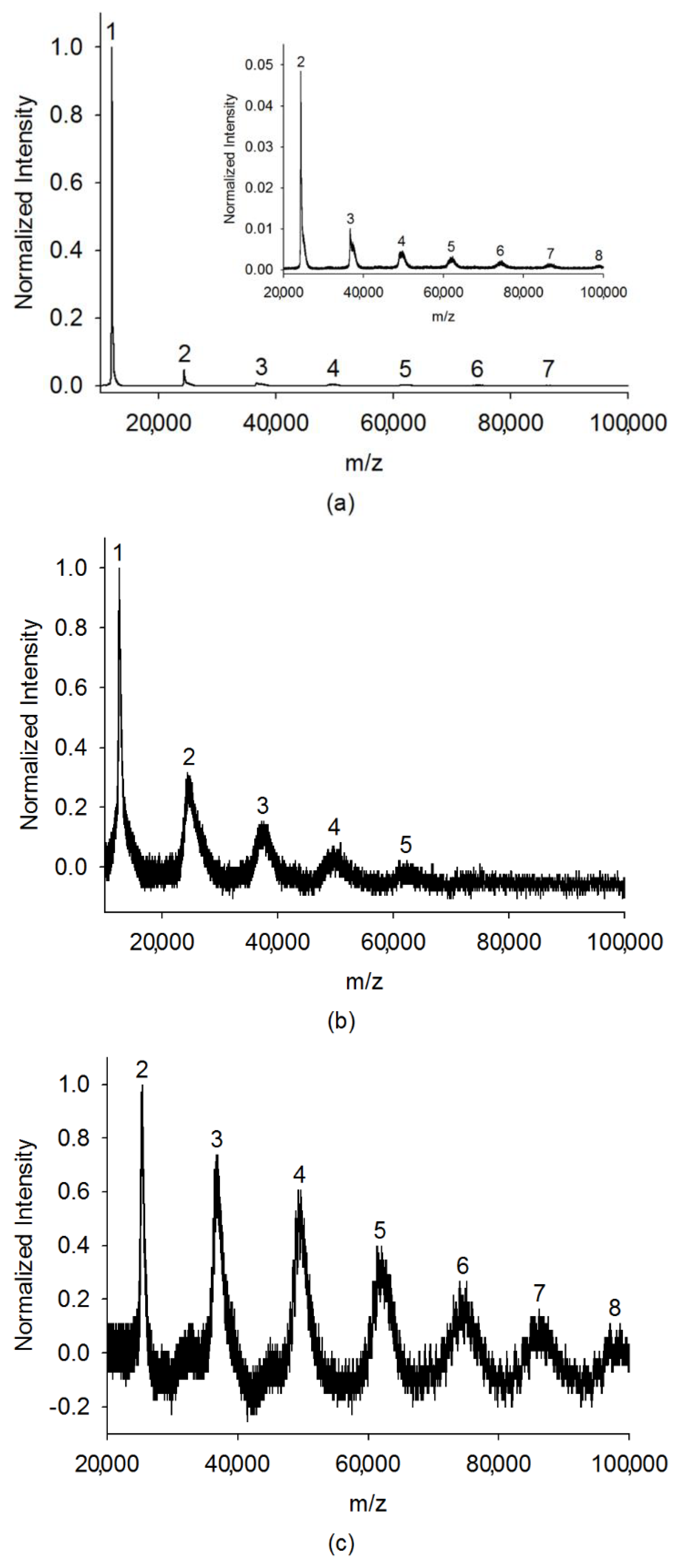
2. Results and Discussion
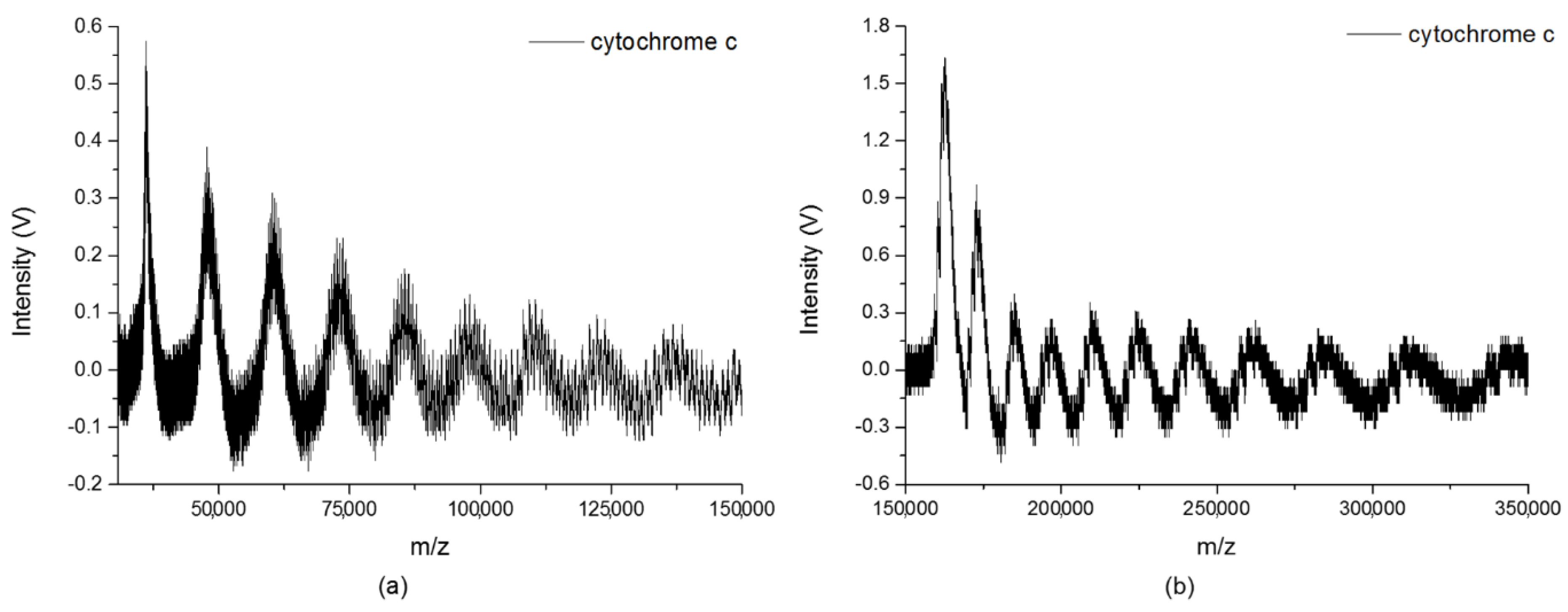
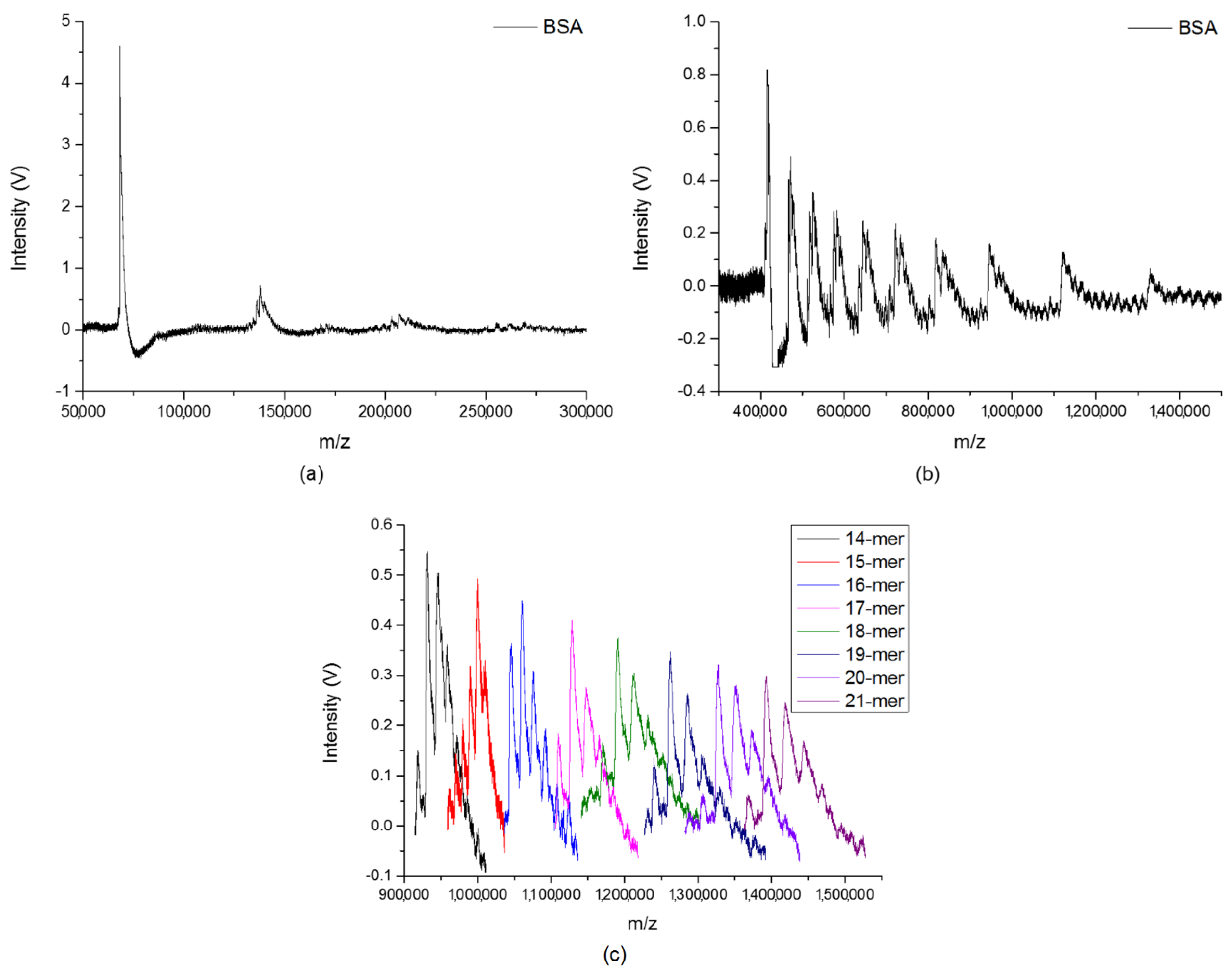
2.1. Large Clusters of the Analytes
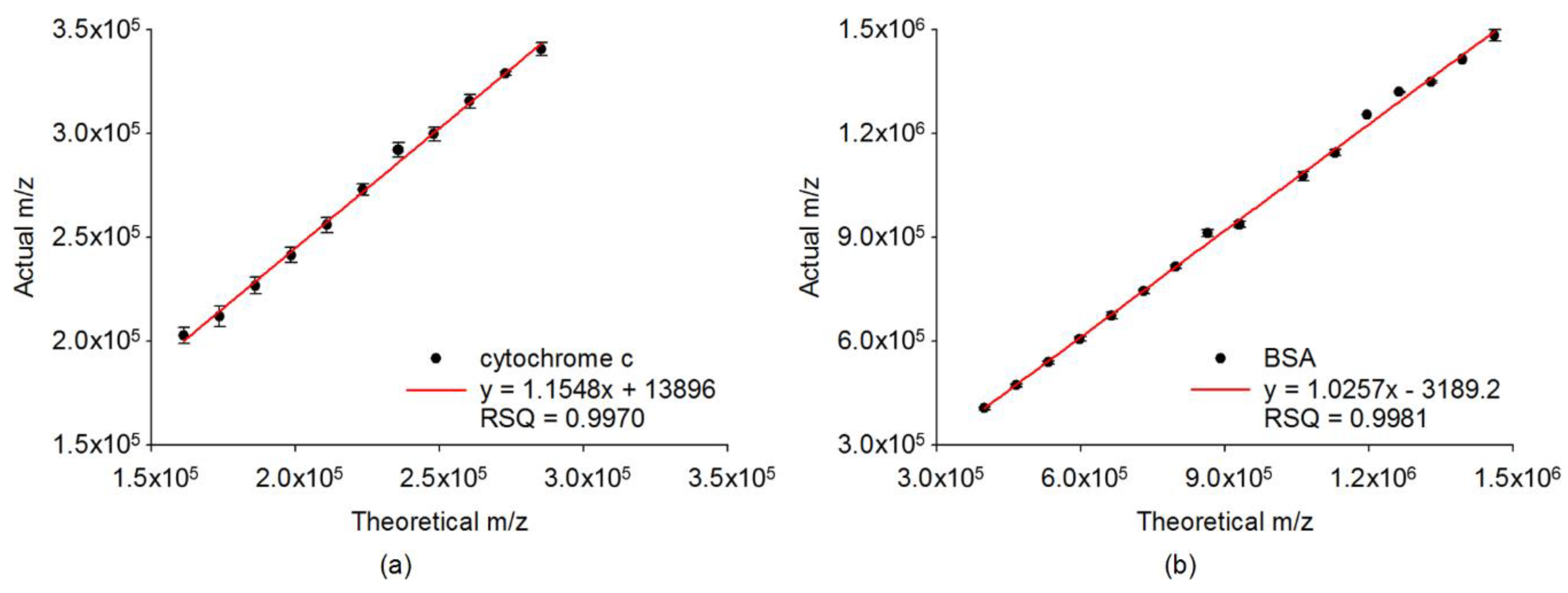
2.2. Quantitative Analysis on Cluster Distributions of the Analytes
3. Materials and Methods
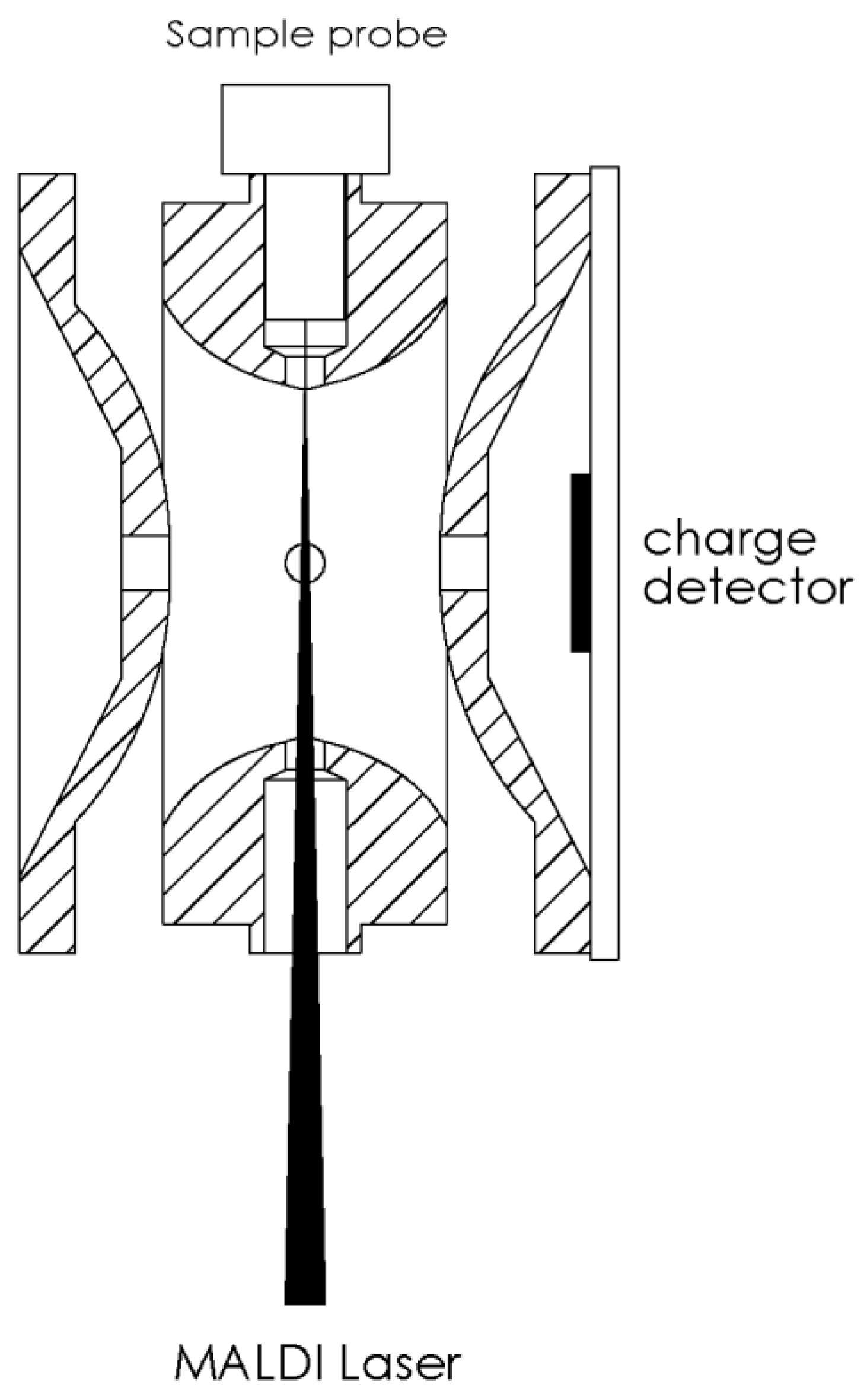
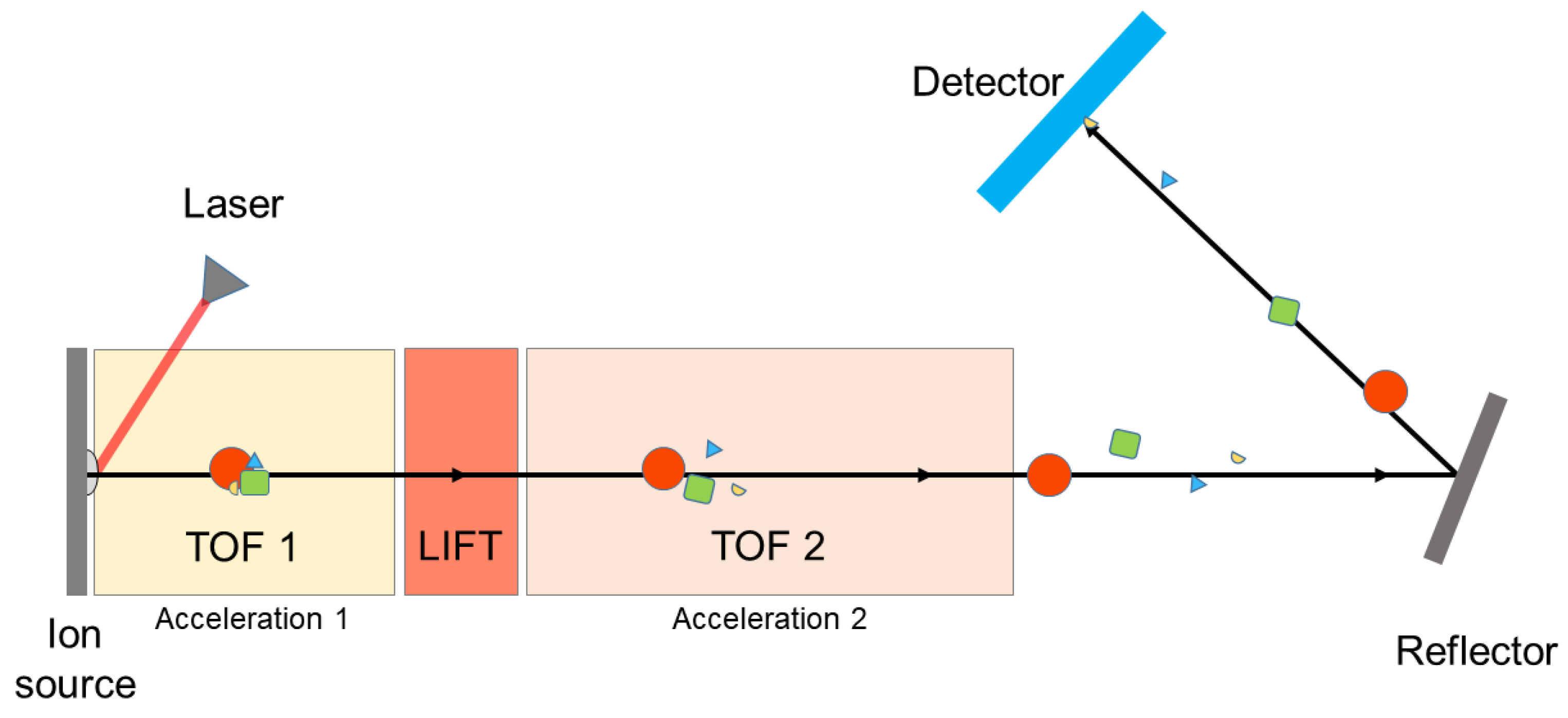
3.1. Instrumentation
3.2. Sample Preparation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Karas, M.; Bachmann, D.; Bahr, U.; Hillenkamp, F. Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int. J. Mass Spectrom. Ion Process. 1987, 78, 53–68. [Google Scholar] [CrossRef]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and polymer analyses up to m/z 100,000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Chen, C.-H.W. Review of a current role of mass spectrometry for proteome research. Anal. Chim. Acta 2008, 624, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Lu, I.-C.; Lin, J.-L.; Lai, S.-H.; Chen, C.-H. Frequency-scanning MALDI linear ion trap mass spectrometer for large biomolecular ion detection. Anal. Chem. 2011, 83, 8273–8277. [Google Scholar] [CrossRef] [PubMed]
- Taranenko, N.I.; Potter, N.T.; Allman, S.L.; Golovlev, V.V.; Chen, C.H. Detection of trinucleotide expansion in neurodegenerative disease by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Genet. Anal. Biomol. Eng. 1999, 15, 25–31. [Google Scholar] [CrossRef]
- Trimpin, S.; Rouhanipour, A.; Az, R.; Räder, H.J.; Müllen, K. New aspects in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: A universal solvent-free sample preparation. Rapid Commun. Mass Spectrom. 2001, 15, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Petricoin, E.F., III; Ardekani, A.M.; Hitt, B.A.; Levine, P.J.; Fusaro, V.A.; Steinberg, S.M.; Mills, G.B.; Simone, C.; Fishman, D.A.; Kohn, E.C.; et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet 2002, 359, 572–577. [Google Scholar] [CrossRef]
- Ehring, H.; Karas, M.; Hillenkamp, F. Role of photoionization and photochemistry in ionization processes of organic molecules and relevance for matrix-assisted laser desorption lonization mass spectrometry. Org. Mass Spectrom. 1992, 27, 472–480. [Google Scholar] [CrossRef]
- Dreisewerd, K.; Schürenberg, M.; Karas, M.; Hillenkamp, F. Matrix-assisted laser desorption/ionization with nitrogen lasers of different pulse widths. Int. J. Mass Spectrom. Ion Processes 1996, 154, 171–178. [Google Scholar] [CrossRef]
- Krause, J.; Stoeckli, M.; Schlunegger, U.P. Studies on the selection of new matrices for ultraviolet matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 1927–1933. [Google Scholar] [CrossRef]
- Lehmann, E.; Knochenmuss, R.; Zenobi, R. Ionization mechanisms in matrix-assisted laser desorption/ionization mass spectrometry: Contribution of pre-formed ions. Rapid Commun. Mass Spectrom. 1997, 11, 1483–1492. [Google Scholar] [CrossRef]
- Harrison, A.G. The gas-phase basicities and proton affinities of amino acids and peptides. Mass Spectrom. Rev. 1997, 16, 201–217. [Google Scholar] [CrossRef]
- McCarley, T.D.; McCarley, R.L.; Limbach, P.A. Electron-transfer ionization in matrix-assisted laser desorption/ionization mass spectrometry. Anal. Chem. 1998, 70, 4376–4379. [Google Scholar] [CrossRef]
- Zenobi, R.; Knochenmuss, R. Ion formation in MALDI mass spectrometry. Mass Spectrom. Rev. 1998, 17, 337–366. [Google Scholar] [CrossRef]
- Karbach, V.; Knochenmuss, R. Do single matrix molecules generate primary ions in ultraviolet matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 1998, 12, 968–974. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, Y.; Zhang, J.-Y. Ionization mechanism of oligonucleotides in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 57–64. [Google Scholar] [CrossRef]
- Knitchinsky, A.N.; Dolguine, A.I.; Khodorkovski, M.A. Matrix-assisted laser desorption ionization of neutral clusters composed of matrix and analyte molecules. Anal. Chem. 1995, 67, 1963–1967. [Google Scholar] [CrossRef]
- Hankin, S.M.; John, P. Microscopic cluster formation during the laser desorption of chrysene-d12. J. Phys. Chem. B 1999, 103, 4566–4569. [Google Scholar] [CrossRef]
- Karas, M.; Glückmann, M.; Schäfer, J. Ionization in matrix-assisted laser desorption/ionization: Singly charged molecular ions are the lucky survivors. J. Mass Spectrom. 2000, 35, 1–12. [Google Scholar] [CrossRef]
- Livadaris, V.; Blais, J.-C.; Tabet, J.-C. Formation of non-specific protein cluster ions in matrix-assisted laser desorption/ionization: Abundances and dynamical aspects. Eur. J. Mass Spectrom. 2000, 6, 409–413. [Google Scholar] [CrossRef]
- Krüger, R.; Pfenninger, A.; Fournier, I.; Glückmann, M.; Karas, M. Analyte incorporation and ionization in matrix-assisted laser desorption/ionization visualized by pH indicator molecular probes. Anal. Chem. 2001, 73, 5812–5821. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Krüger, R. Ion formation in MALDI: The cluster ionization mechanism. Chem. Rev. 2003, 103, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Bahr, U.; Fournier, I.; Glückmann, M.; Pfenninger, A. The initial-ion velocity as a marker for different desorption-ionization mechanisms in MALDI. Int. J. Mass Spectrom. 2003, 226, 239–248. [Google Scholar] [CrossRef]
- Karas, M.; Bahr, U.; Hillenkamp, F. UV laser matrix desorption/ionization mass spectrometry of proteins in the 100000 Dalton range. Int. J. Mass Spectrom. Ion Process. 1989, 92, 231–242. [Google Scholar] [CrossRef]
- Dreisewerd, K.; Schürenberg, M.; Karas, M.; Hillenkamp, F. Influence of the laser intensity and spot size on the desorption of molecules and ions in matrix-assisted laser desorption/ionization with a uniform beam profile. Int. J. Mass Spectrom. Ion Process. 1995, 141, 127–148. [Google Scholar] [CrossRef]
- Ehring, H.; Costa, C.; Demirev, P.A.; Sundqvist, B.U.R. Photochemical versus thermal mechanisms in matrix-assisted laser desorption/ionization probed by back side desorption. Rapid Commun. Mass Spectrom. 1996, 10, 821–824. [Google Scholar] [CrossRef]
- Allwood, D.A.; Dyer, P.E.; Dreyfus, R.W. Ionization modelling of matrix molecules in ultraviolet matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 1997, 11, 499–503. [Google Scholar] [CrossRef]
- Krutchinsky, A.N.; Chait, B.T. On the nature of the chemical noise in MALDI mass spectra. J. Am. Soc. Mass Spectrom. 2002, 13, 129–134. [Google Scholar] [CrossRef]
- Fournier, I.; Brunot, A.; Tabet, J.C.; Bolbach, G. Delayed extraction experiments using a repulsive potential before ion extraction: Evidence of clusters as ion precursors in UV-MALDI. Part I: Dynamical effects with the matrix 2,5-dihydroxybenzoic acid. Int. J. Mass Spectrom. 2002, 213, 203–215. [Google Scholar] [CrossRef]
- Dreisewerd, K. The desorption process in MALDI. Chem. Rev. 2003, 103, 395–426. [Google Scholar] [CrossRef] [PubMed]
- Knochenmuss, R.; Zenobi, R. MALDI ionization: The role of in-plume processes. Chem. Rev. 2003, 103, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, G.J. An overview of some recent developments in ionization methods for mass spectrometry. Eur. J. Mass Spectrom. 2003, 9, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Setz, P.D.; Knochenmuss, R. Exciton mobility and trapping in a MALDI matrix. J. Phys. Chem. A 2005, 109, 4030–4037. [Google Scholar] [CrossRef] [PubMed]
- Fournier, I.; Brunot, A.; Tabet, J.C.; Bolbach, G. Delayed extraction experiments using a repulsing potential before ion extraction: Evidence of non-covalent clusters as ion precursor in UV matrix-assisted laser desorption/ionization. Part II-dynamic effects with α-cyano-4-hydroxycinnamic acid matrix. J. Mass Spectrom. 2005, 40, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Knochenmuss, R. Ion formation mechanisms in UV-MALDI. Analyst 2006, 131, 966–986. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Fournier, F.; Afonso, C.; Wind, F.; Tabet, J.-C. Gas-phase ionization/desolvation processes and their effect on protein charge state distribution under matrix-assisted laser desorption/ionization conditions. Eur. J. Mass Spectrom. 2006, 12, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Huang, L.-C.L.; Wang, Y.-S.; Peng, W.-P.; Chang, H.-C.; Hsu, N.-Y.; Yang, W.-B.; Chen, C.-H. Matrix-assisted laser desorption/ionization (MALDI) mechanism revisited. Anal. Chim. Acta 2007, 582, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jaskolla, T.W.; Karas, M. Compelling evidence for lucky survivor and gas phase protonation: The unified MALDI analyte protonation mechanism. J. Am. Soc. Mass Spectrom. 2011, 22, 976–988. [Google Scholar] [CrossRef] [PubMed]
- Trimpin, S.; Wang, B.; Inutan, E.D.; Li, J.; Lietz, C.B.; Harron, A.; Pagnotti, V.S.; Sardelis, D.; McEwen, C.N. A mechanism for ionization of nonvolatile compounds in mass spectrometry: Considerations from MALDI and inlet ionization. J. Am. Soc. Mass Spectrom. 2012, 23, 1644–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhigilei, L.V.; Kodali, P.B.S.; Garrison, B.J. Molecular dynamics model for laser ablation and desorption of organic solids. J. Phys. Chem. B 1997, 101, 2028–2037. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Kodali, P.B.S.; Garrison, B.J. A microscopic view of laser ablation. J. Phys. Chem. B 1998, 102, 2845–2853. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Garrison, B.J. Microscopic mechanisms of laser ablation of organic solids in the thermal and stress confinement irradiation regimes. J. Appl. Phys. 2000, 88, 1281–1298. [Google Scholar] [CrossRef]
- Itina, T.E.; Zhigilei, L.V.; Garrison, B.J. Microscopic mechanisms of matrix assisted laser desorption of analyte molecules: Insights from molecular dynamics simulation. J. Phys. Chem. B 2002, 106, 303–310. [Google Scholar] [CrossRef]
- Zhigilei, L.V. Dynamics of the plume formation and parameters of the ejected clusters in short-pulse laser ablation. Appl. Phys. A-Mater. Sci. Process. 2003, 76, 339–350. [Google Scholar] [CrossRef]
- Zhigilei, L.V.; Leveugle, E.; Garrison, B.J.; Yingling, Y.G.; Zeifman, M.I. Computer simulations of laser ablation of molecular substrates. Chem. Rev. 2003, 103, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Zhigilei, L.V.; Yingling, Y.G.; Itina, T.E.; Schoolcraft, T.A.; Garrison, B.J. Molecular dynamics simulations of matrix-assisted laser desorption-connections to experiment. Int. J. Mass Spectrom. 2003, 226, 85–106. [Google Scholar] [CrossRef]
- Knochenmuss, R.; Zhigilei, L.V. Molecular dynamics model of ultraviolet matrix-assisted laser desorption/ionization including ionization processes. J. Phys. Chem. B 2005, 109, 22947–22957. [Google Scholar] [CrossRef] [PubMed]
- Leveugle, E.; Zhigilei, L.V. Molecular dynamics simulation study of the ejection and transport of polymer molecules in matrix-assisted pulsed laser evaporation. J. Appl. Phys. 2007, 102, 074914-1–074914-19. [Google Scholar] [CrossRef]
- Knochenmuss, R.; Zhigilei, L.V. Molecular dynamics simulations of MALDI: Laser fluence and pulse width dependence of plume characteristics and consequences for matrix and analyte ionization. J. Mass Spectrom. 2010, 45, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Xu, X. Explosive phase transformation in excimer laser ablation. Appl. Surf. Sci. 1998, 127–129, 111–116. [Google Scholar] [CrossRef]
- Kelly, R.; Miotello, A. Does normal boiling exist due to laser-pulse or ion bombardment? J. Appl. Phys. 2000, 87, 3177–3179. [Google Scholar] [CrossRef]
- Yoo, J.H.; Jeong, S.H.; Mao, X.L.; Greif, R.; Russo, R.E. Evidence for phase-explosion and generation of large particles during high power nanosecond laser ablation of silicon. Appl. Phys. Lett. 2000, 76, 783–785. [Google Scholar] [CrossRef]
- Yoo, J.H.; Jeong, S.H.; Greif, R.; Russo, R.E. Explosive change in crater properties during high power nanosecond laser ablation of silicon. J. Appl. Phys. 2000, 88, 1638–1649. [Google Scholar] [CrossRef]
- Bulgakova, N.M.; Bulgakov, A.V. Pulsed laser ablation of solids: Transition from normal vaporization to phase explosion. Appl. Phys. A-Mater. Sci. Process. 2001, 73, 199–208. [Google Scholar] [CrossRef]
- Leveugle, E.; Zhigilei, L.V.; Sellinger, A.; Fitz-Gerald, J.M. Computational and experimental study of the cluster size distribution in MAPLE. Appl. Surf. Sci. 2007, 253, 6456–6460. [Google Scholar] [CrossRef]
- Lai, S.-H.; Chang, K.-H.; Lin, J.-L.; Wu, C.-L.; Chen, C.-H. Sinapinic acid clusters distribution from monomer to mega Dalton’s region in MALDI process. Chem. Phys. Lett. 2013, 561–562, 142–146. [Google Scholar] [CrossRef]
- Westmacott, G.; Ens, W.; Standing, K.G. Secondary ion and electron yield measurements for surfaces bombarded with large molecular ions. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 1996, 108, 282–289. [Google Scholar] [CrossRef]
- Twerenbold, D. Cryogenic particle detectors. Rep. Prog. Phys. 1996, 59, 349–426. [Google Scholar] [CrossRef]
- Benner, W.H. A gated electrostatic ion trap to repetitiously measure the charge and m/z of large electrospray ions. Anal. Chem. 1997, 69, 4162–4168. [Google Scholar] [CrossRef]
- Wenzel, R.J.; Matter, U.; Schultheis, L.; Zenobi, R. Analysis of megadalton ions using cryodetection MALDI time-of-flight mass spectrometry. Anal. Chem. 2005, 77, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Bahr, U.; Röhling, U.; Lautz, C.; Strupat, K.; Schürenberg, M.; Hillenkamp, F. A charge detector for time-of-flight mass analysis of high mass ions produced by matrix-assisted laser desorption/ionization (MALDI). Int. J. Mass Spectrom. Ion Process. 1996, 153, 9–21. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, J.-L.; Chu, M.-L.; Chen, C.-H. MALDI ion trap mass spectrometer with charge detector for large biomolecule detection. Anal. Chem. 2010, 82, 10125–10128. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Kalberer, M.; Zenobi, R. Direct detection of particles formed by laser ablation of matrices during matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 2003, 17, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.N.; Mishra, S.; Murray, K.K. Characterization of coarse particles formed by laser ablation of MALDI matrixes. J. Phys. Chem. B 2003, 107, 13106–13110. [Google Scholar] [CrossRef]
- Chou, C.-W.; Nelson, R.W.; Williams, P. Dependence of the ejection velocities of laser-ablated ions on the laser wavelength and fluence. Eur. J. Mass Spectrom. 2009, 15, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kinsel, G.R.; Edmondson, R.D.; Russell, D.H. Profile and flight time analysis of bovine insulin clusters as a probe of matrix-assisted laser desorption/ionization ion formation dynamics. J. Mass Spectrom. 1997, 32, 714–722. [Google Scholar] [CrossRef]
- Pfann, W.G. Zone melting this technique offers unique advantages in purification and in control of composition in various substances. Science 1962, 135, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Schlunegger, U.P.; Stoeckli, M.; Caprioli, R.M. Frequency scan for the analysis of high mass ions generated by matrix-assisted laser desorption/ionization in a Paul trap. Rapid Commun. Mass Spectrom. 1999, 13, 1792–1796. [Google Scholar] [CrossRef]
- Stafford, G.C., Jr.; Kelley, P.E.; Syka, J.E.P.; Reynolds, W.E.; Todd, J.F.J. Recent improvements in and analytical applications of advanced ion trap technology. Int. J. Mass Spectrom. Ion Processes 1984, 60, 85–98. [Google Scholar] [CrossRef]
- March, R.E. An introduction to quadrupole ion trap mass spectrometry. J. Mass Spectrom. 1997, 32, 351–369. [Google Scholar] [CrossRef]
- Nie, Z.; Cui, F.; Chu, M.; Chen, C.-H.; Chang, H.-C.; Cai, Y. Calibration of a frequency-scan quadrupole ion trap mass spectrometer for microparticle mass analysis. Int. J. Mass Spectrom. 2008, 270, 8–15. [Google Scholar] [CrossRef]
- Sudakov, M. Effective potential and the ion axial beat motion near the boundary of the first stable region in a nonlinear ion trap. Int. J. Mass Spectrom. 2001, 206, 27–43. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, Y.-K.; Lai, S.-H.; Lin, J.-L.; Chen, C.-H. Biomolecular Clusters Distribution up to Mega Dalton Region Using MALDI-Quadrupole Ion Trap Mass Spectrometer. Int. J. Mol. Sci. 2018, 19, 2789. https://doi.org/10.3390/ijms19092789
Chuang Y-K, Lai S-H, Lin J-L, Chen C-H. Biomolecular Clusters Distribution up to Mega Dalton Region Using MALDI-Quadrupole Ion Trap Mass Spectrometer. International Journal of Molecular Sciences. 2018; 19(9):2789. https://doi.org/10.3390/ijms19092789
Chicago/Turabian StyleChuang, Yung-Kun, Szu-Hsueh Lai, Jung-Lee Lin, and Chung-Hsuan Chen. 2018. "Biomolecular Clusters Distribution up to Mega Dalton Region Using MALDI-Quadrupole Ion Trap Mass Spectrometer" International Journal of Molecular Sciences 19, no. 9: 2789. https://doi.org/10.3390/ijms19092789
APA StyleChuang, Y.-K., Lai, S.-H., Lin, J.-L., & Chen, C.-H. (2018). Biomolecular Clusters Distribution up to Mega Dalton Region Using MALDI-Quadrupole Ion Trap Mass Spectrometer. International Journal of Molecular Sciences, 19(9), 2789. https://doi.org/10.3390/ijms19092789





