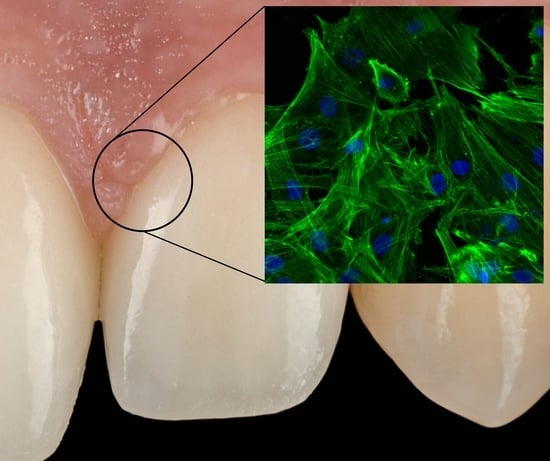
Comparison of Cytomorphometry and Early Cell Response of Human Gingival Fibroblast (HGFs) between Zirconium and New Zirconia-Reinforced Lithium Silicate Ceramics (ZLS)
Abstract
1. Introduction
2. Results
2.1. Surface Characterization
2.1.1. Surface Composition Analysis
2.1.2. Profilometry
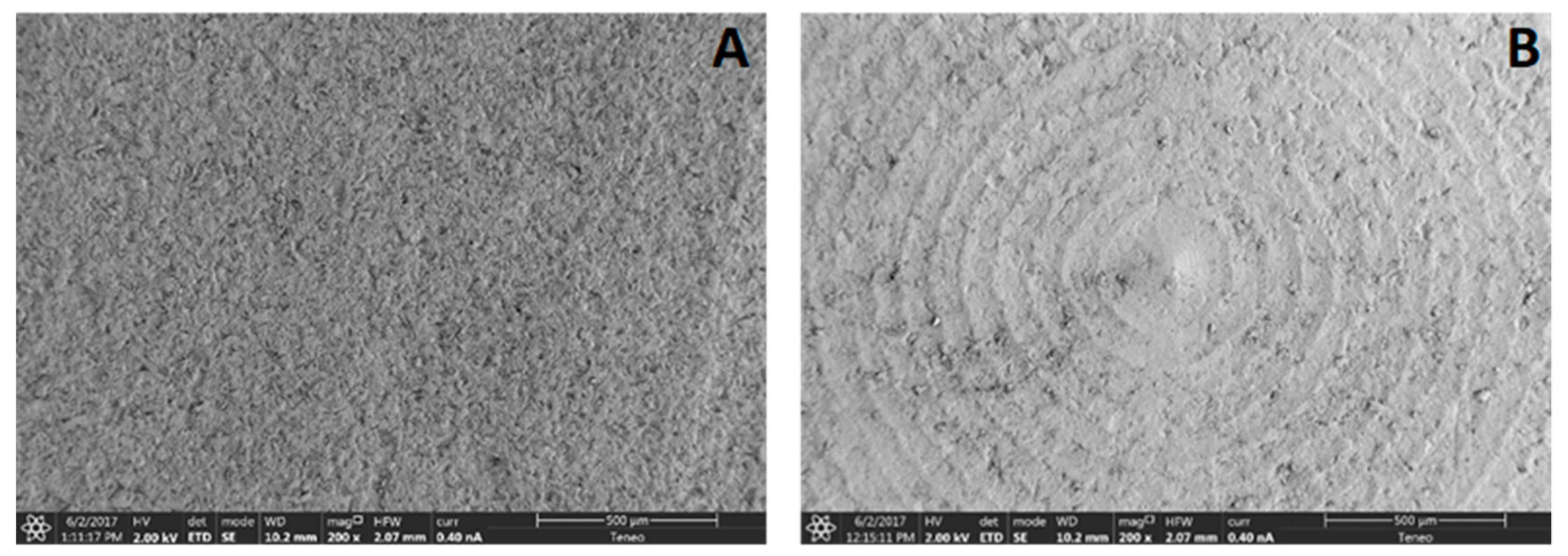
2.1.3. Surface Topography
2.2. Cellular Study
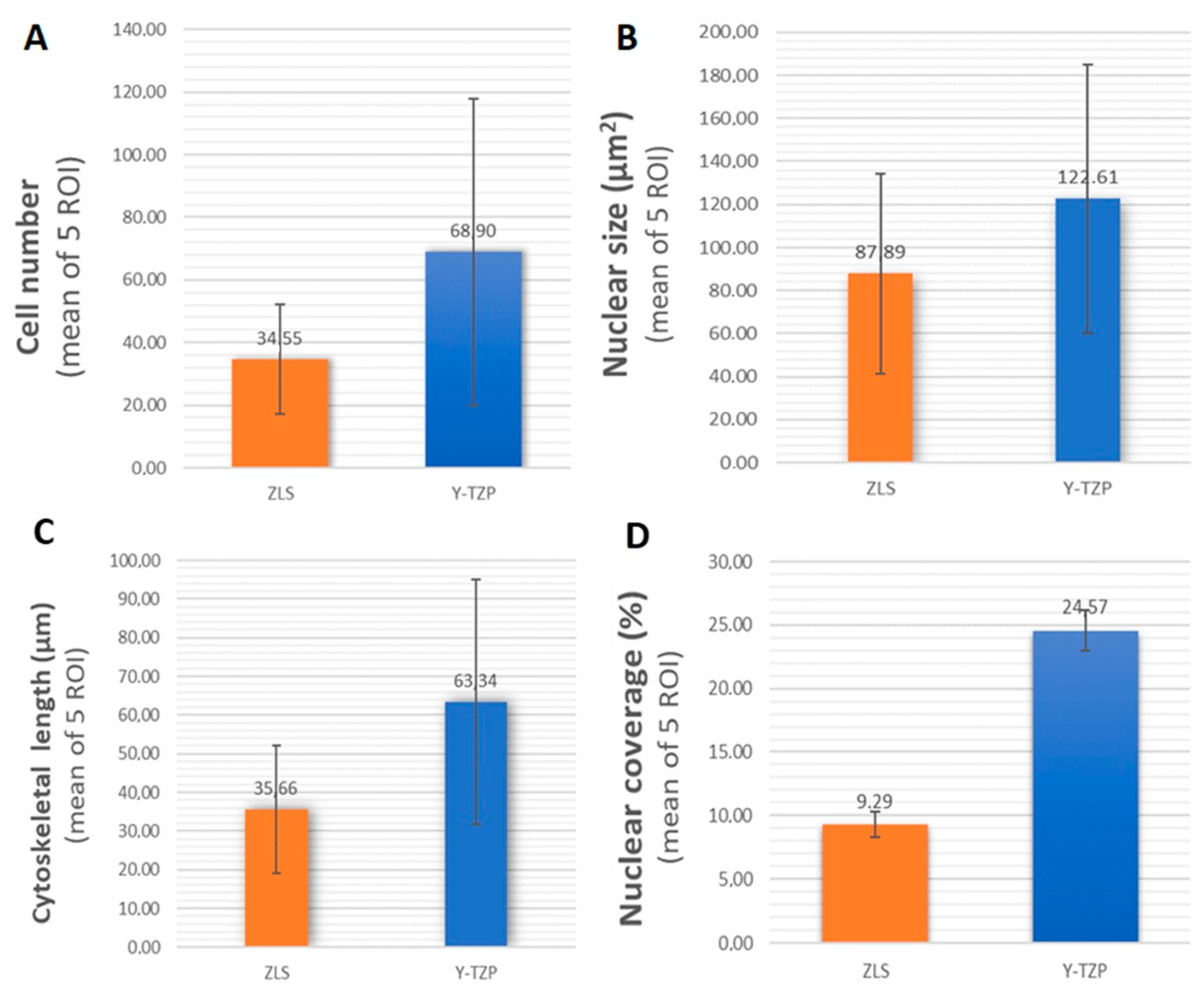
2.2.1. Cell Proliferation
2.2.2. Cytomorphometry
2.2.3. Nuclear Coverage
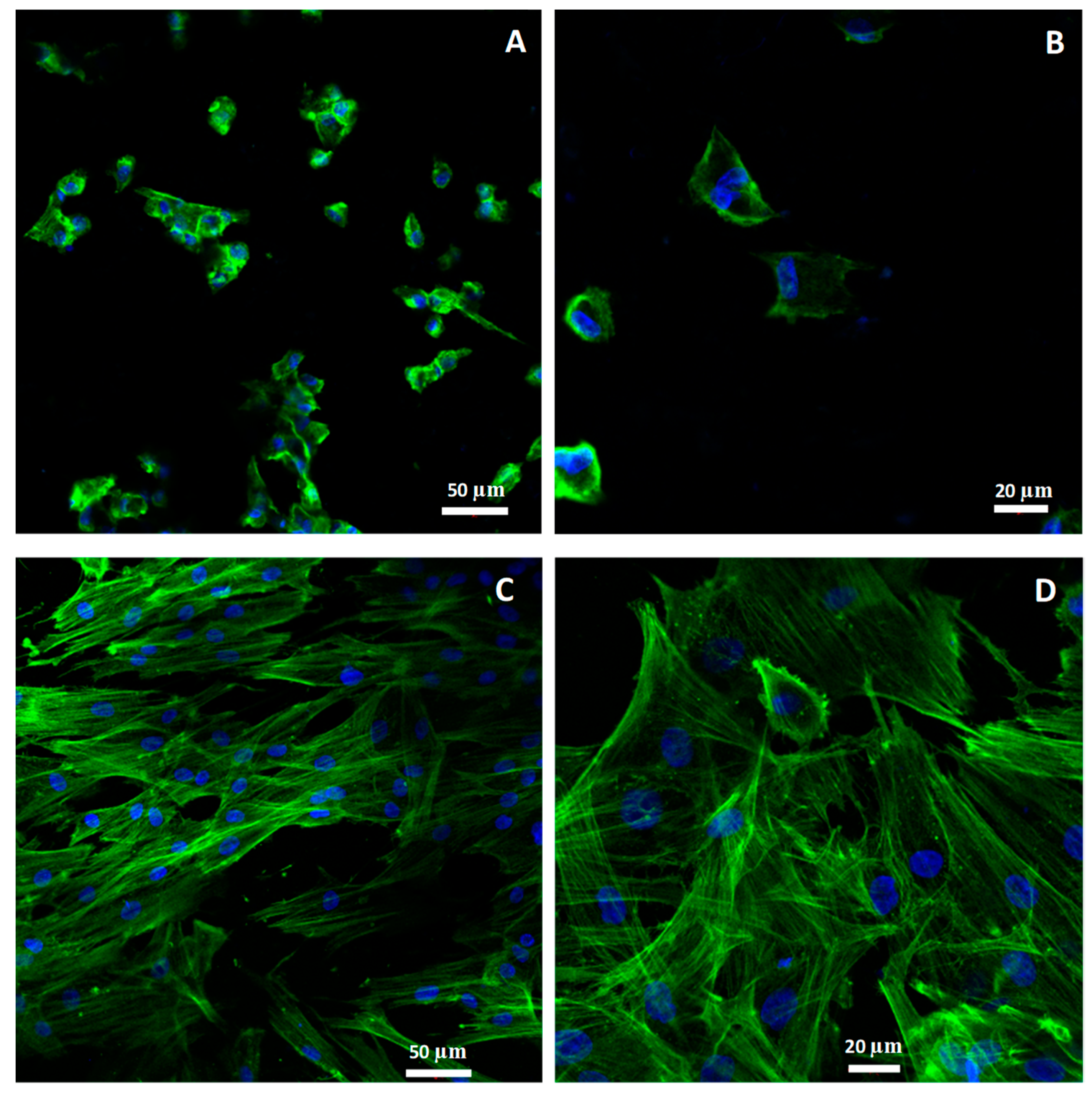
2.2.4. Morphology and Cellular Attachment
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Surface Characterization
4.2.1. Surface Composition Analysis
4.2.2. Profilometry
4.2.3. Scanning Electron Microscopy
4.3. Cell Culture
4.3.1. Immunocitochemical Staining
4.3.2. Confocal Microscopy
4.3.3. Image Processing: Image J
4.3.4. Cellular Parameters Analyzed
4.4. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD/CAM | Computer Aided Design/Computer Aided Manufacturing |
| Y-TZP | Yttria-stabilized Tetragonal Zirconia Polycrystal |
| HGFs | Human Gingival Fibroblasts |
| ZLS | Zirconia Lithium Silicate |
| EDS | Energy Dispersive Spectroscopy |
| SEM | Scanning Electron Microscopy |
References
- Pabst, A.M.; Walter, C.; Bell, A.; Weyhrauch, M.; Schmidtmann, I.; Scheller, H.; Lehmann, K.M. Influence of CAD/CAM zirconia for implant-abutment manufacturing on gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2016, 20, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Dal Piva, A.; Contreras, L.; Ribeiro, F.C.; Anami, L.C.; Camargo, S.; Jorge, A.; Bottino, M.A. Monolithic Ceramics: Effect of Finishing Techniques on Surface Properties, Bacterial Adhesion and Cell Viability. Oper. Dent. 2018, 43, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Pae, A.; Lee, H.; Kim, H.S.; Kwon, Y.D.; Woo, Y.H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4, 025005. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential Behavior of Fibroblasts and Epithelial Cells on Structured Implant Abutment Materials: A Comparison of Materials and Surface Topographies. Clin. Implant Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.D.; Shin, J.C.; Yoon, H.I.; Ku, Y.; Ryoo, H.M.; Kim, D.J.; Kim, D.G.; Han, J.S. Characterization of human gingival fibroblasts on zirconia surfaces containing niobium oxide. Materials 2015, 8, 6018–6028. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, L.; Rossi Iommetti, P.; Palomas, E.; Toesca, A.; Serini, S.; Resci, F.; Missori, M.; De Spirito, M.; Manicone, P.F.; Calviello, G. Crecimiento, viabilidad, adhesiónpotencial y la expresión de fibronectina en fibroblastos cultivados con zirconia o cerámica feldespática in vitro. J. Biomed. Mater. Res. A 2008, 86, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, V.; Borelli, B.; De Colli, M.; Tumedei, M.; Di Iorio, D.; Zara, S.; Sorrentino, R.; Cataldi, A.; Gherlone, E.F.; Zarone, F.; et al. Evaluación SEM de la gingival humana crecimiento de fibroblastos en CAD/CAM zirconia y recubrimiento de cerámica para zirconia. Ann. Stomatol. 2014, 4, 244–249. [Google Scholar]
- Yamano, S.; Ma, A.K.; Shanti, R.M.; Kim, S.W.; Wada, K.; Sukotjo, C. The influence of different implant materials on human gingival fibroblast morphology, proliferation, and gene expression. Int. J. Oral Maxillofac. Implants 2011, 26, 1247–1255. [Google Scholar] [PubMed]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, O.S.A.; Sherief, A.H. Zirconia based ceramics, some clinical and biological aspects: Review. Futur. Dent. J. 2016, 2, 55–64. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P.; Kern, M. Effect of microstructure on the mechanical properties of lithium disilicate glass-ceramics. J. Mech. Behav. Biomed. Mater. 2018, 82, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Gracis, S.; Thompson, V.P.; Ferencz, J.L.; Silva, N.R.; Bonfante, E.A. A new classification system for all-ceramic and ceramic-like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Kimmich, M.; Stappert, C.F.J. Intraoral treatment of veneering porcelain chipping of fixed dental restorations: A review and clinical application. J. Am. Dent. Assoc. 2013, 144, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Vaitelis, J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: A systematic review and meta-analysis. Clin. Oral Implants Res. 2015, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.D.; Lima, E.; Miranda, R.B.P.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental ceramics: A review of new materials and processing methods. Braz. Oral Res. 2017, 31, e58. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Sinjari, B.; Pascetta, R.; Serafini, N.; Perfetti, G.; Trisi, P.; Caputi, S. The zirconia-reinforced lithium silicate ceramic: Lights and shadows of a new material. Dent. Mater. J. 2016, 35, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E.; Elnaghy, A.M. Mechanical properties of zirconia reinforced lithium silicate glass-ceramic. Dent. Mater. 2016, 32, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Sieper, K.; Wille, S.; Kern, M. Fracture strength of lithium disilicate crowns compared to polymer-infiltrated ceramic-network and zirconia reinforced lithium silicate crowns. J. Mech. Behav. Biomed. Mater. 2017, 74, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Vita Suprinity®. Technical and Scientific Documentation; Vita Zahnfabrik: BadSäckingen, Germany, 2014. [Google Scholar]
- Celtra® Duo Zirconia-Reinforced Lithium Silicate (ZLS) Block. Technical Monograph; Dentsply Sirona: Hanau-Wolfgang, Germany, 2016. [Google Scholar]
- Riquieri, H.; Monteiro, J.B.; Viegas, D.C.; Campos, T.M.B.; de Melo, R.M.; de Siqueira Ferreira Anzaloni Saavedra, G. Impact of crystallization firing process on the microstructure and flexural strength of zirconia-reinforced lithium silicate glass-ceramics. Dent. Mater. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Ramos Nde, C.; Campos, T.M.; Paz, I.S.; Machado, J.P.; Bottino, M.A.; Cesar, P.F.; Melo, R.M. Microstructure characterization and SCG of newly engineered dental ceramics. Dent. Mater. 2016, 32, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Alao, A.R.; Stoll, R.; Song, X.F.; Miyazaki, T.; Hotta, Y.; Shibata, Y.; Yin, L. Surface quality of yttria-stabilized tetragonal zirconia polycrystal in CAD/CAM milling, sintering, polishing and sandblasting processes. J. Mech. Behav. Biomed. Mater. 2017, 65, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, M.; Hahnel, S.; Preis, V.; Rosentritt, M. Polishing effects and wear performance of chairside CAD/CAM materials. Clin. Oral Investig. 2018. [Google Scholar] [CrossRef] [PubMed]
- Atay, A.; Gürdal, I.; Bozok Çetıntas, V.; Üşümez, A.; Cal, E. Effects of New Generation All-Ceramic and Provisional Materials on Fibroblast Cells. J. Prosthodont. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Pallant, J. SPSS Survival Manual, 3rd ed.; McGraw Hill Open University Press: New York, NY, USA, 2007; pp. 224–240. [Google Scholar]
- Kwon, Y.D.; Choi, H.J.; Lee, H.; Lee, J.W.; Weber, H.P.; Pae, A. Cellular viability and genetic expression of human gingival fibroblasts to zirconia with enamel matrix derivative (Emdogain®). J. Adv. Prosthodont. 2014, 6, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Grenade, C.; de Pauw-Gillet, M.C.; Gailly, P.; Vanheusden, A.; Mainjot, A. Biocompatibility of polymer-infiltrated-ceramic-network (PICN) materials with Human Gingival Fibroblasts (HGFs). Dent. Mater. 2016, 32, 1152–1164. [Google Scholar] [CrossRef] [PubMed]
- Grenade, C.; de Pauw-Gillet, M.C.; Pirard, C.; Bertrand, V.; Charlier, C.; Vanheusden, A.; Mainjot, A. Biocompatibility of polymer-infiltrated-ceramic-network (PICN) materials with Human Gingival Keratinocytes (HGKs). Dent. Mater. 2017, 33, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P. Influence of abutment material on stability of peri-implant tissues: A systematic review. Int. J. Oral Maxillofac. Implants 2008, 23, 449–456. [Google Scholar] [PubMed]
- Kent, L.W.; Dyken, R.A.; Rahemtulla, F.; Allison, A.C.; Michalek, S.M. Effect of in vitropassage of healthy human gingival fibroblasts on cellular morphology and cytokineexpression. Arch. Oral Biol. 1996, 41, 263–270. [Google Scholar] [CrossRef]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implants Res. 2008, 19, 635–641. [Google Scholar] [PubMed]
- Soon, G.; Pingguan-Murphy, B.; Wee Lai, K.; Ali Akbar, S. Review of zirconia-based bioceramic: Surface modification and cellular response. Ceram. Int. 2016, 42, 12543–12555. [Google Scholar] [CrossRef]
- Denry, I. How and when does fabrication damage adversely affect the clinical performance of ceramic restorations? Dent. Mater. 2013, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Tetè, S.; Mastrangelo, F.; Bianchi, A.; Zizzari, V.; Scarano, A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. Int. J. Oral Maxillofac. Implants 2009, 24, 52–58. [Google Scholar] [PubMed]
- Brunette, D.M.; Chehroudi, B. The effects of the surface topography of micromachined titanium substrata on cell behavior in vitro and in vivo. J. Biomech. Eng. 1999, 121, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.; Simpson, J.; Weber, H.P.; Buser, D. Attachment and growth of periodontal cells on smooth and rough titanium. Int. J. Oral Maxillofac. Surg. 1994, 9, 289–297. [Google Scholar]
- Fischer, N.G.; Wong, J.; Baruth, A.; Cerutis, D.R. Effect of Clinically Relevant CAD/CAM Zirconia Polishing on Gingival Fibroblast Proliferation and Focal Adhesions. Materials 2017, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Pendegrass, C.J.; Gordon, D.; Middleton, C.A.; Sun, S.N.; Blunn, G.W. Sealing the skin barrier around transcutaneous implants: In vitro study of keratinocyte proliferation and adhesion in response to surface modifications of titanium alloy. J. Bone Jt. Surg. Br. 2008, 90, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Rutkunas, V.; Bukelskiene, V.; Sabaliauskas, V.; Balciunas, E.; Malinauskas, M.; Baltriukiene, D. Assessment of human gingival fibroblast interaction with dental implant abutment materials. J. Mater. Sci. Mater. Med. 2015, 26, 169. [Google Scholar] [CrossRef] [PubMed]
- Kournetas, N.; Spintzyk, S.; Schweizer, E.; Sawada, T.; Said, F.; Schmid, P.; Geis-Gerstorfer, J.; Eliades, G.; Rupp, F. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent. Mater. 2017, 33, e317–e327. [Google Scholar] [CrossRef] [PubMed]
- Hopp, I.; Michelmore, A.; Smith, L.E.; Robinson, D.E.; Bachhuka, A.; Mierczynska, A.; Vasilev, K. The influence of substrate stiffness gradients on primary human dermal fibroblasts. Biomaterials 2013, 34, 5070–5077. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Wang, H.B.; Dembo, M.; Wang, Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Donno, R. Nanomechanical Characterisation of Cells and Biocompatible Substrates. Ph.D. Thesis, School of Medicine, The University of Manchester, Causeway Bay, UK, 2013. [Google Scholar]
- Freytes, D.O.; Wan, L.Q.; Vunjak-Novakovic, G. Geometry and force control of cell function. J. Cell. Biochem. 2009, 10, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, V. Evaluation of Material Mechanical Properties Influencenon Single Cell Mechanics. In Research Doctorate in Ingegneria dei Materiali e Delle Strutture XXIII Cycle, Facoltà di Ingegneria Dipartimento di Ingegneria dei Materiali e Della Produzione; Università degli studi di Napoli “Federico II”: Napoli, Italia, 2011. [Google Scholar]
- Vita Zahnfabrik BadSäckingen. VITA YZ Technical and Scientific Documentation; Vita Zahnfabrik BadSäckingen: Bad Säckingen, Germany, 2014. [Google Scholar]
- Lawson, N.C.; Bansal, R.; Burgess, J.O. Wear, strength, modulus and hardness of CAD/CAM restorative materials. Dent. Mater. 2016, 32, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Durand, J.C.; Jacquot, B.; Fages, M. Dental biomaterials for chairside CAD/CAM: State of the art. J. Adv. Prosthodont. 2017, 9, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Elson, E.L. Cellular Mechanics as an Indicator of Cytoskeletal Structure and Function. Annu. Rev. Biophys. Biophys. Chem. 1988, 17, 397–430. [Google Scholar] [CrossRef] [PubMed]
- Jean, R.P.; Gray, D.S.; Spector, A.A.; Chen, C.S. Characterization of the nuclear deformation caused by changes in endothelial cell shape. J. Biomech. Eng. 2004, 126, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Vishavkarma, R.; Raghavan, S.; Kuyyamudi, C.; Majumder, A.; Dhawan, J.; Pullarkat, P.A. Role of actin filaments in correlating nuclear shape and cell spreading. PLoS ONE 2014, 9, e107895. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E.; Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 16, 1157–1173. [Google Scholar] [CrossRef]
- Nebe, B.; Forster, C.; Pommerenke, H.; Fulda, G.; Behrend, D.; Bernewski, U.; Schmitz, K.P.; Rychly, J. Structural alterations of adhesion mediating components in cells cultured on poly-beta-hydroxy butyric acid. Biomaterials 2001, 22, 2425–2434. [Google Scholar] [CrossRef]
- Swanson, J.A.; Lee, M.; Knapp, P.E. Cellular dimensions affecting the nucleocytoplasmic volume ratio. J. Cell Biol. 1991, 115, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S. Machine Learning Algorithms for Problem Solving in Computational Applications: Intelligent Techniques; IGI Global: Hershey, PA, USA, 2012; pp. 1–464. [Google Scholar] [CrossRef]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachments and proliferation of osteoblasts and fibroblasts on biomaterials for orthopedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef]
- Ismail, M.; Rohanizadeh, R.; Atwa, S.; Mason, R.; Ruys, A.; Martin, P.; Bendavid, A. The influence of surface chemistry and topography on the contact guidance of MG63 osteoblast cells. J. Mater. Sci. Mater. Med. 2007, 18, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Valenciano, D. Análisis del software ImageJ para el análisis científico de imágenes. In Proyecto fin de Grado en Ingeniería de Sonido e Imagen, Escuela Técnica Superior de Ingeniería y Sistemas de Telecomunicación; Universidad Politécnica de Madrid: Madrid, Spain, 2014. [Google Scholar]
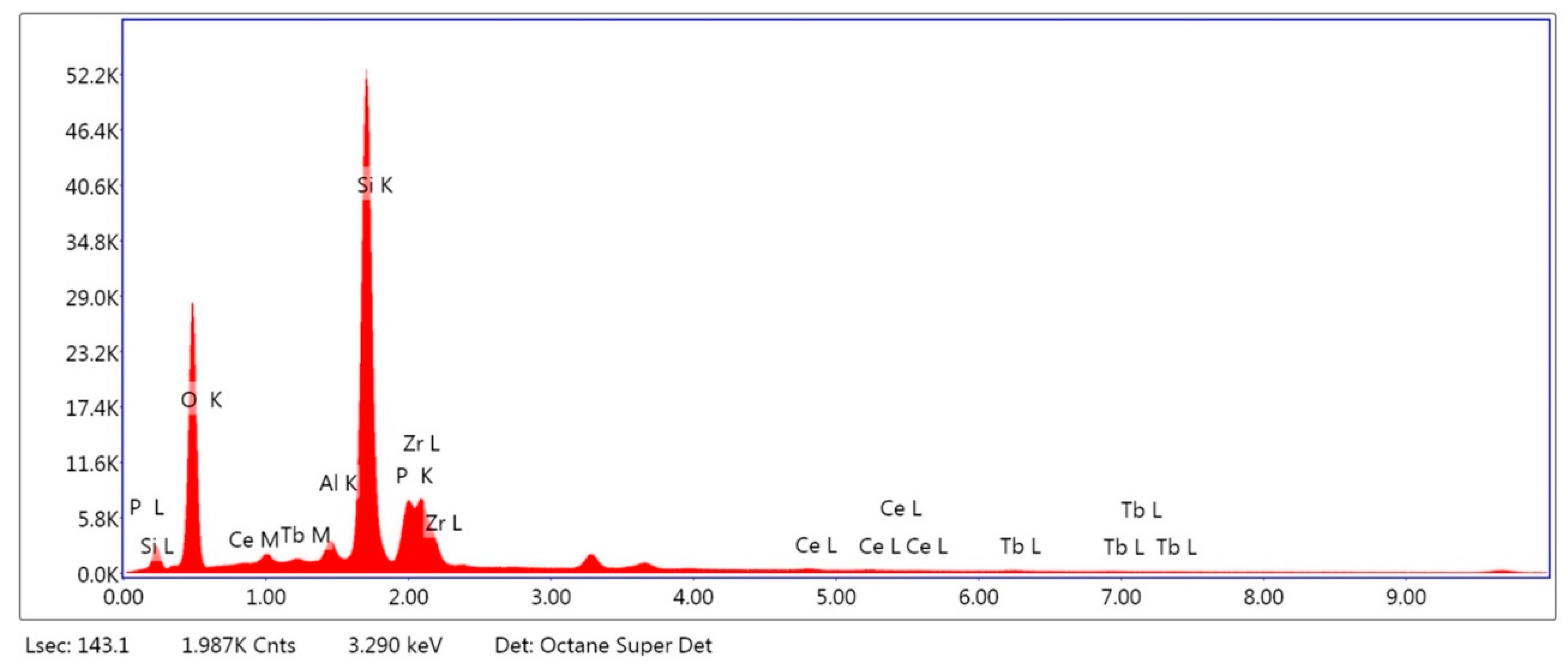
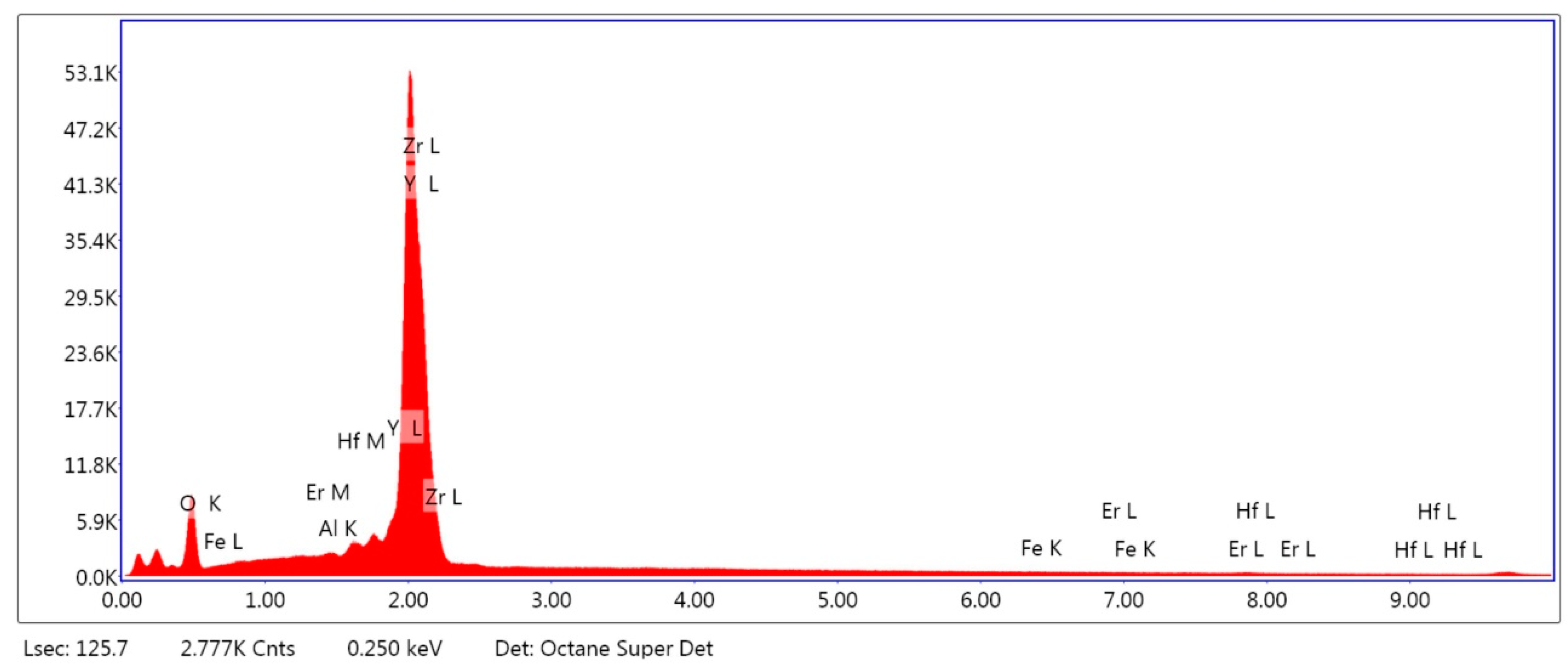

| Material | CELTRA DUO (ZLS) [wt %] | Material | Y-TZP [wt %] |
|---|---|---|---|
| Al2O3 | 2.85 | Al2O3 | 0.08 |
| SiO2 | 71.01 | ZrO2 | 88.66 |
| P2O5 | 7.44 | Y2O3 | 10.41 |
| ZrO2 | 16.72 | Fe2O3 | 0.16 |
| Ce2O3 | 1.10 | Er2O3 | 0.26 |
| Tb2O3 | 0.89 | Hf2O3 | 0.43 |
| Name Material Type Manufacturer Ref./Lot Number | |||
|---|---|---|---|
| VITA YZ® (Y-TZP) | Zirconium dioxide partially stabilized with yttrium oxide | VITA Zahnfabrik, Bad Säckinger, Germany | YZ Twhite/63320 |
| Celtra® Duo (ZLS) | Lithium silicate vitreous ceramic reinforced with zirconium | Degudent GmbH, Hanau-Wolfgang, Germany | HT-A1/18027341 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.-Á.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. Comparison of Cytomorphometry and Early Cell Response of Human Gingival Fibroblast (HGFs) between Zirconium and New Zirconia-Reinforced Lithium Silicate Ceramics (ZLS). Int. J. Mol. Sci. 2018, 19, 2718. https://doi.org/10.3390/ijms19092718
Rizo-Gorrita M, Luna-Oliva I, Serrera-Figallo M-Á, Gutiérrez-Pérez J-L, Torres-Lagares D. Comparison of Cytomorphometry and Early Cell Response of Human Gingival Fibroblast (HGFs) between Zirconium and New Zirconia-Reinforced Lithium Silicate Ceramics (ZLS). International Journal of Molecular Sciences. 2018; 19(9):2718. https://doi.org/10.3390/ijms19092718
Chicago/Turabian StyleRizo-Gorrita, María, Irene Luna-Oliva, María-Ángeles Serrera-Figallo, José-Luis Gutiérrez-Pérez, and Daniel Torres-Lagares. 2018. "Comparison of Cytomorphometry and Early Cell Response of Human Gingival Fibroblast (HGFs) between Zirconium and New Zirconia-Reinforced Lithium Silicate Ceramics (ZLS)" International Journal of Molecular Sciences 19, no. 9: 2718. https://doi.org/10.3390/ijms19092718
APA StyleRizo-Gorrita, M., Luna-Oliva, I., Serrera-Figallo, M.-Á., Gutiérrez-Pérez, J.-L., & Torres-Lagares, D. (2018). Comparison of Cytomorphometry and Early Cell Response of Human Gingival Fibroblast (HGFs) between Zirconium and New Zirconia-Reinforced Lithium Silicate Ceramics (ZLS). International Journal of Molecular Sciences, 19(9), 2718. https://doi.org/10.3390/ijms19092718