Molecular Characterisation and Functions of Fis1 and PDCD6 Genes from Echinococcus granulosus
Abstract
1. Introduction
2. Results
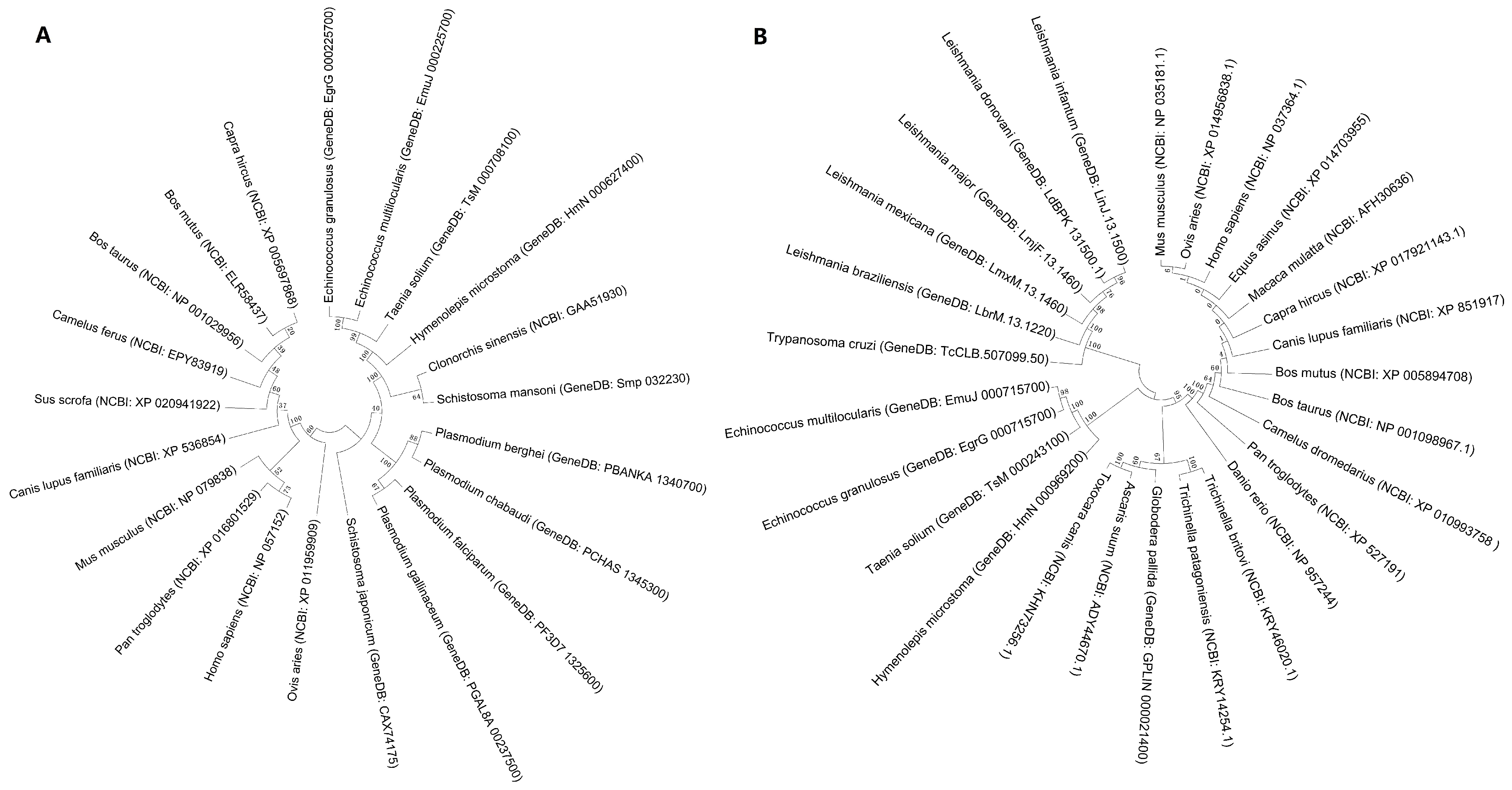
2.1. Bioinformatic Analysis of Eg-Fis1 and Eg-PDCD6
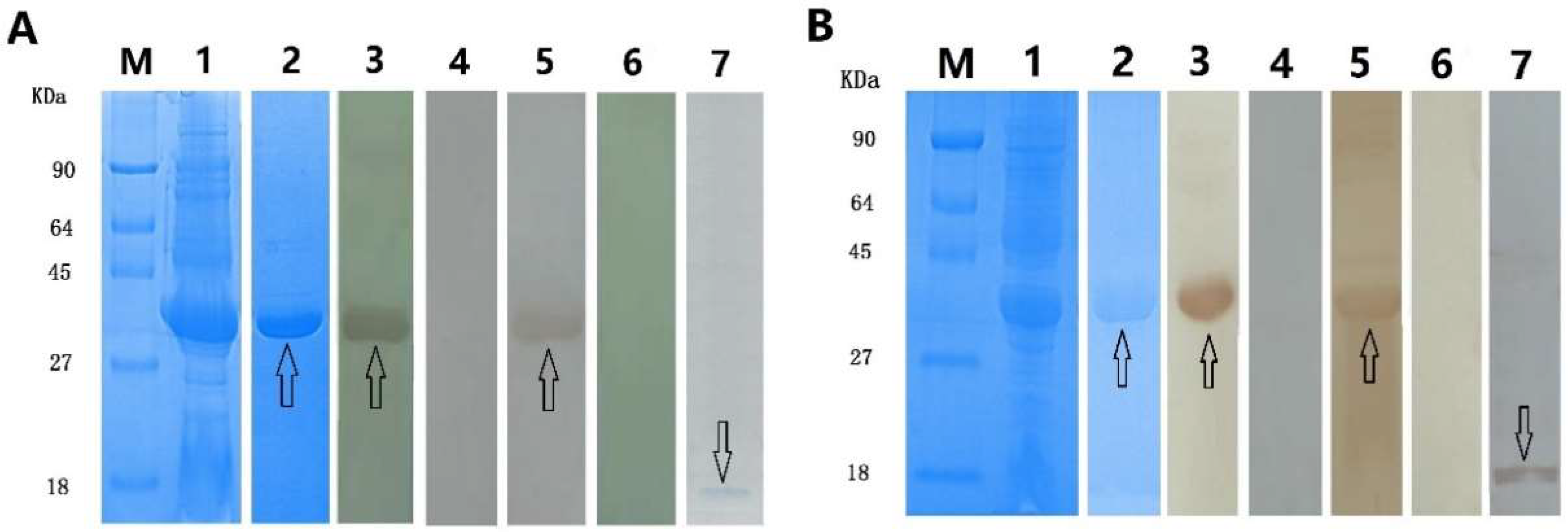
2.2. Expression of Recombinant rEg-Fis1 and rEg-PDCD
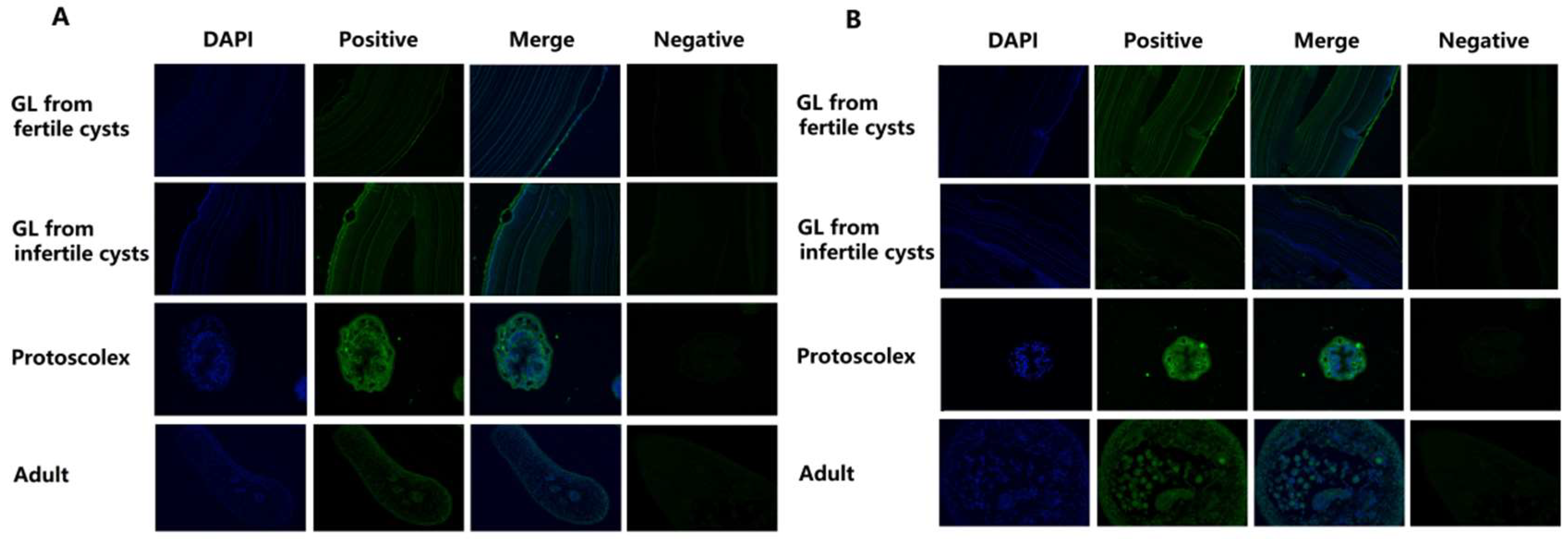
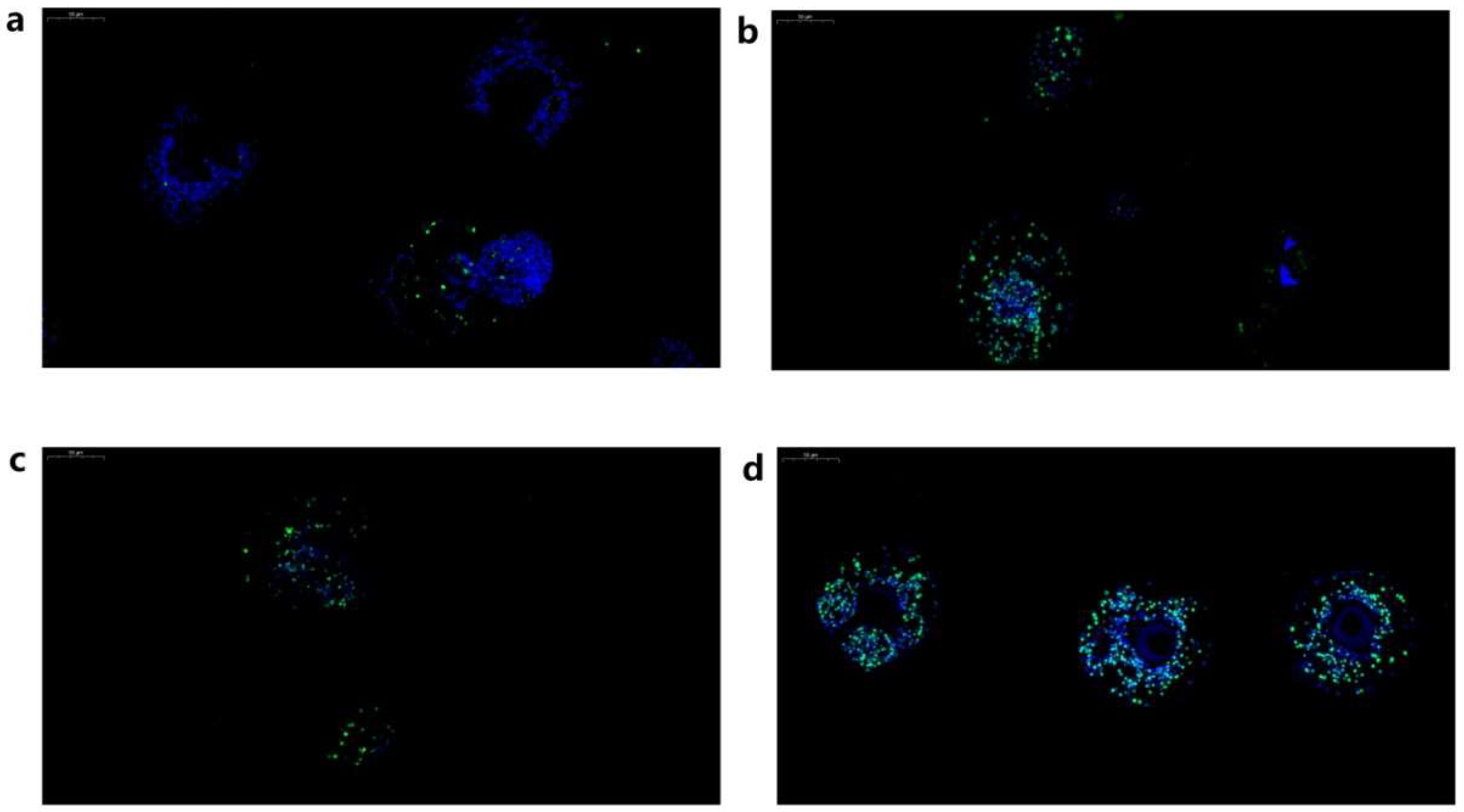
2.3. Localisation of Eg-Fis1 and Eg-PDCD6 during Different E. granulosus Life Cycle Stages
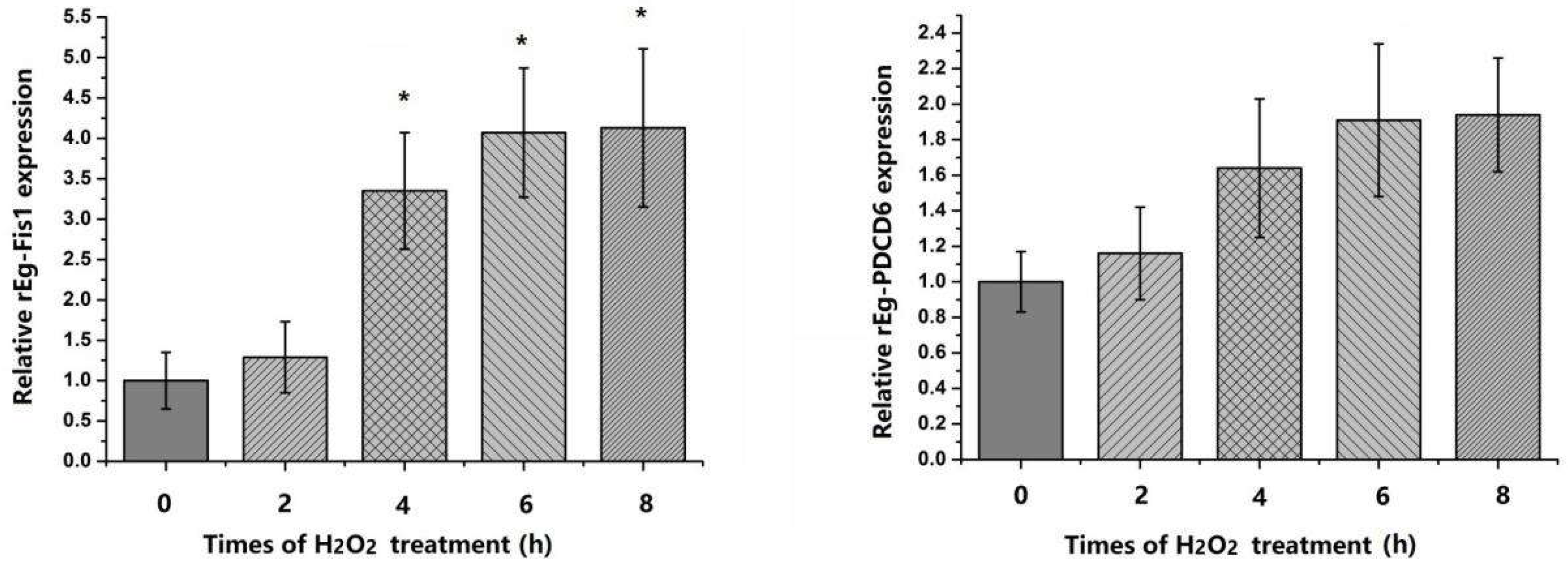
2.4. Expression of Eg-Fis1 and Eg-PDCD6 in Response to Oxidative Stress
2.5. Small Interfering RNA (siRNA)-Mediated Knockdown of Eg-Fis1 and Eg-PDCD6
2.6. Measurement of Apoptotic Rate in PSCs Following RNAi Treatment
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Parasites
4.3. Bioinformatic Analysis
4.4. Cloning and Expression of Recombinant rEg-Fis1 and rEg-PDCD6
4.5. Preparation of Polyclonal Antibodies
4.6. Immunoblotting and Immunolocalisation
4.7. Eg-Fis1 and Eg-PDCD6 Expression in PSCs Following H2O2 Treatment
4.8. RNA Interference Assay
4.9. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eckert, J.; Gemmell, M.A.; Meslin, F.X.; Pawłowski, Z.S. WHO/OIE manual on echinococcosis in humans and animals: A public health problem of global concern. Int. J. Parasitol. 2001, 31, 1717–1718. [Google Scholar]
- Cardona, G.A.; Carmena, D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet. Parasitol. 2013, 192, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Daumerie, D.; Savioli, L. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases; WHO Organization: Geneva, Switzerland, 2011; Volume 7, p. 596. [Google Scholar]
- Alessandra, S.; Federica, D.; Antonella, T.; Elena, O. Host-Parasite Relationship in Cystic Echinococcosis: An Evolving Story. Clin. Dev. Immunol. 2011, 2012, 639362. [Google Scholar]
- Daryani, A.; Sharif, M.; Amouei, A.; Nasrolahei, M. Fertility and viability rates of hydatid cysts in slaughtered animals in the Mazandaran Province, Northern Iran. Trop. Anim. Health Prod. 2009, 34, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Kamenetzky, L.; Canova, S.G.; Guarnera, E.A.; Rosenzvit, M.C. Echinococcus granulosus: DNA extraction from germinal layers allows strain determination in fertile and nonfertile hydatid cysts. Exp. Parasitol. 2000, 95, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Mcmanus, D.P.; Souissi, A.; Chelly, S.; Torgerson, P.R. Transmission dynamics of the Echinococcus granulosus sheep-dog strain (G1 genotype) in camels in Tunisia. Vet. Parasitol. 2004, 121, 151–156. [Google Scholar]
- Yoon, Y.; Pitts, K.R.; Mcniven, M.A. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol. Biol. Cell 2001, 12, 2894. [Google Scholar] [CrossRef] [PubMed]
- James, D.I.; Parone, P.Y.; Martinou, J.C. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 2003, 278, 36373–36379. [Google Scholar] [CrossRef] [PubMed]
- Stojanovski, D.; Koutsopoulos, O.S.; Okamoto, K.; Ryan, M.T. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J. Cell Sci. 2004, 117, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Narayana, S.V.; Hitomi, K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem. J. 1997, 328, 718. [Google Scholar] [PubMed]
- Krebs, J.; Saremaslani, P.; Caduff, R. ALG-2: A Ca2+-binding modulator protein involved in cell proliferation and in cell death. Biochim. Biophys. Acta Proteins Proteom. 2002, 1600, 68–73. [Google Scholar] [CrossRef]
- Vito, P.; Lacaná, E.; D’Adamio, L. Interfering with Apoptosis: Ca2+-Binding Protein ALG-2 and Alzheimer’s Disease Gene ALG-3. Science 1996, 271, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Tarabykina, S.; Møller, A.L.; Durussel, I.; Cox, J.; Berchtold, M.W. Two forms of the apoptosis-linked protein ALG-2 with different Ca2+ affinities and target recognition. J. Biol. Chem. 2000, 275, 10514. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Hu, R.; Lacaná, E.; D’Adamio, L.; Gu, H. Apoptosis-linked gene 2-deficient mice exhibit normal T-cell development and function. Mol. Cell. Biol. 2002, 22, 4094. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Huang, M.S.; Yang, C.J.; Hung, J.Y.; Wu, L.Y.; Kuo, P.L. Lung tumor-associated osteoblast-derived bone morphogenetic protein-2 increased epithelial-to-mesenchymal transition of cancer by Runx2/Snail signaling pathway. J. Biol. Chem. 2011, 286, 37335. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, S.; Karbowski, M.; Smith, C.L.; Youle, R.J. Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis. Mol. Biol. Cell 2004, 15, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Dashzeveg, N.; Lu, Z.G.; Taira, N.; Miki, Y.; Yoshida, K. Programmed cell death 6, a novel p53-responsive gene, targets to the nucleus in the apoptotic response to DNA damage. Cancer Sci. 2012, 103, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, G.; Cabrejos, M.E.; Morassutti, A.L.; Cabezón, C.; Orellana, J.; Hellman, U.; Zaha, A.; Galanti, N. DNA damage, RAD9 and fertility/infertility of Echinococcus granulosus hydatid cysts. J. Cell. Physiol. 2008, 216, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Yoon, Y.; Bonekamp, N.A.; Mcniven, M.A.; Schrader, M. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 2005, 16, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Jeong, S.Y.; Karbowski, M.; Youle, R.J.; Tjandra, N. The Solution Structure of Human Mitochondria Fission Protein Fis1 Reveals a Novel TPR-like Helix Bundle. J. Mol. Biol. 2003, 334, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Mozdy, A.D.; Mccaffery, J.M.; Shaw, J.M. Dnm1p GTPase-Mediated Mitochondrial Fission Is a Multi-Step Process Requiring the Novel Integral Membrane Component Fis1p. J. Cell Biol. 2000, 151, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.; Zhang, Q.; Li, M.; Zhang, M. Apoptosis-linked gene product ALG-2 is a new member of the Calpain small subunit subfamily of Ca2+-binding proteins. Biochemistry 1999, 38, 7498–7508. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Yamaguchi, K.; Kitaura, Y.; Satoh, H.; Hitomi, K. Calcium-induced exposure of a hydrophobic surface of mouse ALG-2, which is a member of the penta-EF-hand protein family. J. Biochem. 1998, 124, 1170. [Google Scholar] [CrossRef] [PubMed]
- D’Adamio, L.; Lacanà, E.; Vito, P. Functional cloning of genes involved in T-cell receptor-induced programmed cell death. Semin. Immunol. 1997, 9, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Bereiter-Hahn, J.; Vöth, M.; Mai, S.; Jendrach, M. Structural implications of mitochondrial dynamics. Biotechnol. J. 2010, 3, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Jofuku, A.; Eura, Y.; Mihara, K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 2003, 301, 891–898. [Google Scholar] [CrossRef]
- Busch, K.B.; Bereiter-Hahn, J.; Wittig, I.; Schagger, H.; Jendrach, M. Mitochondrial dynamics generate equal distribution but patchwork localization of respiratory Complex I. Mol. Membr. Biol. 2006, 23, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Isobe, K.; Nakada, K.; Hayashi, J.I. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat. Genet. 2001, 28, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Hajyehia, A.; Levischaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Kobayashi, C.; Agata, K.; Ikeo, K.; Gojobori, T. Detection of apoptosis during planarian regeneration by the expression of apoptosis-related genes and TUNEL assay. Gene 2004, 333, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.; Jiménez, V.; Cabrera, G.; Iragüen, D.; Galanti, N. Apoptosis as a possible mechanism of infertility in Echinococcus granulosus hydatid cysts. J. Cell. Biochem. 2007, 100, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Spotin, A.; Majdi, M.M.A.; Sankian, M.; Varasteh, A. The study of apoptotic bifunctional effects in relationship between host and parasite in cystic echinococcosis: A new approach to suppression and survival of hydatid cyst. Parasitol. Res. 2012, 110, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, L.; Wu, S.; Xing, D. Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation-induced apoptosis. FASEB J. 2015, 30, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Khadidja, H.; Achour, Y.; Vasile, C. Study of the fertility and morphostructural of Echinococcus granulosus in Algeria. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca 2014, 71, 92–96. [Google Scholar]
- Khan, A.H.; El-Buni, A.A.; Ali, M.Y. Fertility of the cysts of Echinococcus granulosus in domestic herbivores from Benghazi, Libya, and the reactivity of antigens produced from them. Pathog. Glob. Health 2001, 95, 337–342. [Google Scholar]
- Kitani, F.A.A.; Riyami, S.A.; Yahyai, S.A.; Awahi, A.H.A.; Aawali, M.A.; Hussain, M.H. Abattoir based surveillance of cystic echinococcosis (CE) in the Sultanate of Oman during 2010–2013. Vet. Parasitol. 2015, 211, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Koskei, P.; Janitschke, K.; Feseha, G. Prevalence of Echinococcus granulosus in some selected sites of Ethiopia. East Afr. J. Public Health 2011, 8, 170. [Google Scholar] [PubMed]
- Latif, A.A.; Tanveer, A.; Maqbool, A.; Siddiqi, N.; Kyaw-Tanner, M.; Traub, R.J. Morphological and molecular characterisation of Echinococcus granulosus in livestock and humans in Punjab, Pakistan. Vet. Parasitol. 2010, 170, 44–49. [Google Scholar] [CrossRef] [PubMed]

| Group Name | Number of Apoptotic Cells | Total Cells | Apoptosis Rate (%) |
|---|---|---|---|
| NCG | 42 | 301 | 13.95% |
| NIG | 76 | 119 | 63.87% |
| Fis1-IG | 182 | 338 | 53.85% |
| PDCD6-IG | 285 | 438 | 65.07% |
| siRNA Name | Sequences | |
|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |
| FIS-12 | GGAUUUGAAUGAGCCGGUUTT | AACCGGCUCAUUCAAAUCCTT |
| FIS-253 | GCAAUCGAAUGUUGCGAUATT | UAUCGCAACAUUCGAUUGCTT |
| FIS-364 | GCUGUUGGUUUAGCUGUCGTT | CGACAGCUAAACCAACAGCTT |
| PDCD-153 | CGACCUCUUUAGUCCUAAUTT | AUUAGGACUAAAGAGGUCGTT |
| PDCD-286 | GAGCUACAGUCCGCUAUAUTT | AUAUAGCGGACUGUAGCUCTT |
| PDCD-375 | GGGCGUUGUUUACUUCGAUTT | AUCGAAGUAAACAACGCCCTT |
| siRNA-CON | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Zhan, J.; Guo, C.; Li, C.; Shen, N.; Gu, X.; Xie, Y.; Peng, X.; Yang, G. Molecular Characterisation and Functions of Fis1 and PDCD6 Genes from Echinococcus granulosus. Int. J. Mol. Sci. 2018, 19, 2669. https://doi.org/10.3390/ijms19092669
Wang N, Zhan J, Guo C, Li C, Shen N, Gu X, Xie Y, Peng X, Yang G. Molecular Characterisation and Functions of Fis1 and PDCD6 Genes from Echinococcus granulosus. International Journal of Molecular Sciences. 2018; 19(9):2669. https://doi.org/10.3390/ijms19092669
Chicago/Turabian StyleWang, Ning, Jiafei Zhan, Cheng Guo, Chunyan Li, Nengxing Shen, Xiaobin Gu, Yue Xie, Xuerong Peng, and Guangyou Yang. 2018. "Molecular Characterisation and Functions of Fis1 and PDCD6 Genes from Echinococcus granulosus" International Journal of Molecular Sciences 19, no. 9: 2669. https://doi.org/10.3390/ijms19092669
APA StyleWang, N., Zhan, J., Guo, C., Li, C., Shen, N., Gu, X., Xie, Y., Peng, X., & Yang, G. (2018). Molecular Characterisation and Functions of Fis1 and PDCD6 Genes from Echinococcus granulosus. International Journal of Molecular Sciences, 19(9), 2669. https://doi.org/10.3390/ijms19092669






