Mechanism of Lakoochin A Inducing Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS and MAPKs Pathway
Abstract
1. Introduction
2. Results
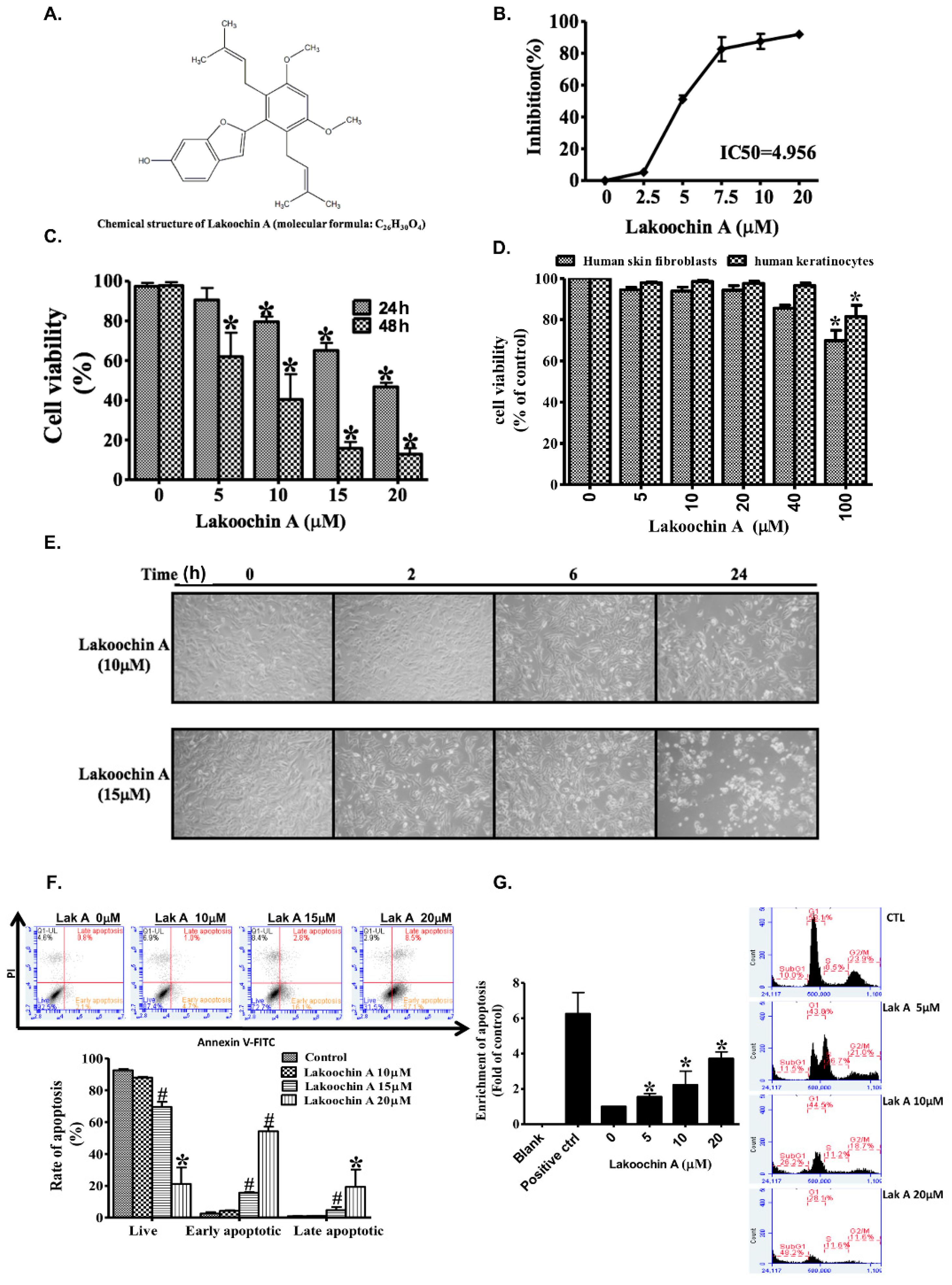
2.1. Lakoochin A Inhibits Proliferation and Viability of A375.S2 Melanoma Cells
2.2. Lakoochin A Promotes Apoptosis and Cell Cycle Arrest in A375.S2 Melanoma Cells
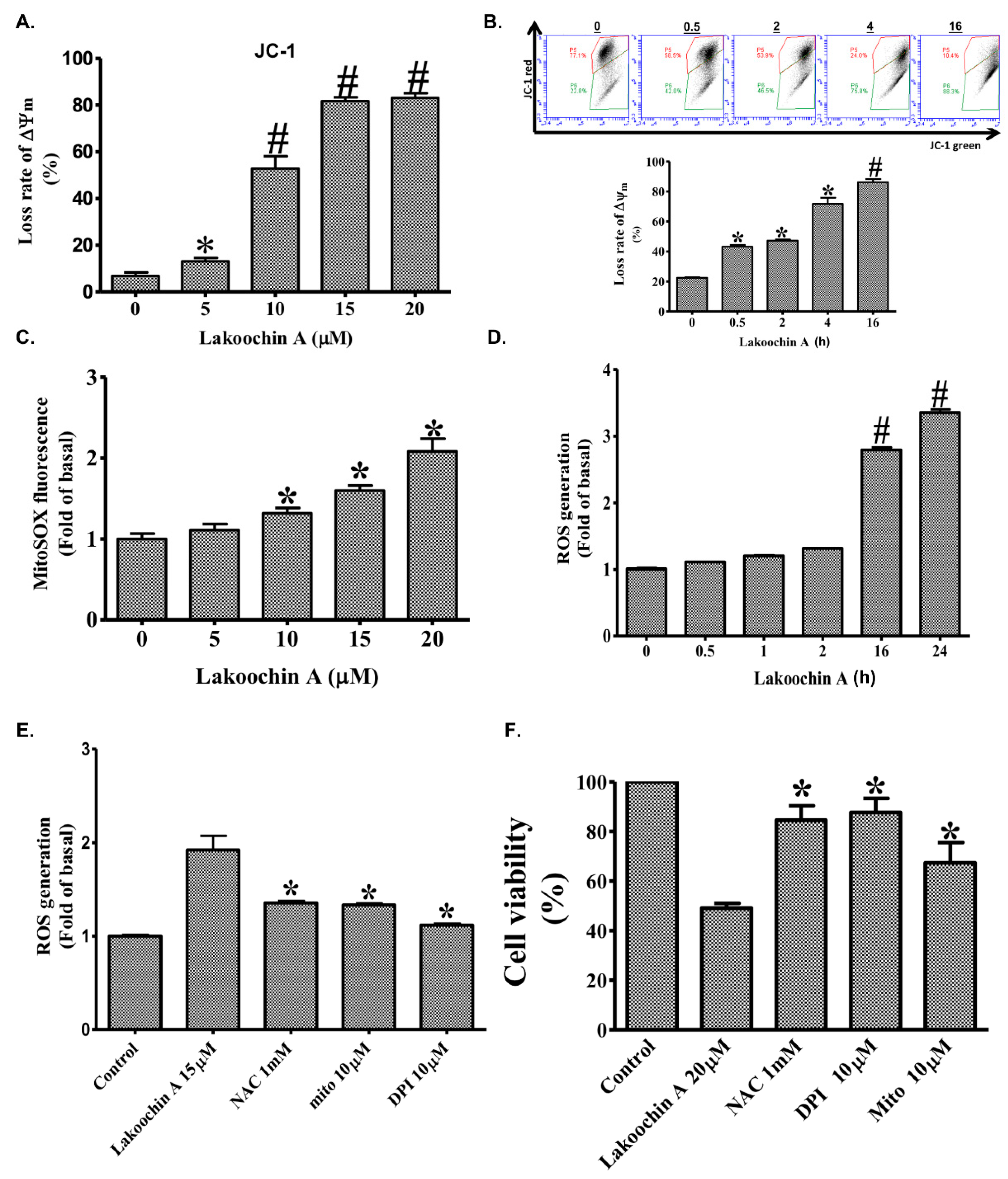
2.3. Lakoochin A Increases Apoptosis of A375.S2 Cells through the Mitochondrial Pathway
2.4. Lakoochin A Induces Cellular and Mitochondrial ROS Production in A375.S2 Cells
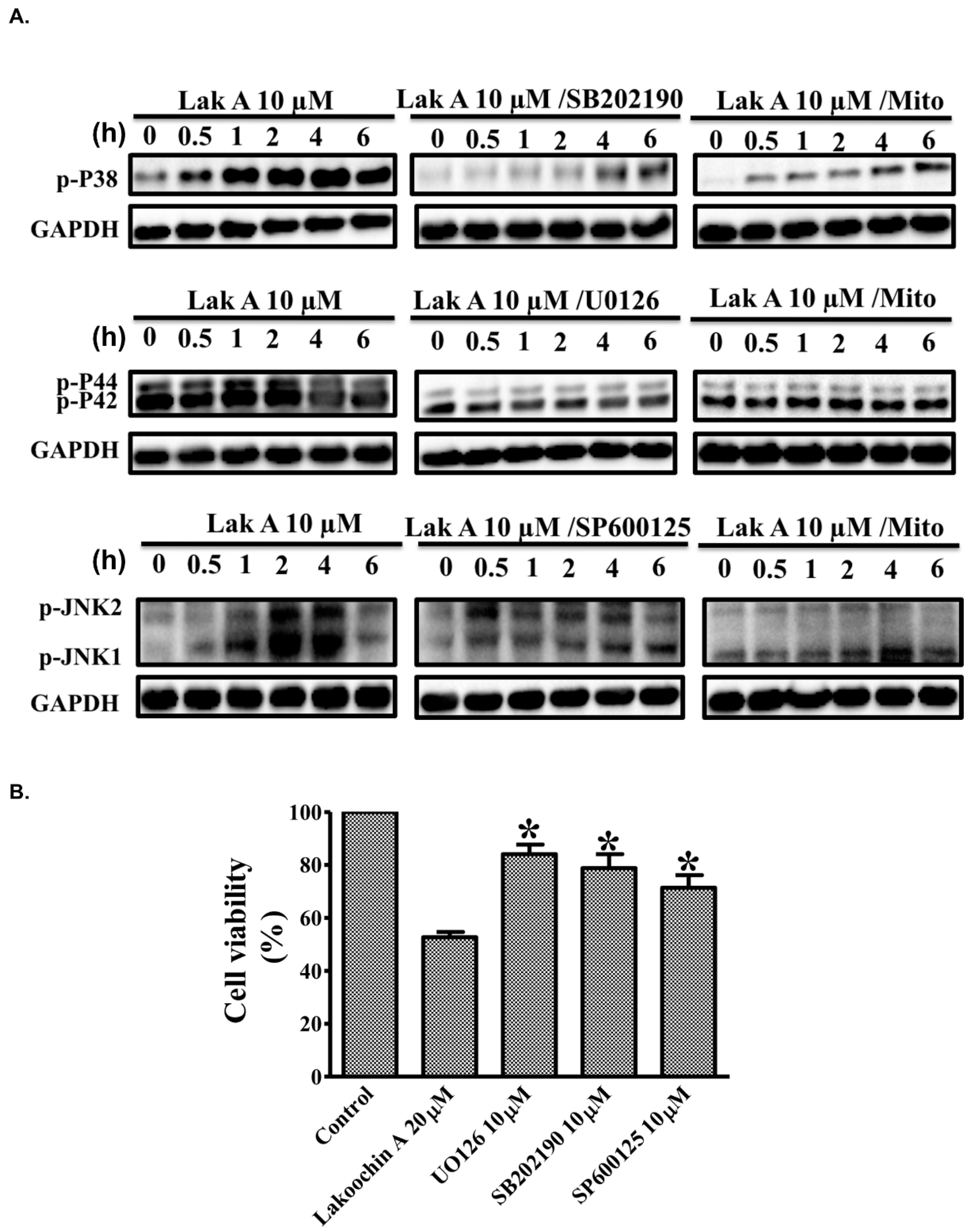
2.5. Lakoochin A Induces Mitochondrial ROS Generation to Activate the MAPK Pathway in A375.S2 Cells
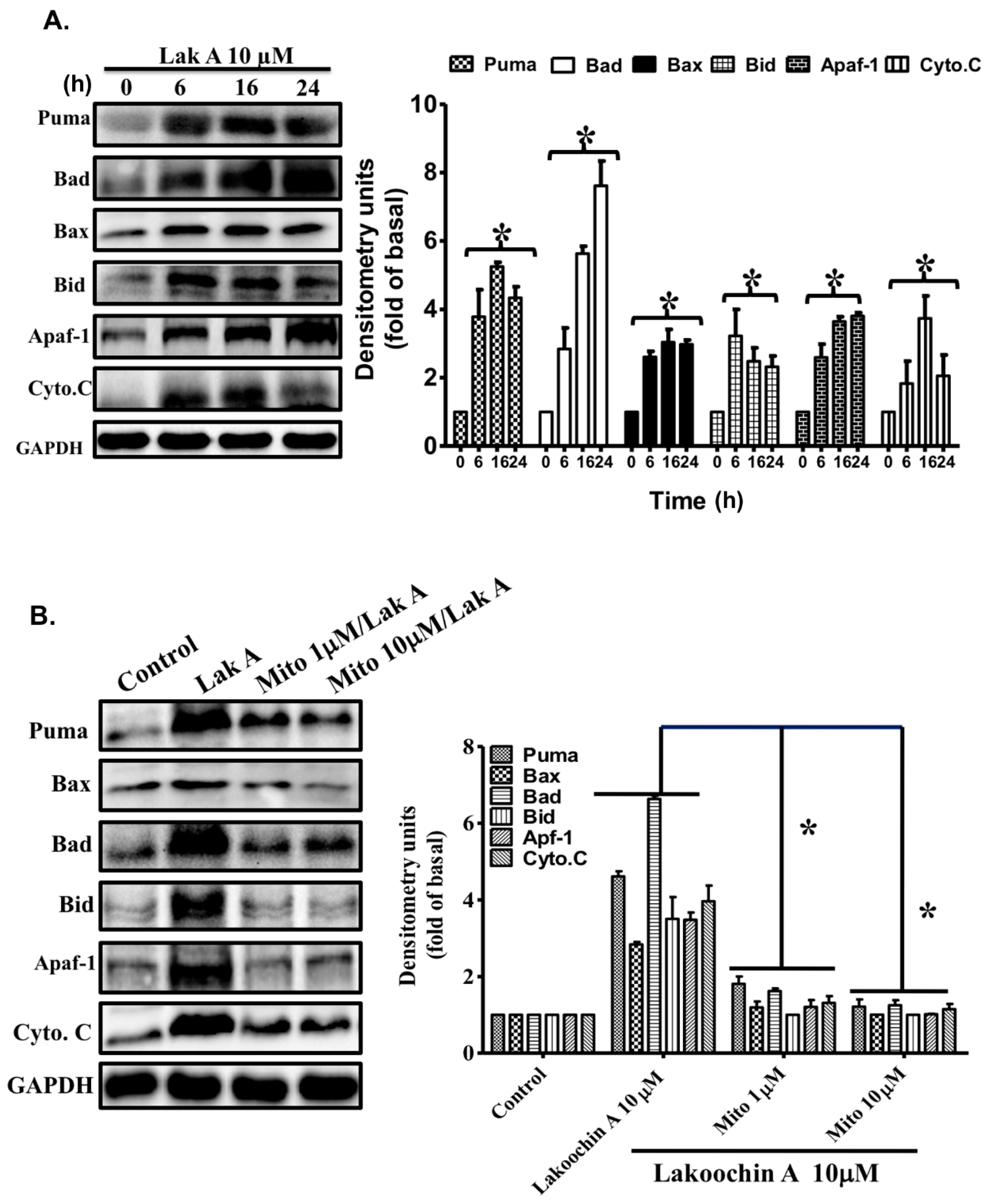
2.6. Lakoochin A Induces Apoptosis-Related Protein Expression in A375.S2 Cells via Mitochondrial ROS Generation
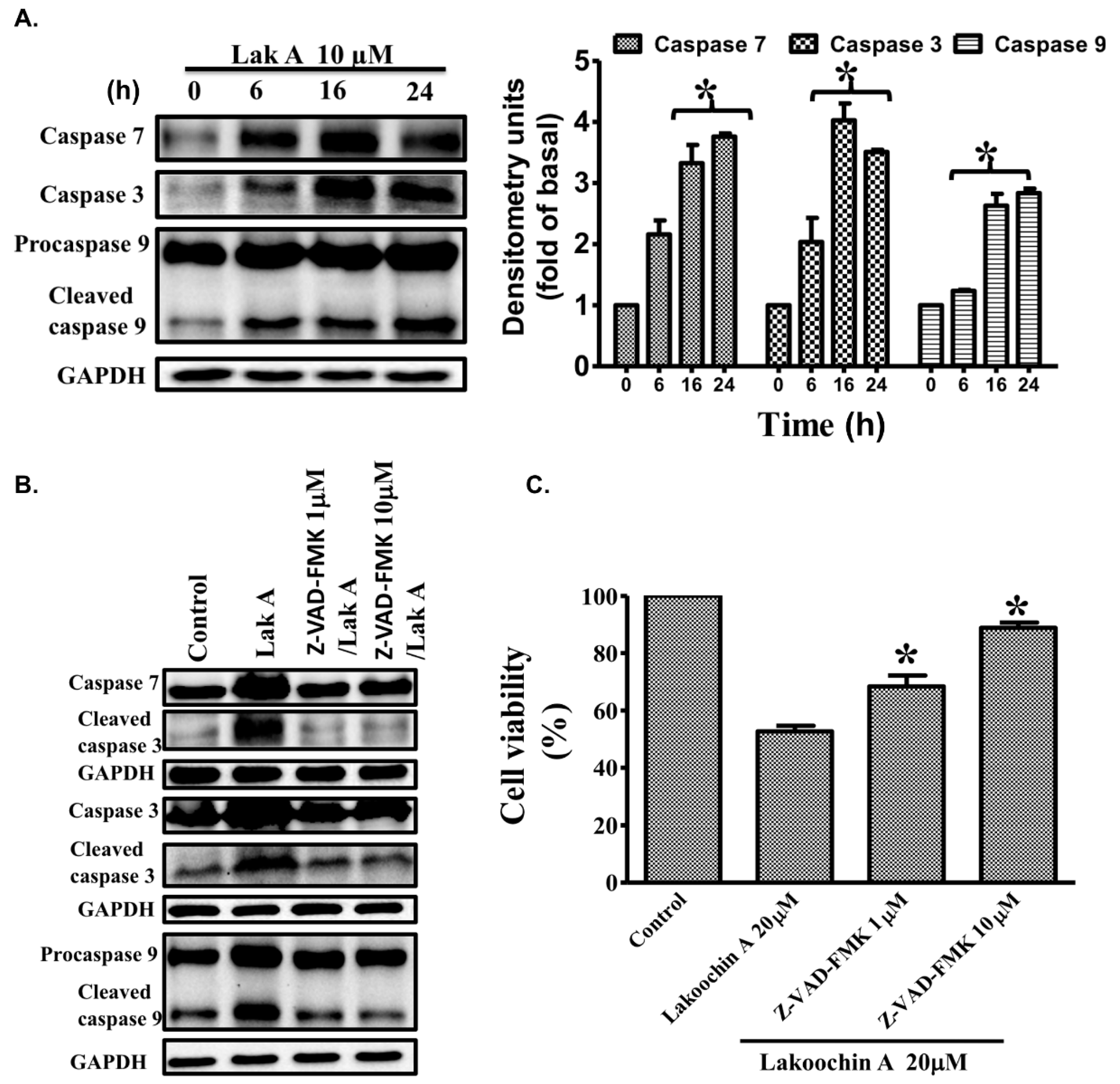
2.7. Lakoochin A Induces the Activation of Caspase-3, Caspase-7, and Caspase-9 in A375.S2 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Proliferation Assay
4.4. Cell Viability Assay
4.5. Cell Apoptosis Measurement
4.6. Cell Cycle Analysis
4.7. Assessment of Mitochondrial Membrane Potential
4.8. Determination of Cellular and Mitochondrial ROS Production
4.9. Immunoblotting Analysis for MAPKs, Apoptosis-Related Proteins, and Caspases
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Apaf-1 | apoptotic protease activating factor 1 |
| Bad | Bcl-2-associated death promoter |
| Bax | BH3-interacting domain death agonist (Bid), BCL2-associated X protein |
| DPI | diphenylene iodonium |
| ERK | extracellular regulated protein kinase |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinases |
| NAC | N-acetylcysteine |
| ROS | reactive oxygen species |
| Puma | p53 upregulated modulator of apoptosis |
References
- McCourt, C.; Dolan, O.; Gormley, G. Malignant melanoma: A pictorial review. Ulster Med. J. 2014, 83, 103–110. [Google Scholar] [PubMed]
- Miller, A.J.; Mihm, M.C., Jr. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- Puntumchai, A.; Kittakoop, P.; Rajviroongit, S.; Vimuttipong, S.; Likhitwitayawuid, K.; Thebtaranonth, Y. Lakoochins A and B, new antimycobacterial stilbene derivatives from Artocarpus lakoocha. J. Nat. Prod. 2004, 67, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Lin, C.C.; Lu, T.M.; Li, J.H.; Chen, I.S.; Kuo, Y.H.; Ko, H.H. Chemical constituents derived from Artocarpus xanthocarpus as inhibitors of melanin biosynthesis. Phytochemistry 2015, 117, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Le, T.H.; Nguyen, H.X.; Dang, P.H.; Do, T.N.V.; Abe, M.; Takagi, R.; Nguyen, N.T. Artocarmins G–M, Prenylated 4-Chromenones from the Stems of Artocarpus rigida and Their Tyrosinase Inhibitory Activities. J. Nat. Prod. 2017, 80, 3172–3178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yan, G.R.; Pan, S.L.; Wang, H.Y.; Hou, A.J. New isoprenylated 2-arylbenzofurans and pancreatic lipase inhibitory constituents from Artocarpus nitidus. Chem. Biodivers. 2009, 6, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Sritularak, B.; Tantrakarnsakul, K.; Lipipun, V.; Likhitwitayawuid, K. Flavonoids with anti-HSV activity from the root bark of Artocarpus lakoocha. Nat. Prod. Commun. 2013, 8, 1079–1080. [Google Scholar] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2010, 129, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Yoshikawa, K.; Shimizu, K.; Kondo, R. Isoprenoid-substituted flavonoids from wood of Artocarpus heterophyllus on B16 melanoma cells: Cytotoxicity and structural criteria. Fitoterapia 2010, 81, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.W.; Tzeng, W.S.; Lin, L.T.; Lee, C.W.; Yen, M.H.; Yen, F.L.; Lin, C.C. Artocarpus communis Induces Autophagic Instead of Apoptotic Cell Death in Human Hepatocellular Carcinoma Cells. Am. J. Chin. Med. 2015, 43, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.P.; Qiao, X.; Pan, S.; Shen, H.; Zhu, G.F.; Hou, A.J. New isoprenylated flavonoids and cytotoxic constituents from Artocarpus tonkinensis. J. Asian Nat. Prod. Res. 2010, 12, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Soo, H.C.; Chung, F.F.; Lim, K.H.; Yap, V.A.; Bradshaw, T.D.; Hii, L.W.; Tan, S.H.; See, S.J.; Tan, Y.F.; Leong, C.O.; et al. Cudraflavone C Induces Tumor-Specific Apoptosis in Colorectal Cancer Cells through Inhibition of the Phosphoinositide 3-Kinase (PI3K)-AKT Pathway. PLoS ONE 2017, 12, e0170551. [Google Scholar] [CrossRef] [PubMed]
- Nuntawong, P.; Kongkatitham, V.; Likhitwitayawuid, K.; Mekboonsonglarp, W.; Sukrong, S.; Tanasupawat, S.; Sritularak, B. New 2-arylbenzofurans from the root bark of Artocarpus gomezianus and their alpha-glucosidase inhibitory activity. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Yen, F.L.; Ko, H.H.; Li, S.Y.; Chiang, Y.C.; Lee, M.H.; Tsai, M.H.; Hsu, L.F. Cudraflavone C Induces Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS Production and MAPK Activation. Int. J. Mol. Sci. 2017, 18, 1508. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Liu, J.F.; Chiang, Y.C.; Hu, S.C.; Hsu, L.F.; Lin, Y.C.; Lin, Z.C.; Lee, H.C.; Chen, M.C.; Huang, C.L.; et al. Artocarpin, an isoprenyl flavonoid, induces p53-dependent or independent apoptosis via ROS-mediated MAPKs and Akt activation in non-small cell lung cancer cells. Oncotarget 2017, 8, 28342–28358. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef] [PubMed]
- Day, B.J. Antioxidant therapeutics: Pandora’s box. Free Radic. Biol. Med. 2014, 66, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Goto, S.; Koltai, E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol. Aspects Med. 2011, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.J.; Limagne, E.; Ragot, K.; Lizard, G.; Ghiringhelli, F.; Solary, E.; Chauffert, B.; Latruffe, N.; Delmas, D. The role of reactive oxygen species and subsequent DNA-damage response in the emergence of resistance towards resveratrol in colon cancer models. Cell Death Dis. 2014, 5, e1533. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, B.; Beevers, C.S.; Odaka, Y.; Chen, L.; Liu, L.; Luo, Y.; Zhou, H.; Chen, W.; Shen, T.; et al. Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Carcinogenesis 2012, 33, 868–875. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Svedman, F.C.; Pillas, D.; Taylor, A.; Kaur, M.; Linder, R.; Hansson, J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—A systematic review of the literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Harries, M.; Malvehy, J.; Lebbe, C.; Heron, L.; Amelio, J.; Szabo, Z.; Schadendorf, D. Treatment patterns of advanced malignant melanoma (stage III-IV)—A review of current standards in Europe. Eur. J. Cancer 2016, 60, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Beberok, A.; Wrzesniok, D.; Szlachta, M.; Rok, J.; Rzepka, Z.; Respondek, M.; Buszman, E. Lomefloxacin Induces Oxidative Stress and Apoptosis in COLO829 Melanoma Cells. Int. J. Mol. Sci. 2017, 18, 2194. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Boutros, T.; Chevet, E.; Metrakos, P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol. Rev. 2008, 60, 261–310. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Ferrara, F.; Diana, G.; Fulgenzi, A.; Corsi, M.; Ponti, W.; Ferrero, M.E.; Bertelli, A. Resveratrol, a natural stilbene in grapes and wine, enhances intraphagocytosis in human promonocytes: A co-factor in antiinflammatory and anticancer chemopreventive activity. Int. J. Tissue React. 1999, 21, 93–104. [Google Scholar] [PubMed]
- Platella, C.; Guida, S.; Bonmassar, L.; Aquino, A.; Bonmassar, E.; Ravagnan, G.; Montesarchio, D.; Roviello, G.N.; Musumeci, D.; Fuggetta, M.P. Antitumour activity of resveratrol on human melanoma cells: A possible mechanism related to its interaction with malignant cell telomerase. Biochim. Biophys. Acta 2017, 1861, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Darjatmoko, S.R.; Polans, A.S. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011, 21, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, B.; E, C.; Liu, J.; Zhang, Q.; Liu, J.; Chen, N.; Chen, R.; Zhu, R. Resveratrol inhibits the proliferation of human melanoma cells by inducing G1/S cell cycle arrest and apoptosis. Mol. Med. Rep. 2015, 11, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Niles, R.M.; McFarland, M.; Weimer, M.B.; Redkar, A.; Fu, Y.M.; Meadows, G.G. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Cancer Lett. 2003, 190, 157–163. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, J.Y.; Kuo, C.H.; Pai, P.Y.; Ho, T.J.; Chen, T.S.; Tsai, F.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Mitochondrial ROS-induced ERK1/2 Activation and HSF2-mediated AT1 R Upregulation Are Required for Doxorubicin-induced Cardiotoxicity. J. Cell. Physiol. 2018, 233, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Molecular signaling in death receptor and mitochondrial pathways of apoptosis (Review). Int. J. Oncol. 2003, 22, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Hu, Q.; Bai, X.C.; Huang, W.; Yan, C.; Scheres, S.H.; Shi, Y. Atomic structure of the apoptosome: Mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev. 2015, 29, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lee, S.; Malladi, S.; Chen, M.D.; Mastrandrea, N.J.; Zhang, Z.; Bratton, S.B. The Apaf-1 apoptosome induces formation of caspase-9 homo- and heterodimers with distinct activities. Nat. Commun. 2016, 7, 13565. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Kornbluth, S. The apoptosome: Physiological, developmental, and pathological modes of regulation. Dev. Cell 2006, 10, 549–561. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.-T.; Chiang, Y.-C.; Ko, H.-H.; Chi, P.-L.; Tsai, C.-L.; Ko, M.-I.; Lee, M.-H.; Hsu, L.-F.; Lee, C.-W. Mechanism of Lakoochin A Inducing Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS and MAPKs Pathway. Int. J. Mol. Sci. 2018, 19, 2649. https://doi.org/10.3390/ijms19092649
Peng K-T, Chiang Y-C, Ko H-H, Chi P-L, Tsai C-L, Ko M-I, Lee M-H, Hsu L-F, Lee C-W. Mechanism of Lakoochin A Inducing Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS and MAPKs Pathway. International Journal of Molecular Sciences. 2018; 19(9):2649. https://doi.org/10.3390/ijms19092649
Chicago/Turabian StylePeng, Kuo-Ti, Yao-Chang Chiang, Horng-Huey Ko, Pei-Ling Chi, Chia-Lan Tsai, Ming-I Ko, Ming-Hsueh Lee, Lee-Fen Hsu, and Chiang-Wen Lee. 2018. "Mechanism of Lakoochin A Inducing Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS and MAPKs Pathway" International Journal of Molecular Sciences 19, no. 9: 2649. https://doi.org/10.3390/ijms19092649
APA StylePeng, K.-T., Chiang, Y.-C., Ko, H.-H., Chi, P.-L., Tsai, C.-L., Ko, M.-I., Lee, M.-H., Hsu, L.-F., & Lee, C.-W. (2018). Mechanism of Lakoochin A Inducing Apoptosis of A375.S2 Melanoma Cells through Mitochondrial ROS and MAPKs Pathway. International Journal of Molecular Sciences, 19(9), 2649. https://doi.org/10.3390/ijms19092649





