Synthesis, Evaluation for Cytotoxicity and Molecular Docking Studies of Benzo[c]furan-Chalcones for Potential to Inhibit Tubulin Polymerization and/or EGFR-Tyrosine Kinase Phosphorylation
Abstract
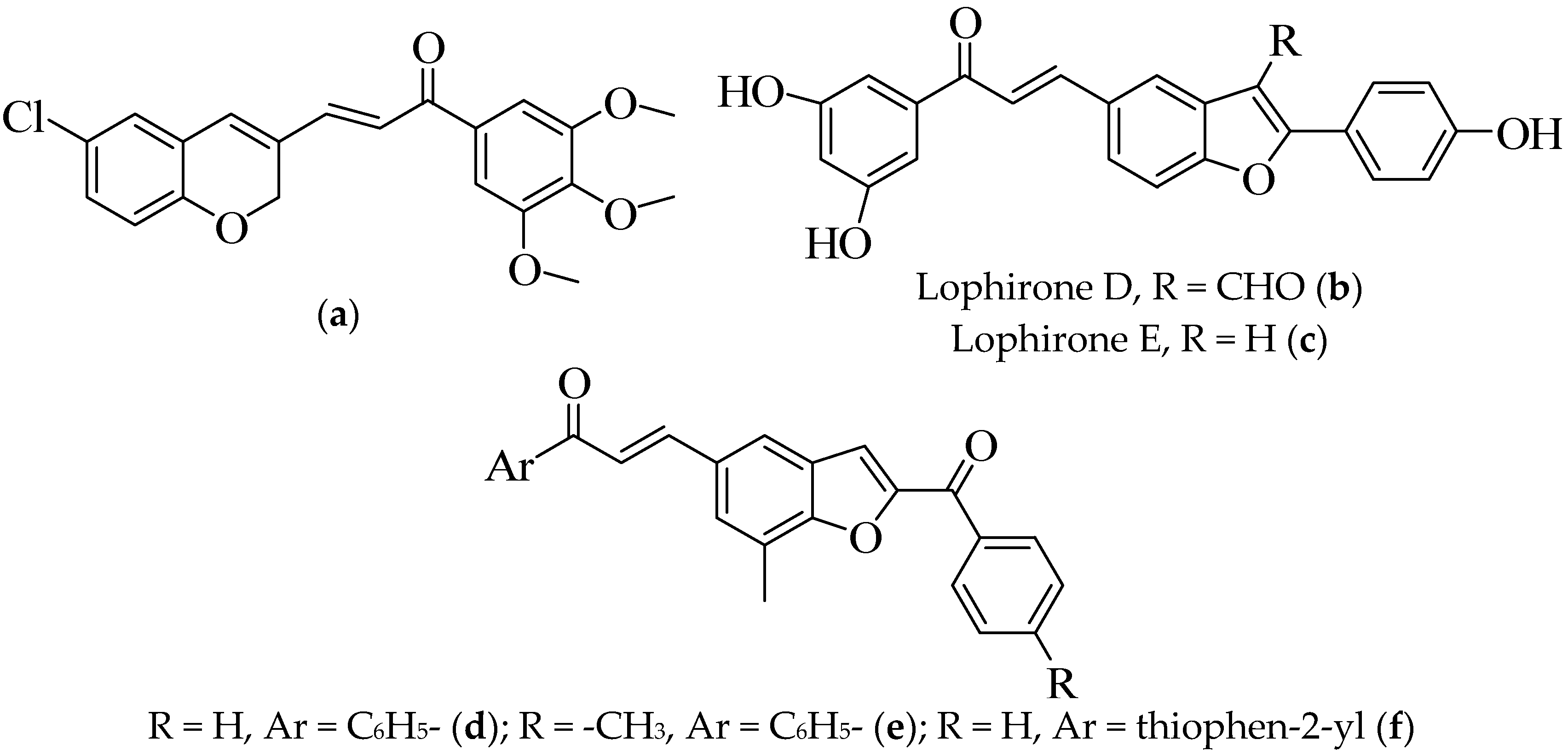
1. Introduction
2. Results and Discussion
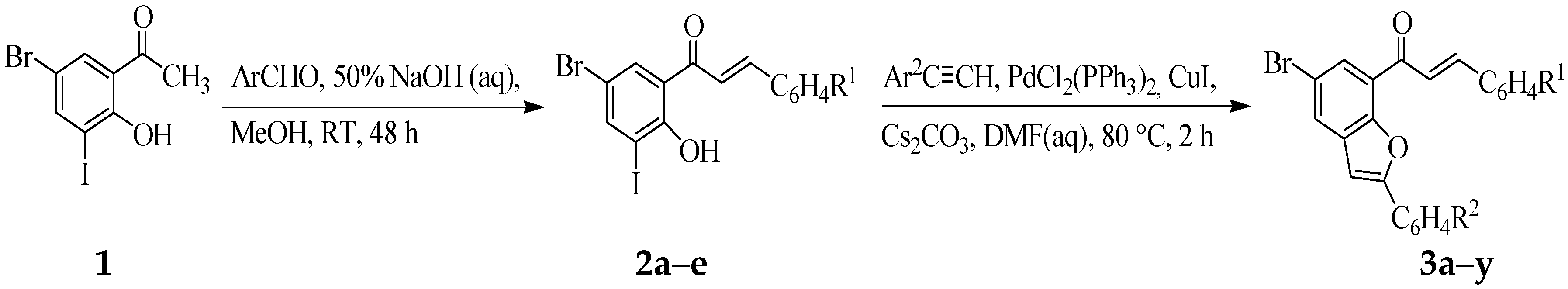
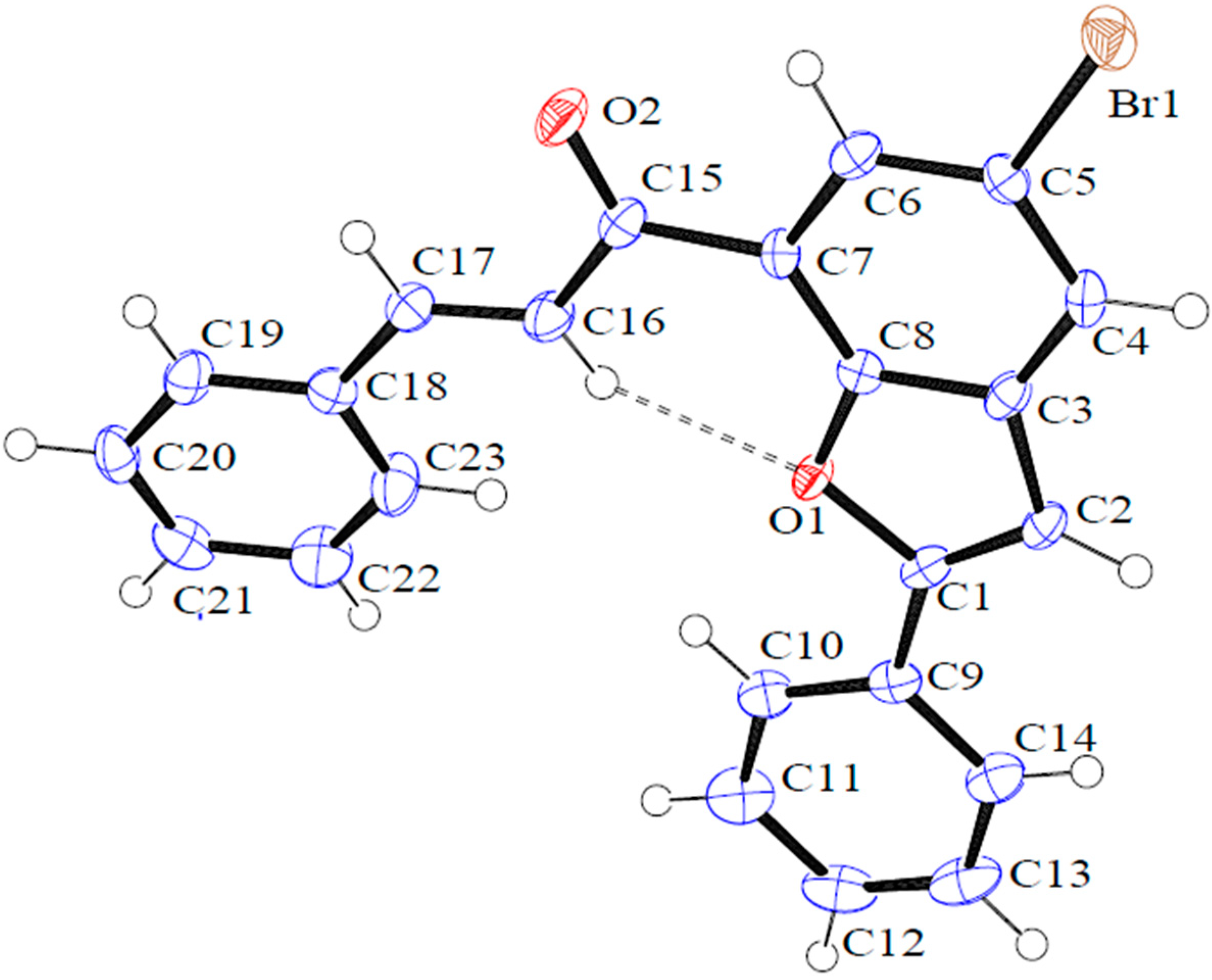
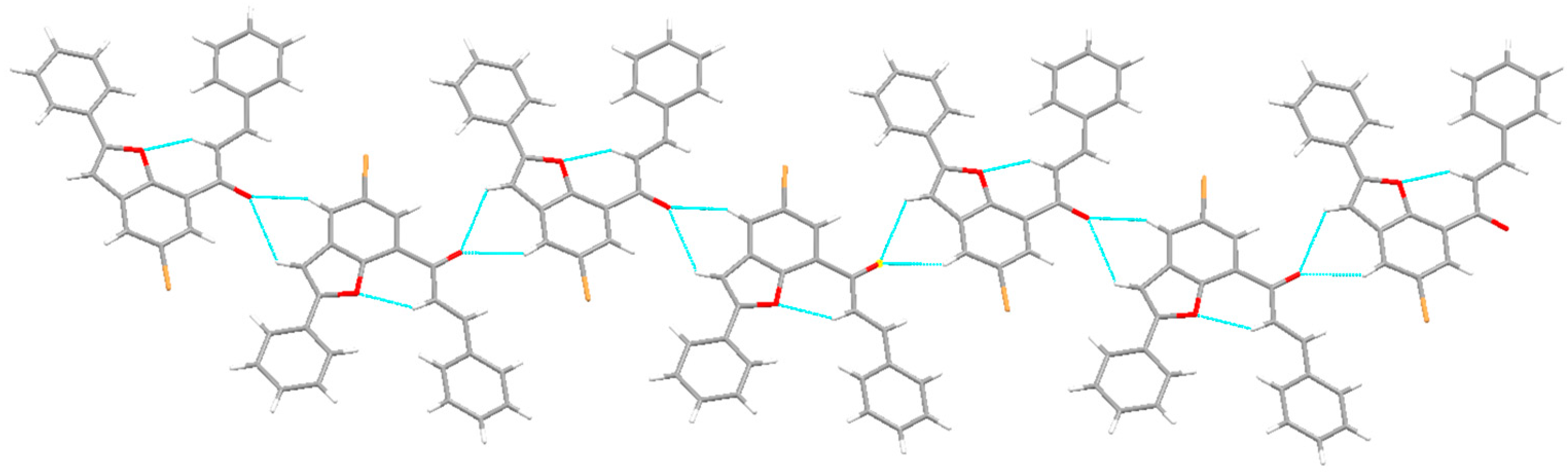
2.1. Chemistry
2.2. Biological Studies
2.2.1. Cytotoxicity Studies and SAR Analysis
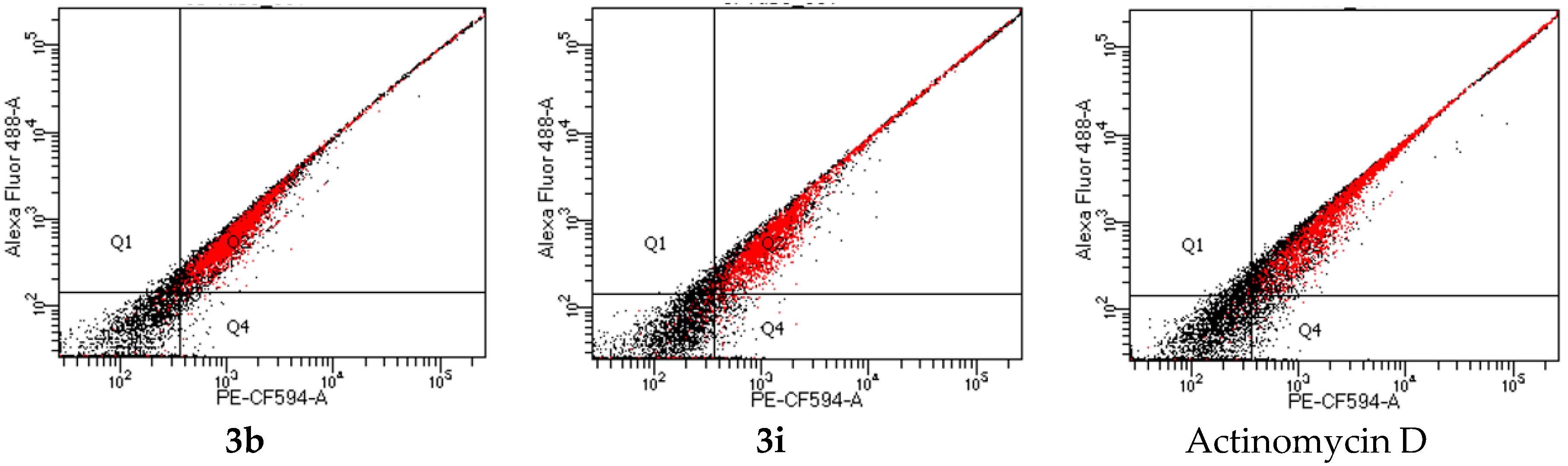
2.2.2. Evaluation of Cell Death Pathway by Annexin V-Cy3 SYTOX Staining Assay
2.2.3. Effect of Compounds 3a–y on Tubulin Polymerization
2.2.4. Evaluation of Compounds 3b, 3i, 3o, 3p, and 3v for Potential to Inhibit EGFR-TK Phosphorylation
2.3. Molecular Docking Studies
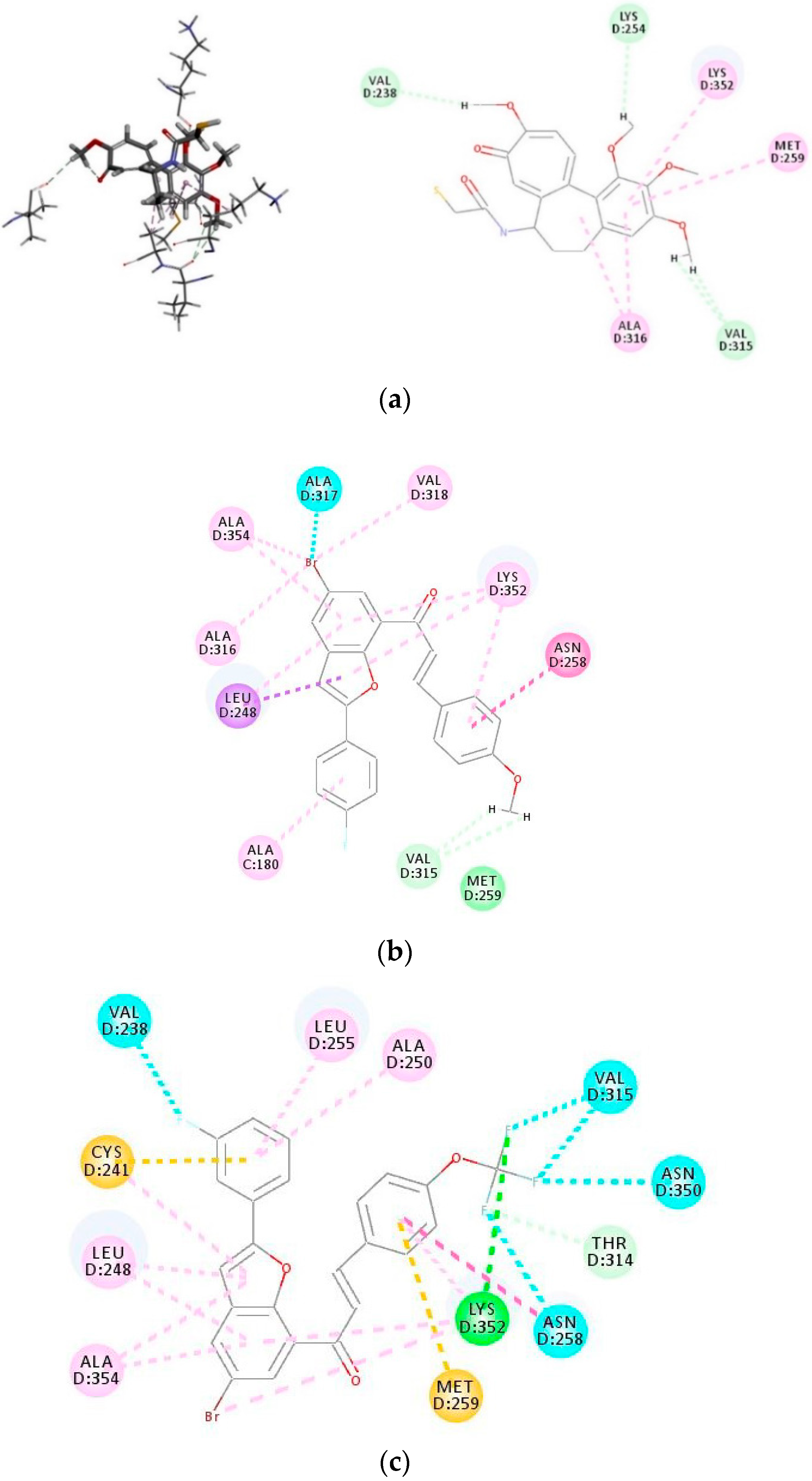
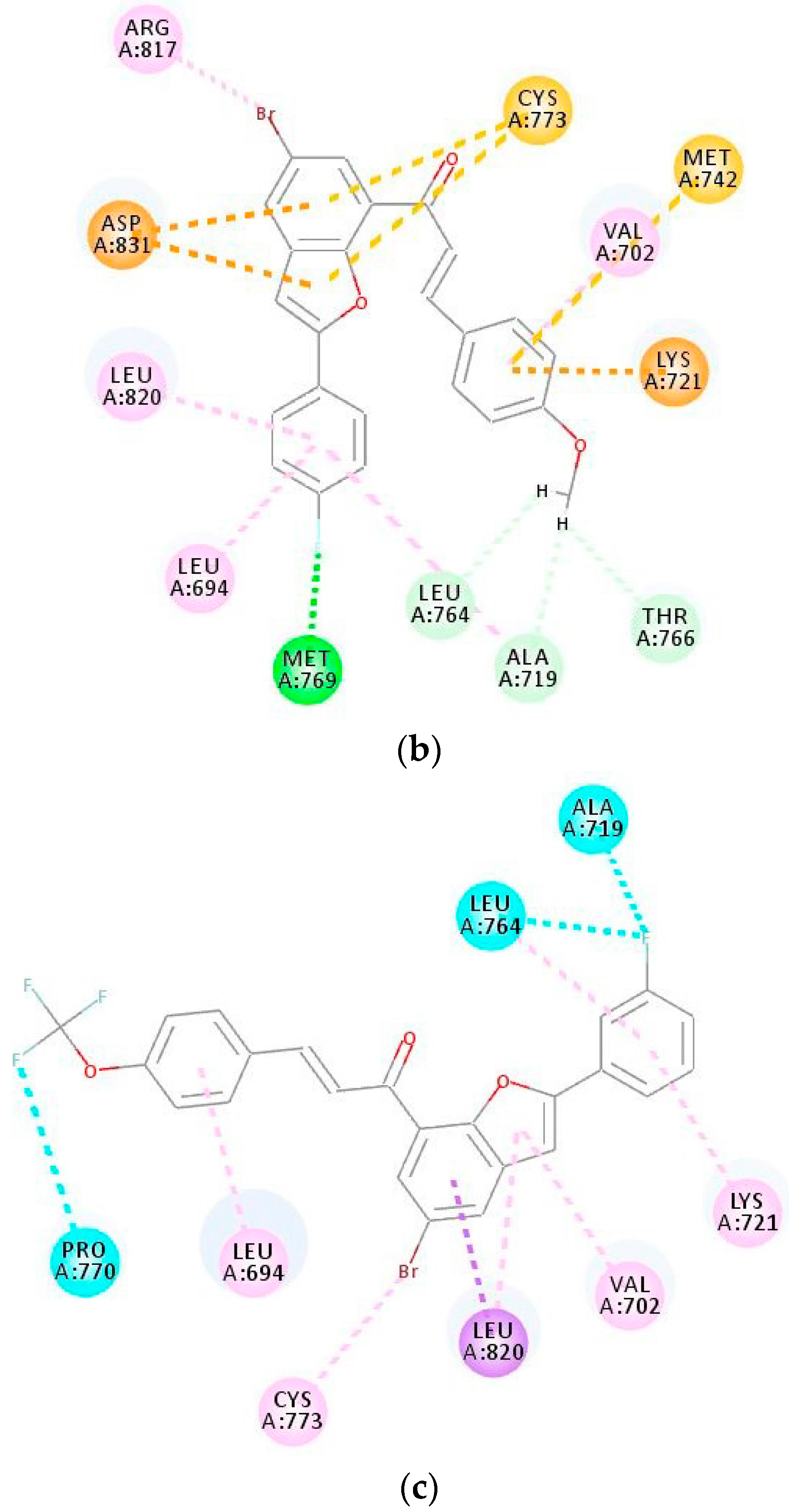
2.3.1. Molecular Docking of 3i and 3o into Tubulin
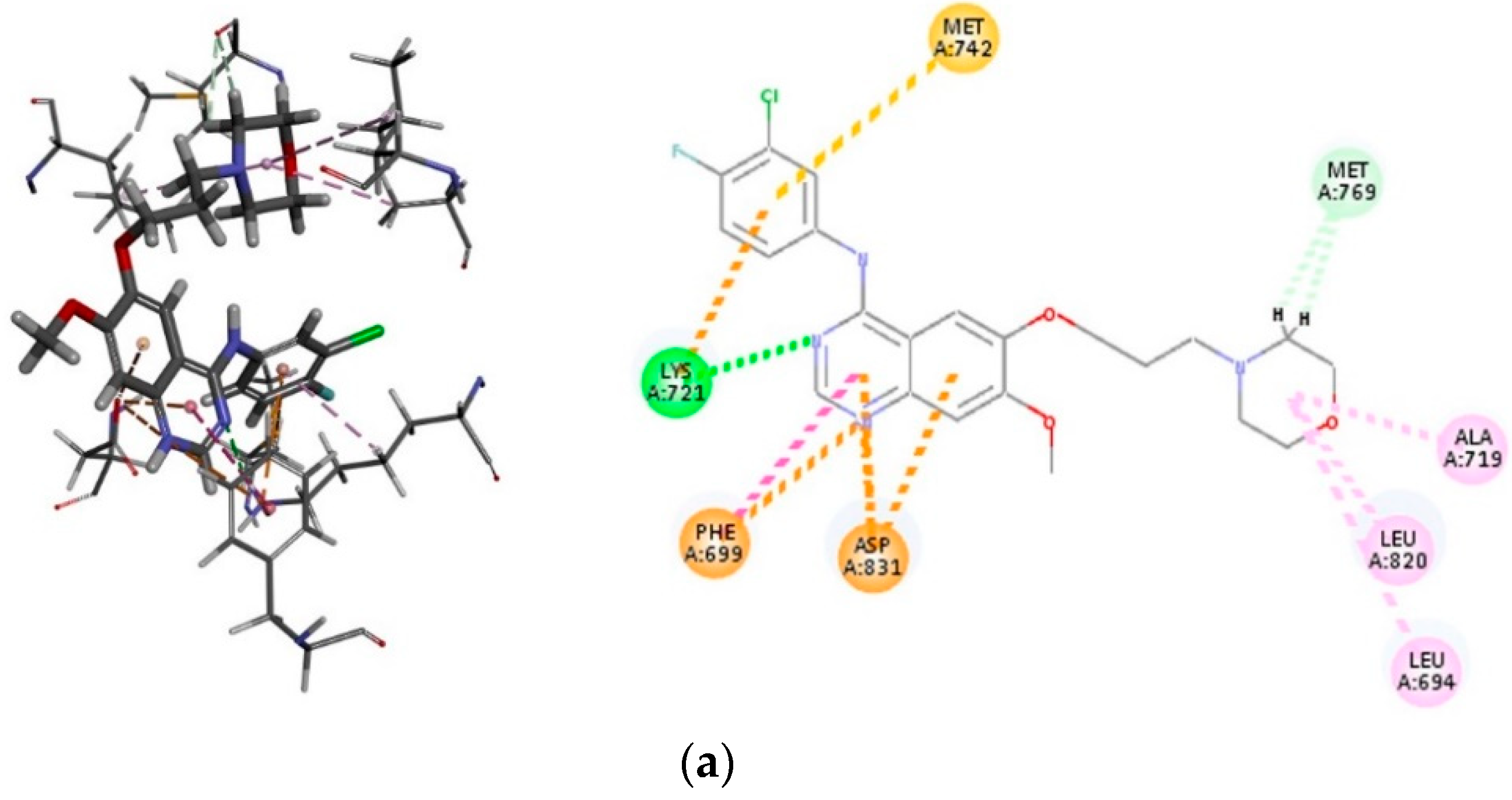
2.3.2. Molecular Docking of Compounds 3i and 3o into the EGFR-TK Active Site
3. Materials and Methods
3.1. General
3.2. One-Pot Synthesis of 5-Bromo-2-hydroxy-3-iodoacetophenone (1)
3.3. Typical Procedure for the Synthesis of Chalcone Derivatives 2a–e
3.4. Typical Procedure for the Synthesis of Benzofuran-Chalcone Hydrids 3a–y
3.5. Materials and Methods for the In Vitro Cytotoxicity Assay
3.5.1. Cell Culturing
3.5.2. Cytotoxicity against the MCF-7 Cell Line
3.6. Apoptosis Assays
3.6.1. Determination of Apoptotic Cell Death by Annexin V-Cy3 SYTOX Staining Assay
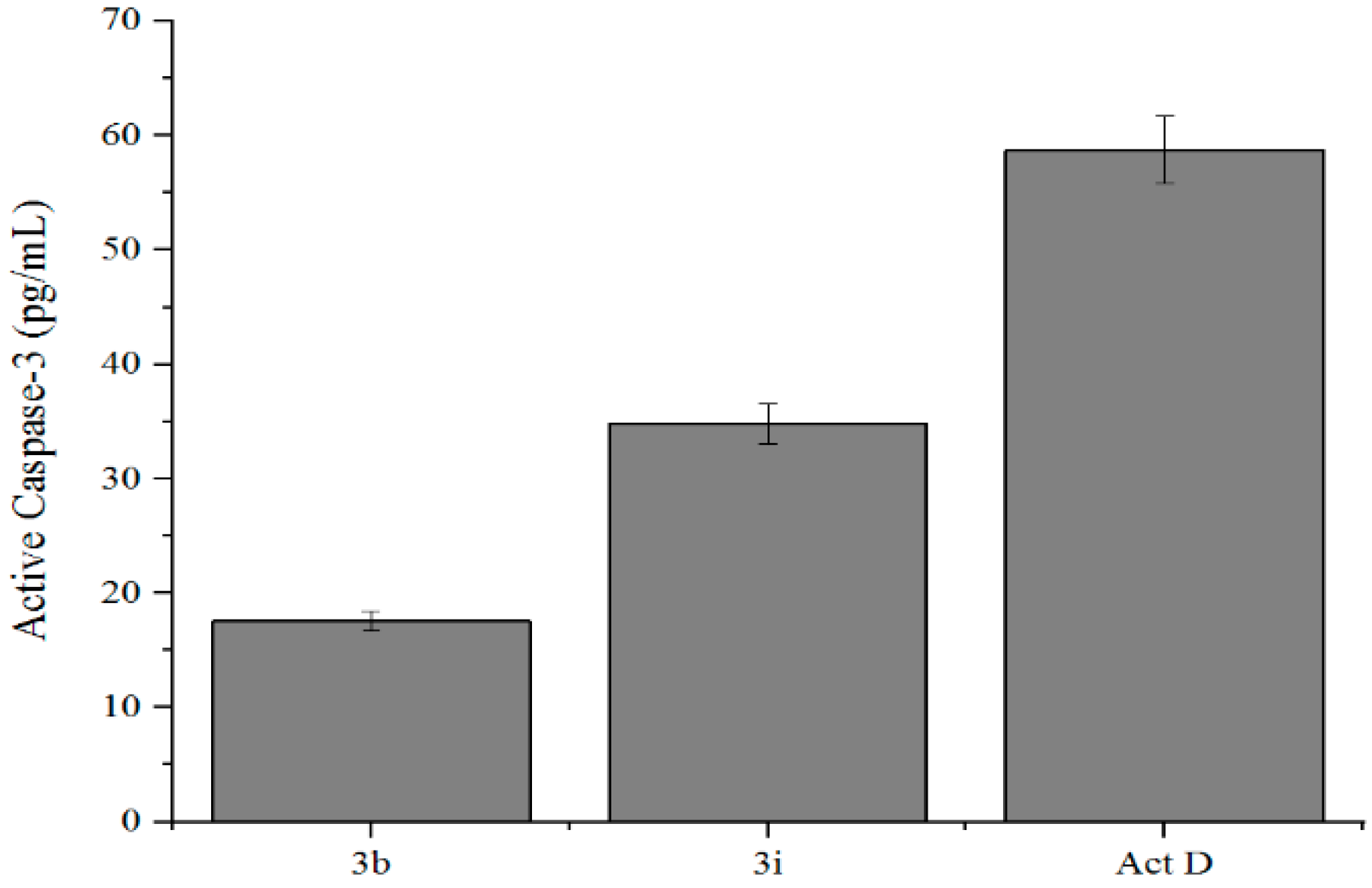
3.6.2. Caspase-3 Activation
3.7. Tubulin Polymerization Assay
3.8. EGFR-TK Phosphorylation Inhibition Assays
3.9. Molecular Docking of Compounds 3i and 3o against Tubulin and EGFR
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.J.; Donehower, R.C.; Rowinsky, E.K. Treatment of metastatic breast cancer with combination paclitaxel/cyclophosphamide. Semin. Oncol. 1995, 22, 23–27. [Google Scholar] [PubMed]
- Singh, P.; Anand, A.; Kumar, P. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Ducki, S. The development of chalcones as promising anticancer agents. Idrugs 2007, 10, 42–46. [Google Scholar] [PubMed]
- Mirzaei, H.; Emami, S. Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity. Eur. J. Med. Chem. 2016, 121, 610–639. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Aryapour, H.; Riazi, G.H.; Ahmadian, S.; Foroumadi, A.; Mahdavi, M.; Emami, S. Induction of apoptosis through tubulin inhibition in human cancer cells by new chromene-based chalcones. Pharm. Biol. 2012, 50, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Cara, C.L.; Cruz-Lopez, O.; Tolomeo, M.; Grimaudo, S.; Cristina, A.D.; Pipitone, M.R.; Balzarini, J.; et al. Design, synthesis and structure-activity relationship of 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]furan derivatives as a novel class of inhibitors of tubulin polymerization. Bioorg. Med. Chem. 2009, 17, 6862–6871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xing, C. Diverse molecular targets for chalcones with varied bioactivities. Med. Chem. 2015, 5, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Galal, S.A.; Abd El-All, A.S.; Abdallah, M.M.; El Diwani, H.I. Synthesis of potent antitumor and antiviral benzofuran derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Karim, S.S.; Anwar, M.M.; Mohamed, N.A.; Nasr, T.; Elseginy, S.A. Design, synthesis, biological evaluation and molecular docking studies of novel benzofuran-pyrazole derivatives as anticancer agents. Bioorg. Chem. 2015, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M.F. Synthesis, characterization, and anticancer activity of new benzofuran substituted chalcones. J. Chem. 2016, 2016, 7678486. [Google Scholar] [CrossRef]
- Coskun, D.; Erkisa, M.; Ulukaya, E.; Coskun, M.F.; Ari, F. Novel 1-(7-ethoxy-1-benzofuran-2-yl) substituted chalcone derivatives: Synthesis, characterization and anticancer activity. Eur. J. Med. Chem. 2017, 136, 212–222. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- More, K.R.; Mali, R.S. An alternate method for the synthesis of 2-aryl/alkyl-5-bromo-7-methoxy benzofurans; application to the synthesis of Egonol, Homoegonol, and analogs via Heck reaction. Tetrahedron 2016, 72, 7496–7504. [Google Scholar] [CrossRef]
- Tih, R.G.; Sondengmi, B.L.; Martin, M.T.; Bodo, B. Lophirones D and E: Two new cleaved bioflavonoids from Lophira lanceolate. J. Nat. Prod. 1989, 52, 284–288. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Modukuri, R.M.; Jadiya, P.; Dodda, R.P.; Kumar, M.; Sridhar, B.; Kumar, V.; Haque, R.; Siddiqi, M.I.; Nazir, A. Benzofuran-chalcone hybrids as potential multifunctional agents against Alzheimer’s disease: Synthesis and in vivo studies with transgenic Caenorhabditis elegans. ChemMedChem 2014, 9, 2671–2684. [Google Scholar] [CrossRef] [PubMed]
- Khansole, S.V.; Mokle, S.S.; Sayyed, M.A.; Vibhute, Y.B. Pyridinium iodochloride: An efficient reagent for iodination of hydroxylated aromatic ketones and aldehydes. J. Chin. Chem. Soc. 2008, 55, 871–874. [Google Scholar] [CrossRef]
- Arcadi, A.; Blesi, F.; Cacchi, S.; Fabrizi, G.; Goggiamani, A. 2,5,7-Trisubstituted benzo[b]furans through a copper- and/or palladium-catalyzed assembly and functionalization process. Tetrahedron Lett. 2011, 52, 5149–5152. [Google Scholar] [CrossRef]
- Arcadi, A.; Blesi, F.; Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. Multisubstituted benzo[b]furans through a copper- and/or palladium-catalyzed assembly and functionalization process. Tetrahedron 2013, 69, 1857–1871. [Google Scholar] [CrossRef]
- Thévenin, M.; Thoret, S.; Grellier, P.; Dubois, J. Synthesis of polysubstituted benzofuran derivatives as novel inhibitors of parasitic growth. Bioorg. Med. Chem. 2013, 21, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Hwang, J.W.; Lee, H.S.; Yang, D.M.; Jun, J.-G. Highly effective total synthesis of benzofuran natural product egonol. Bull. Korean Chem. Soc. 2008, 29, 1594–1596. [Google Scholar]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar] [CrossRef]
- Maluleka, M.M.; Mphahlele, M.J. Available online: https://www.ccdc.cam.ac.uk/Community/Depositastructure (accessed on 2 March 2018).
- Kleeff, J.; Kornmann, M.; Sawhney, H.; Korc, M. Actinomycin D induces apoptosis and inhibits growth of pancreatic cancer cells. Int. J. Cancer 2000, 86, 399–407. [Google Scholar] [CrossRef]
- Dyrager, C.; Wickstrom, M.; Friden-Saxin, M.; Friberg, A.; Dahlén, K.; Wallén, E.A.A.; Gullbo, J.; Grøtli, M.; Luthman, K. Inhibitors and promoters of tubulin polymerization: Synthesis and biological evaluation of chalcones and related dienones as potential anticancer agents. Bioorg. Med. Chem. 2011, 19, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.B.; Guo, Y.J.; Zhang, K.; Chen, Y.Z.; Mack, P. Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim. Biophys. Acta 2001, 1550, 144–152. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, C.L. Epidermal growth factor receptor dependence in human tumors: More than just expression? Oncologist 2002, 7, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Comşa, Ş.; Cîmpean, A.M.; Raica, M. The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of chalcones with anticancer activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Janicke, R.U.; Schulze-Osthoff, K. Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 2003, 10, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef] [PubMed]
- Nam, N. Combretastatin A-4 analogues as antimitotic antitumor agents. Curr. Med. Chem. 2003, 10, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Alloatti, D.; Gianini, G.; Cabri, W.; Lustrati, I.; Maezi, M.; Ciacci, A.; Gallo, G.; Tinti, M.O.; Marcellini, M.; Riccioni, T.; et al. Synthesis and biological activity of fluorinated Combretastatin analogues. J. Med. Chem. 2008, 51, 2708–2721. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Nam, J.-Y.; Han, M.Y.; Lee, E.K.; Choi, J.-D.; Bok, S.H.; Kwon, B.-M. Actinomycin D as a novel SH2 domain ligand inhibits Shc/Grb2 interaction in B104-1-1 (neu*-transformed NIH3T3) and SAA (hEGFR-overexpressed NIH3T3) cell. FEBS Lett. 1999, 453, 174–178. [Google Scholar] [CrossRef]
- Ismail, R.S.M.; Ismail, N.S.M.; Abuserii, A.; El Ella, D.A.A. Recent advances in 4-aminoquinazoline based scaffold derivatives targeting EGFR kinases as anticancer agents. Future J. Pharm. Sci. 2016, 2, 9–19. [Google Scholar] [CrossRef]
- Choowongkomon, K.; Orathai Sawatdichaikul, O.; Songtawee, N.; Limtrakul, J. Receptor-based virtual screening of EGFR kinase inhibitors from the NCI diversity database. Molecules 2010, 15, 4041–4054. [Google Scholar] [CrossRef] [PubMed]
- Pemg, Y.-H.; Shiao, H.-Y.; Tu, C.-H.; Liu, P.-M.; Hsu, J.T.-A.; Amancha, P.K.; Wu, J.-S.; Coumar, M.S.; Chen, C.-H.; Wang, S.-Y.; et al. Protein kinase inhibitor design by targeting the Asp-Phe-Gly (DFG) motif: The role of the DFG motif in the design of epidermal growth factor receptor inhibitors. J. Med. Chem. 2013, 56, 3889–3903. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, M.R.; Vander Zanden, C.M.; Carter, M.; Ho, P.S. Halogen bonding (X-bonding): A biological perspective. Protein Sci. 2013, 22, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.J. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mphahlele, M.J.; Paumo, H.K.; Choong, Y.S. Synthesis and in vitro cytotoxicity of the 4-(halogenoanilino)-6-bromoquinazolines and their 6-(4-fluorophenyl) substituted derivatives as potential inhibitors of epidermal growth factor receptor tyrosine kinase. Pharmaceuticals 2017, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E.; Wolf, S.G.; Downing, K.H. Atomic structure of tubulin and FtsZ. Nature 1998, 391, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Robertson, D.H.; Brooks, C.L., III; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Fu, L.-W. Mechanisms of acquired resistance to tyrosine kinase inhibitors. Acta Pharm. Sin. B 2011, 1, 197–207. [Google Scholar] [CrossRef]
| R1 | Substitution Pattern of Compounds 2a–e & 3a–y | |||||
|---|---|---|---|---|---|---|
| 2 | 3a–e (R2) | 3f–j (R2) | 3k–o (R2) | 3p–t (R2) | 3u–y (R2) | |
| 4-H | 2a | 3a (H) | 3f (4-F) | 3k (3-F) | 3p (3-Cl) | 3u (4-OMe) |
| 4-F | 2b | 3b (H) | 3g (4-F) | 3l (3-F) | 3q (3-Cl) | 3v (4-OMe) |
| 4-Cl | 2c | 3c (H) | 3h (4-F) | 3m (3-F) | 3r (3-Cl) | 3w (4-OMe) |
| 4-OMe | 2d | 3d (H) | 3i (4-F) | 3n (3-F) | 3s (3-Cl) | 3x (4-OMe) |
| 4-OCF3 | 2e | 3e (H) | 3j (4-F) | 3o (3-F) | 3t (3-Cl) | 3y (4-OMe) |
| Compounds | MCF-7 Cell Line IC50 (μM) ± SD |
|---|---|
| 3a | 47.96 ± 2.10 |
| 3b | 0.55 ± 0.24 |
| 3c | >100 |
| 3d | 22.78 ± 0.86 |
| 3e | 0.59 ± 0.21 |
| 3f | 35.81 ± 1.20 |
| 3g | 48.59 ± 2.10 |
| 3h | 54.16 ± 0.40 |
| 3i | 3.55 × 10−4 ± 0.07 |
| 3j | 16.00 ± 0.62 |
| 3k | 23.01 ± 0.38 |
| 3l | >100 |
| 3m | 14.75 ± 0.33 |
| 3n | 39.25 ± 0.42 |
| 3o | 7.87 ± 0.25 |
| 3p | 12.63 ± 0.23 |
| 3q | 23.82 ± 0.29 |
| 3r | 66.75 ± 0.84 |
| 3s | 2.69 ± 0.23 |
| 3t | 26.62 ± 0.27 |
| 3u | 48.96 ± 0.30 |
| 3v | 28.43 ± 0.46 |
| 3w | 30.62 ± 0.35 |
| 3x | 69.09 ± 0.76 |
| 3y | 43.17 ± 0.28 |
| Actinomycin D | 37.82 ± 1.30 |
| Compound | %Viable Cells (Q3) | %Early Apoptosis (Q4) | %Late Apoptosis (Q2) | %Necrosis (Q1) |
|---|---|---|---|---|
| 3b | 23.3 | 1.5 | 72 | 3.2 |
| 3i | 32.2 | 3.1 | 61.7 | 3.1 |
| Actinomycin D | 30.7 | 3.4 | 61.4 | 4.5 |
| Compounds | IC50 (µM) | S.D. |
|---|---|---|
| 3b | 26.5 | ±0.17 |
| 3e | 2.54 | ±0.24 |
| 3i | 5.51 × 10−5 | ±0.39 |
| 3j | 8.85 × 10−3 | ±0.42 |
| 3o | 1.76 × 10−4 | ±0.11 |
| 3p | 9.37 × 10−2 | ±0.21 |
| 3s | 0.71 | ±0.30 |
| 3v | 1.62 × 10−2 | ±0.12 |
| Colchicine | 9.88 × 10−2 | ±0.17 |
| Compound | EGFR, IC50 (μM) |
|---|---|
| 3b | 0.17 ± 0.03 |
| 3i | 0.09 ± 0.03 |
| 3o | 0.12 ± 0.04 |
| 3p | 0.17 ± 0.06 |
| 3v | 0.15 ± 0.02 |
| Actinomycin D | 0.04 ± 0.03 |
| Gefitinib | 0.03 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphahlele, M.J.; Maluleka, M.M.; Parbhoo, N.; Malindisa, S.T. Synthesis, Evaluation for Cytotoxicity and Molecular Docking Studies of Benzo[c]furan-Chalcones for Potential to Inhibit Tubulin Polymerization and/or EGFR-Tyrosine Kinase Phosphorylation. Int. J. Mol. Sci. 2018, 19, 2552. https://doi.org/10.3390/ijms19092552
Mphahlele MJ, Maluleka MM, Parbhoo N, Malindisa ST. Synthesis, Evaluation for Cytotoxicity and Molecular Docking Studies of Benzo[c]furan-Chalcones for Potential to Inhibit Tubulin Polymerization and/or EGFR-Tyrosine Kinase Phosphorylation. International Journal of Molecular Sciences. 2018; 19(9):2552. https://doi.org/10.3390/ijms19092552
Chicago/Turabian StyleMphahlele, Malose J., Marole M. Maluleka, Nishal Parbhoo, and Sibusiso T. Malindisa. 2018. "Synthesis, Evaluation for Cytotoxicity and Molecular Docking Studies of Benzo[c]furan-Chalcones for Potential to Inhibit Tubulin Polymerization and/or EGFR-Tyrosine Kinase Phosphorylation" International Journal of Molecular Sciences 19, no. 9: 2552. https://doi.org/10.3390/ijms19092552
APA StyleMphahlele, M. J., Maluleka, M. M., Parbhoo, N., & Malindisa, S. T. (2018). Synthesis, Evaluation for Cytotoxicity and Molecular Docking Studies of Benzo[c]furan-Chalcones for Potential to Inhibit Tubulin Polymerization and/or EGFR-Tyrosine Kinase Phosphorylation. International Journal of Molecular Sciences, 19(9), 2552. https://doi.org/10.3390/ijms19092552






