1. Introduction
Human platelet derived growth factor (PDGF) belongs to the glycoprotein dimer family, and has four isoforms: PDGF-A, PDGF-B, PDGF-C, and PDGF-D [
1]. The PDGFs specifically bind to the PDGF receptor (PDGFR) through a homodimer or heterodimer, such as PDGF-AA, PDGF-BB, or PDGF-AB, for molecular signal transition [
2]. Among these compounds, PDGF-BB, constructed of two PDGF-B chains is the most commonly found dimer in the human body. The molecular weight of PDGF-BB is 14 kDa, and it plays an important role in many processes, such as cell proliferation and wound healing, due to it is a strong activity as a mitogen for various cell types, especially vascular endothelial cells (VECs) and bone marrow mesenchymal stem cell (BMSCs) [
1]. In particular, when angiorrhexis occurs, the platelets can release PDGF-BB to recruit the perivascular cells and vessel smooth muscle cells and to promote VEC proliferation to facilitate vessel remolding. As for bone repairing, PDGF-BB can accelerate the differentiation of BMSCs into osteoblasts which can bring about more osteocytes to achieve bone repair [
3]. Thus, PDGF-BB is regarded as a desirable pharmaceutical and was recently approved by the FDA for osteochondritis (OCT) and cardiovascular disorder treatment in the clinical setting. In addition, through innovation in the cosmetics industry, PDGF-BB has also been considered a main ingredient in solving skin problems, including diminishing wrinkles and moisturizing [
4]. Thus, the clinical demand for PDGF-BB for a wide range of applications in biomedicine and bio-cosmetics has increased, drawing much attention to the recombinant expression of PDGF-BB. For decades, several attempts have been made to produce the recombinant PDGF-BB in the hosts of the yeasts
Pichia Pink [
5] and tobacco
N. tabacum L. [
6]. However, poor yields, complicated processing, and low bioactivity have severely limited their ability to meet the market’s demand, especially with the increasing number of applications for cell therapy and translational medicine. As a result, constructing an efficient strategy for the cost effective and mass production of recombinant PDGF-BB with native bioactivity is urgent and necessary.
The domesticated silkworm possesses a great ability to synthesize abundant silk proteins within the short term in silk glands and secrete them into silk to make cocoons. Thus, the mass production of recombinant proteins by transgenic silkworms is a desirable bioreactor [
7]. Silk proteins mainly consist of fibroin proteins (75%) and sericin proteins (25%) [
8]. The fibroin proteins are synthesized by the posterior silk glands cells which consist of fibroin heavy chains (H-chains), fibroin light chains (L-chains), and fibrohexamerins in a molar ratio of 6:6:1 [
9]. The sericin proteins are mainly encoded by the
sericin-1,
sericin-2, and
sericin-3 genes, respectively [
10]. The genetic transformation tool mediated by the
piggyBac transposon was successfully applied in silkworms in 2000 [
11]. The transgenic silkworm has been genetically engineered as an ideal bioreactor to express recombinant foreign proteins along with the synthesis of their silk proteins. Two major expression systems based on the usage of the fibroin and sericin-1 promoters have been successfully constructed [
12,
13]. Thereafter, more than ten exogenous proteins with various bio-functions and application potential have been successfully expressed in the silk glands of transgenic silkworms and cocoons, including human type III pro-collagen, human serum albumin, human acid fibroblast growth factor, and antibodies [
14,
15,
16]. These efforts have led to the silk glands of transgenic silkworm being very close to the ideal bioreactor organ to massively produce the recombinant pharmaceutical proteins to meet the increased demand of the clinic.
In this study, we provided an effective strategy to produce bioactive recombinant PDGF-BB proteins in silk cocoons of transgenic silkworm using our previous established
sericin-1 expression system [
17]. The recombinant PDGF-BB was specifically synthesized into the middle silk gland cells and secreted into the sericin lumen. The transcriptional and translational expression of the recombinant PDGF-BB was well studied. Furthermore, the recombinant PDGF-BB was purified from cocoons with 82% purity and 50.2% recovery rate, and the bioassays further indicated that the purified recombinant PDGF-BB possesses the bio-function to promote NIH/3T3 cell proliferation and migration. The results strongly suggest the potential of silk gland-based bioreactors of silkworm to cost effectively and massively produce these valuable recombinant proteins with natural bioactivity in silk cocoons.
3. Discussion
hPDGF-BB has shown therapeutic potential in a wide range of biological processes, such as wound healing [
20], vessel remolding [
21], and bone repair [
3]. The daily increased demands and cost expensive production of hPDGF-BB has aroused interest in large-scale production of recombinant hPDGF-BB in biomedicine and therapeutics.
The silk glands of the silkworm, which was domesticated for over thousand years, possess a huge ability to synthesize an abundant number of silk proteins in a very short period of 5 to 6 days in the fifth instar. Thus, it has been regarded as an desirable bioreactor for producing exogenous proteins of interest in the silk glands of transgenic silkworms [
7]. Here, we reported the successful production of recombinant hPDGF-BB in the sericin layer of cocoons by transgenic silkworms. To achieve the adaptative and considerable expression of PDGF-BB in silkworm, the coding sequence of PDGF-BB was optimized according to the silkworm codon usage bias and inserted into the previously-optimized
sericin-1 expression system with potent activity [
17]. The construct DNA was microinjected into the silkworm eggs to hereditably integrate into the silkworm genome, with a transformation frequency of 38% which is considerable higher than several previous reports [
14,
16,
22,
23,
24]. This high transformation frequency was probably caused by the higher activity of the hsp70 promoter used to transiently express the
piggyBac transposase in the silkworm embryos [
25] than that of the commonly used BmAct3 promoter [
11], which has been proven and used to achieve higher expression of Cas9 proteins for efficient genome edit in silkworm elsewhere [
26].
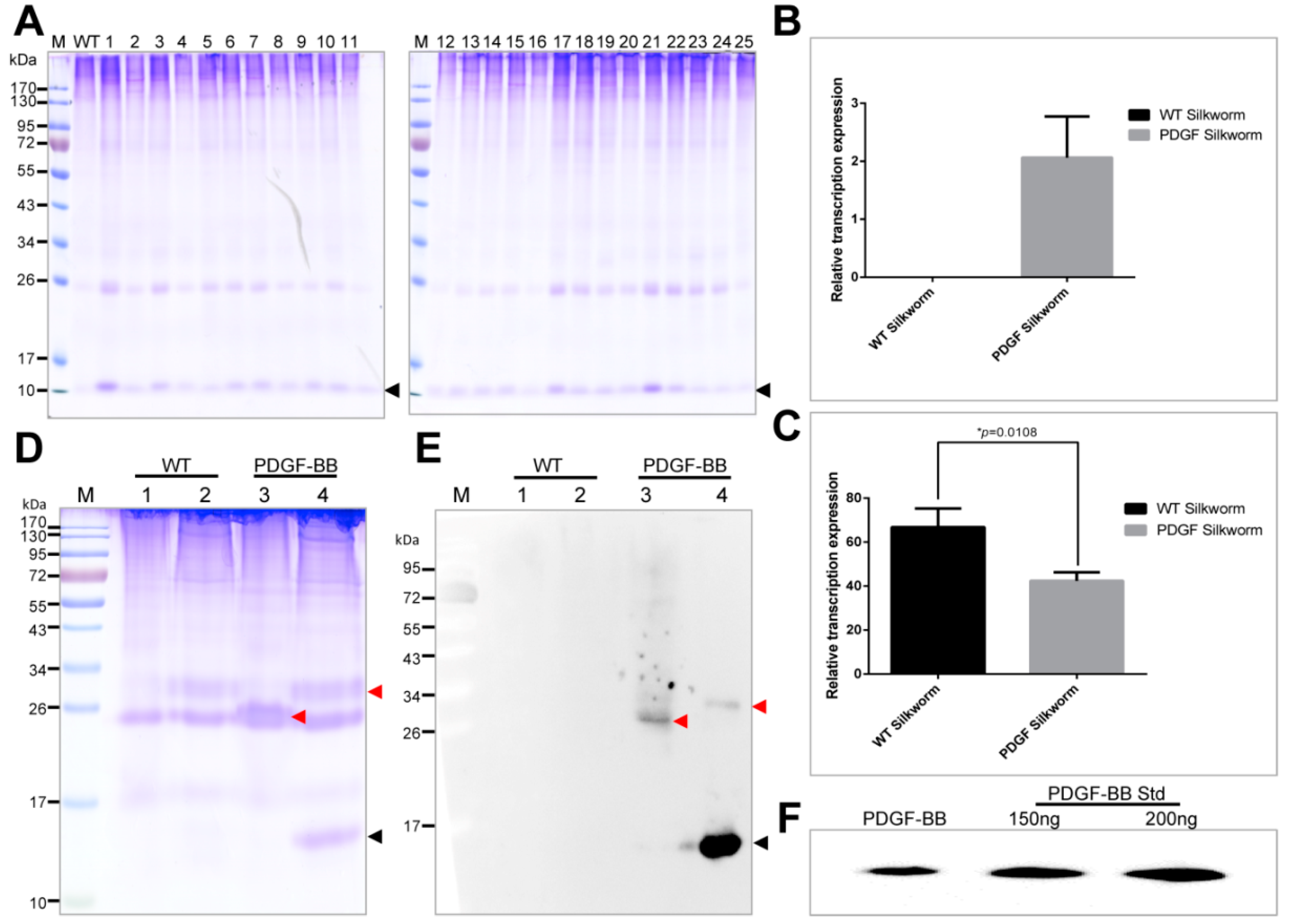
The PDGF-BB was successfully expressed in the cocoons with a molecular weight accordant to the theory from the positive silkworm individuals. However, their expression levels varied dramatically among the 25 positive silkworm individuals, suggesting the expression of PDGF-BB in different silkworm individuals was severely affected by the chromosome “position affect” caused by the
piggyBac transposase-mediated random integration into the silkworm genome, which had been reported in several previous studies [
12,
13,
16,
27,
28]. The PDGF-21 strain with the highest expression of PDGF-BB contained a single copy insertion of
piggyBac at an intergenic region in the silkworm genome far away from the neighboring endogenous genes. This might help to avoid the effect of chromosome “position affect” on the expression of PDGF-BB. The transcription expression of the
PDGF-B gene in the PDGF-21 strain competitively decreased that of the endogenous
sericin-1 gene. However, there are no obvious differences in the protein band intensities of sericins from the normal and transgenic silkworm cocoons in SDS-Page. Since the sericins could lubricate the flow of fibroin during spinning processing of silk fiber [
29]. If the synthesized sericins proteins decrease severely, it may affect the spinning processing of silk fiber and reduce the silk production. However, we did not observe the abnormal spinning processing of silk fiber in the transgenic silkworm, and the decrease of the economic characteristics of cocoons from the transgenic silkworms. Thus, we speculated that the protein level of sericins was not significantly affected or slightly decreased by the over-expression of PDGF-BB in transgenic silkworm and revealed the potential discordant regulations of the
sericin-1 gene on the transcriptional and translational levels.
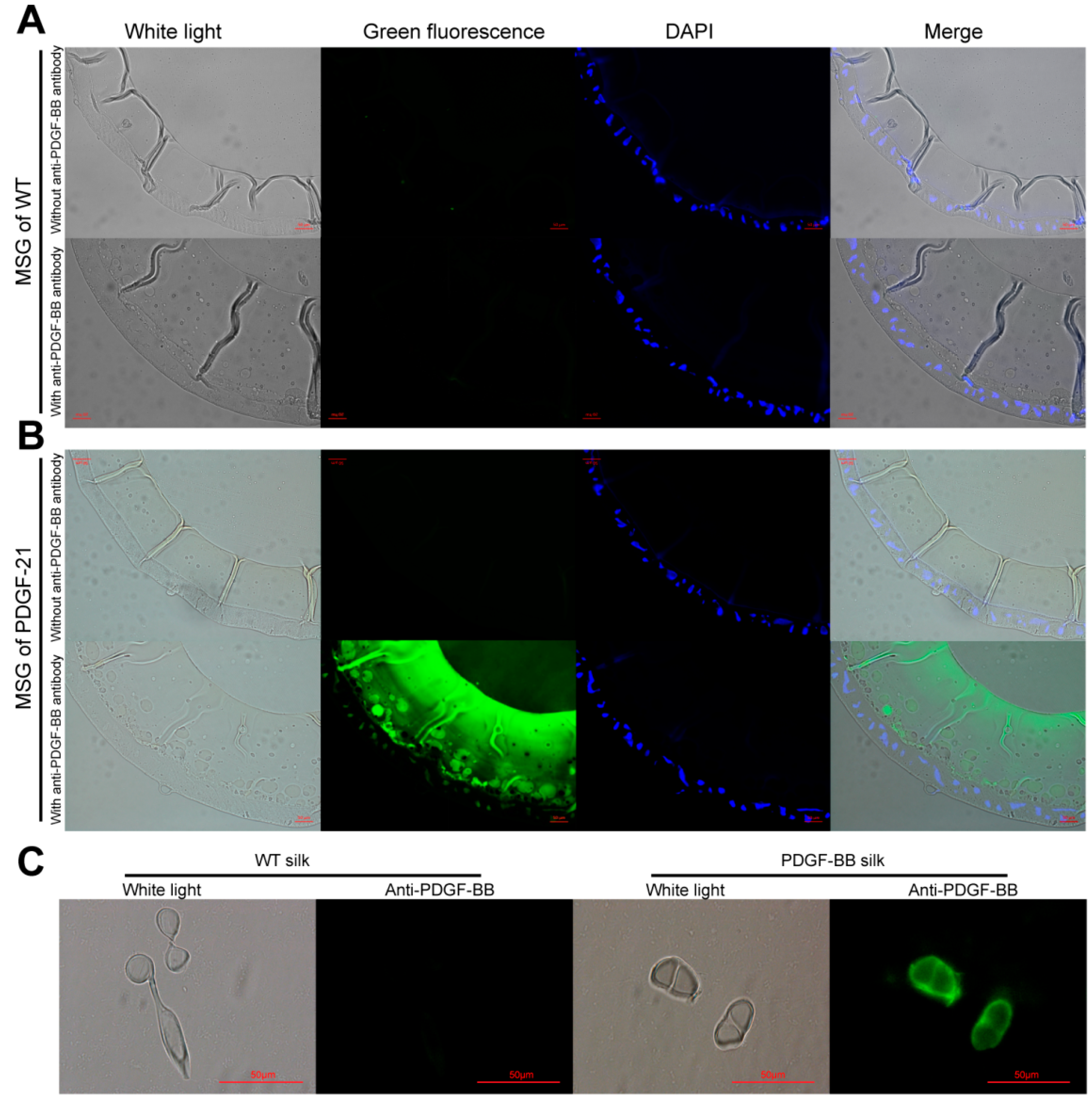
The synthesis, secretion, and spinning processes of the recombinant PDGF-BB proteins were visualized, showing that the synthesized PDGF-BB proteins were specifically secreted into the sericin layer of the MSG lumen, and then spun along with the sericins to form the silk thread of cocoon and finally, they were distributed in the sericin layer of silk. This result is consistent with our previous reports [
15,
30]. The homodimer forms of PDGF-BB in the PDGF-21 strain were analyzed, which strongly indicated that the PDGF-BB recombinant protein synthesized in the middle silk gland of transgenic silkworm could form the homodimer by the intermolecular disulfide bond. Remarkable, the homodimer of the recombinant PDGF-BB was stable even under a harsh extraction conditionof8 M urea and high temperature, which probably benefited from the well protection from the sericin. This phenomenon had also been illustrated by several other literatures which had revealed that the sericin and fibroin in silk fibers might functioned as a stabilizer for the long-term stabilities of the collagen [
31], blood components [
32], and antibodies [
33]. Thus the specific advantage of silk fiber as a stabilizer might be applied for the silk gland based bioreactor for the long-term stability and storage of recombinant proteins in the cocoons, which is lacking in other expression systems such as
E. coli and yeast.
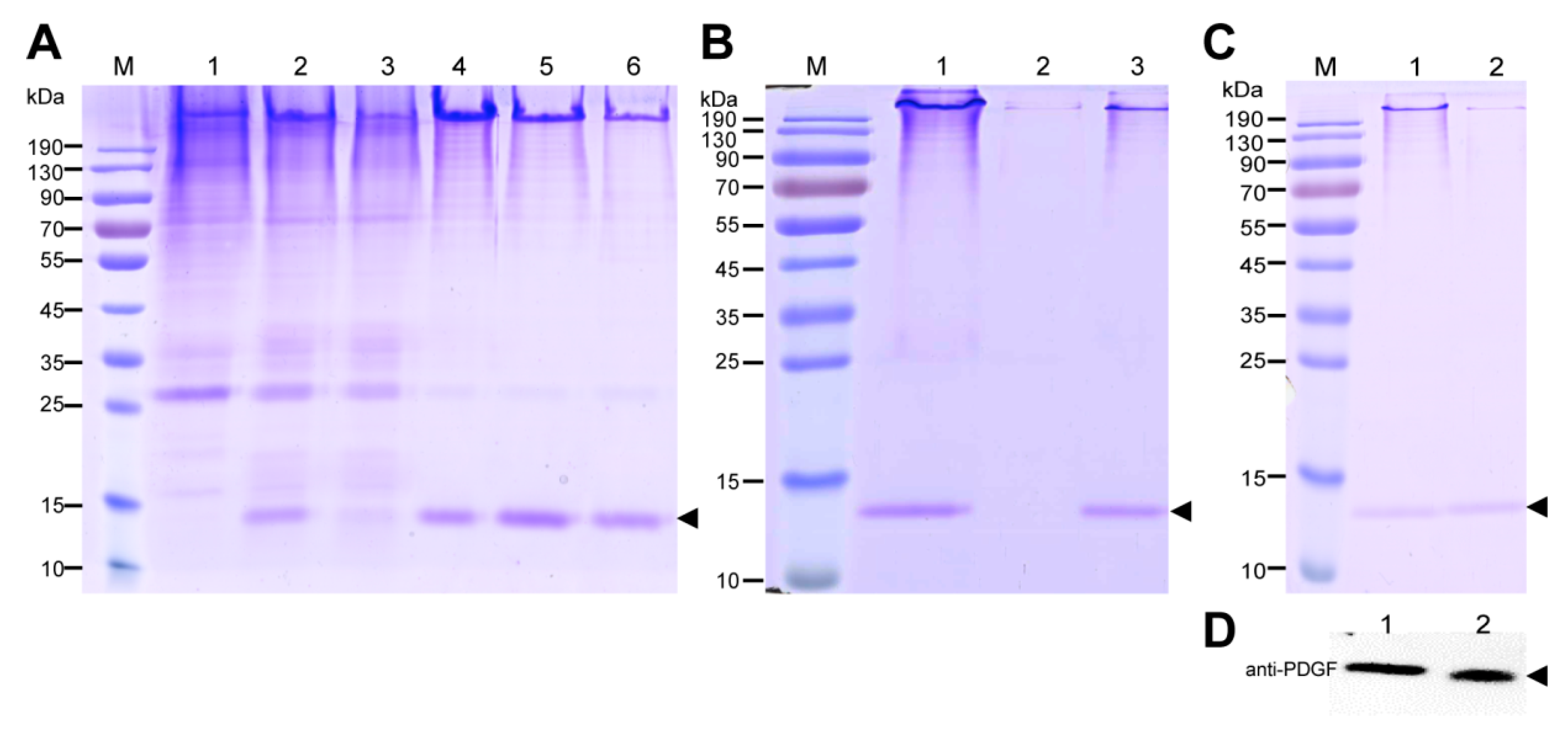
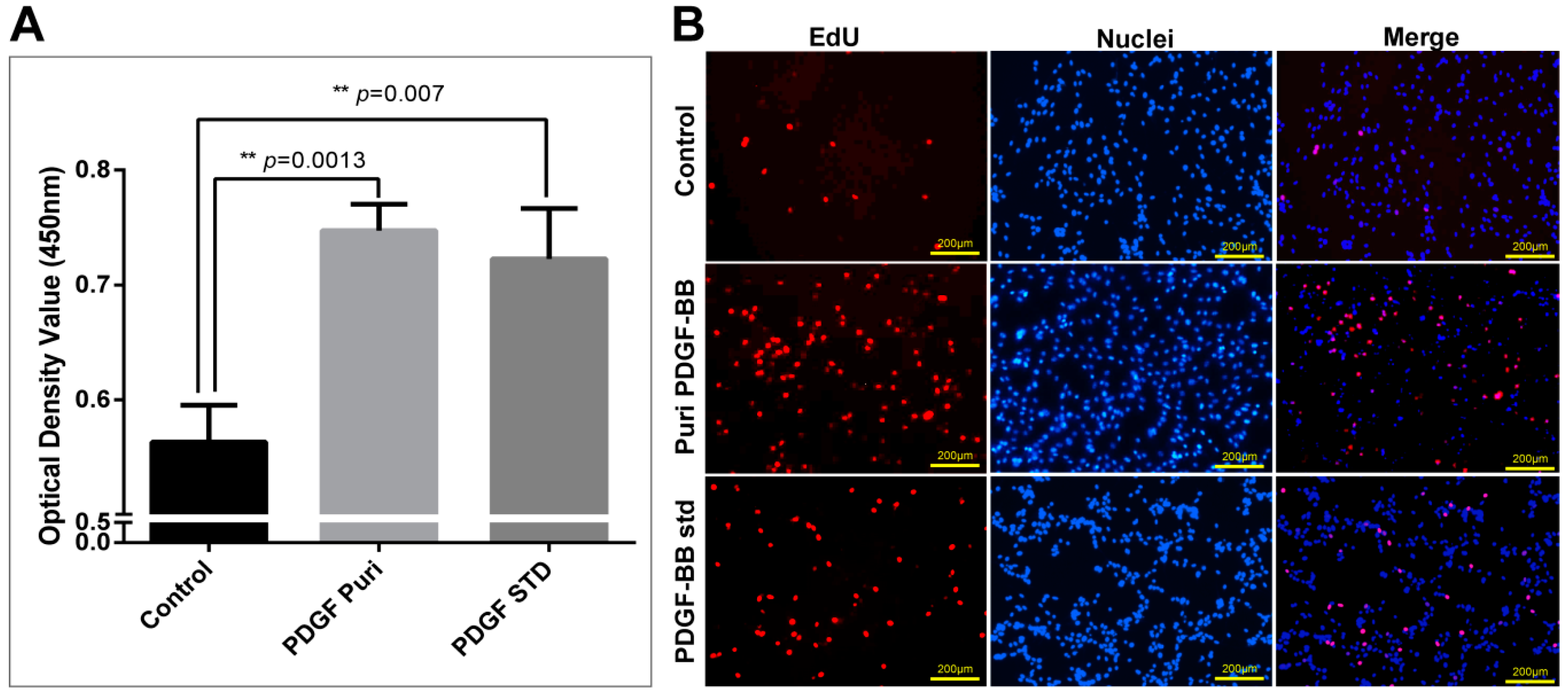
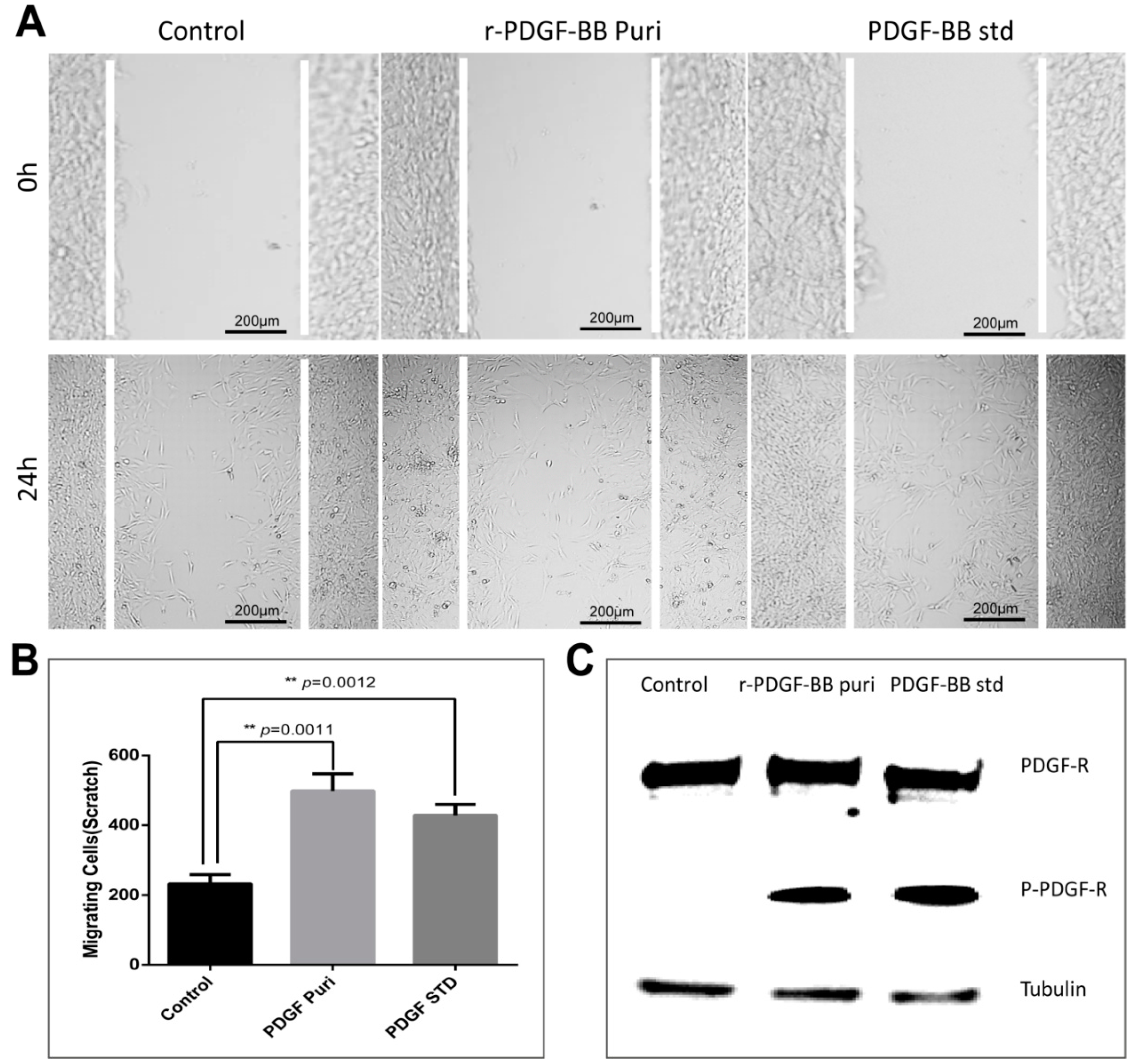
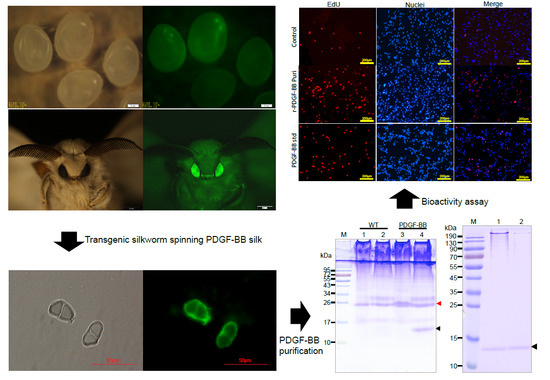
The purification strategy of recombinant PDGF-BB from the cocoons was constructed according to its heparin-binding property and different isoelectric point to that of the endogenous sericins. Processing comprised extraction, specifically by binding its affinity domain to a heparin-binding column, and purity improvement with a cation exchange column (SP-HP) and anion exchange column (Q-HP). Through the purification process, we were able to purify approximately 4.52 mg of the recombinant PDGF-BB with a purity of 82% from 30 g cocoons of the PDGF-21 strain. The recovery rate was estimated to be 50.2%. During the part of the purification process using the heparin column, we also found that a large proportion of the endogenous sericin proteins could also be eluted from the column, and this severely affected the purification efficiency of recombinant PDGF-BB, suggesting that the sericins from the cocoons of silkworm might be capableof binding specifically to the heparin. Thus, more efforts, such as the introduction of a 6× his tag to PDGF-BB and using Nickel ion affinity chromatography technology for its purification, should be made to optimize the purification strategy and process to achieve better purity of the recombinant PDGF-BB to meet its clinical application in the future. The bioactivity of the purified PDGF-BB was estimated which could successfully phosphorylate the PDGF receptor to activate the molecular signal pathway to achieve the promotion of cell proliferation and migration.
Transgenic silkworms are promising tools for producing recombinant proteins at industrial scales which had been discussed in elsewhere [
7]. According to the content of the recombinant PDGF-BB in the cocoons of transgenic silkworm PDGF-21 strain, our calculations showed that it is possible to produce 0.33 kg of PDGF-BB recombinant proteins in a facility with a floor surface of about 500 m
2 and ten workers rearing a total of about 2.0 million PDGF-21 silkworms in one year. These worms will produce a total of approximately 1000 kg of cocoons, which carry a predicted 0.3 kg of total PDGF-BB recombinant protein yield.
4. Materials and Methods
4.1. Silkworm Strains and Cell Lines
The silkworm Dazao strain was applied to generate transgenic silkworms. The mouse embryonic fibroblast cell NIH/3T3 cell line was cultivated with Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Waltham, MA, USA) containing 10% (v/v) fetal bovine serum (FBS, Gibco, Waltham, MA, USA) at 37 °C in a 5% CO2 atmosphere.
4.2. Transgenic Vector Construction
The coding sequence of the
PDGF-B gene was optimized according to the silkworm codon bias and synthesized commercially. It was then inserted in the previously constructed
Sericin-1 expression vector pSL1180 [hSer1spDsRedSer1PA] [
17] to replace
DsRed at the
BamHI and
NotI sites. Thereafter, the open reading frame (ORF) containing the
PDGF-B gene was cut by the AscI restriction enzyme and inserted into the framework transgenic vector, pBac [3xp3EGFP, af] [
11], cut by the same restrictionenzyme to obtain the final transgenic expression vector, pBac [3xp3EGFP, hSPDGFSer1PA].
4.3. Generation of Transgenic Silkworms
The plasmid Mini kit (Qiagen, Hilden, Germany) was used to extract the plasmids for microinjection. After mixture of the pBac [3xp3EGFP, hSPDGFSer1PA] plasmid and the helper plasmid, phsp70PIG [
25], which transiently express the
piggyBac transposase with a 1:1 mole ratio and a final concentration of 500 ng/μL, the mixture plasmid was microinjected (Eppendorf, Hamburg, Germany) into the non-diapause silkworm embryos within 2 h after oviposition, in accordance with a previously reported method [
11]. These G0-hatched larvae were fed to oviposit the G1 eggs, which were fluorescently screened at the ocelli and compound eyes where EGFP expresses specifically on the 6th and 7th day using the Olympus SZX12 fluorescence stereomicroscope (Olympus, Tokyo, Japan). The G1 positive individuals were reared to the moth stage and intraspecifically crossed or backcrossed with moths of wild type silkworms (Dazao strain) to generate stable transgenic silkworms.
4.4. Genetic Analysis—Inverse PCR
Moth genomic DNA was extracted, and 20 µg was digested with HaeIII at 37 °C overnight and purified using the phenol/chloroform method. Then, 2 µg was circularized by ligation using T4 DNA ligase at 16 °C overnight. The ligated DNA (50–100 ng) was amplified using Taq polymerase under standard conditions with primers (
Table 2) designed from the arm region of the
piggyBac vector. Amplified products were separated using agarose gel electrophoresis and recycled using an OMEGA Gel Extraction Kit (Omega Bio-Tek, Guangzhou, China), and then inserted into the TA-clone vector (TAKARA Bio, Dalian, China) for sequencing. The sequences of transgene insertions were analyzed according to the silkworm genome database: SilkDB (
http://www.silkdb.org/silkdb/).
4.5. Histological Examination of PDGF-BB Expression in the Cross-Sections of the Middle Silk Gland and Silk Fibers
The middle silk gland (MSG) of 5th instar silkworms at day 6 and the silk fibers were each fixed overnight with 4% (v/v) formalin and frozen by Tissue-Tek®O.C.T.™ compound (Sakura Finetechnical Co., Ltd., Chuo-ku, Japan) before being crosscut into 10 μm thick sections by a freezing microtome. The sections were then immune-blotted with rabbit anti-PDGF polyclonal antibody (BioVision, Zurich, Switzerland) and detected with green fluoresce isothiocyanate-labeled goat anti-rabbit IgG (Beyotime, Shanghai, China) and fluorescence microscopy at the excitation wavelengths of 488 nm for green fluorescent signals (Nikon, Tokyo, Japan).
4.6. Transcriptional Expression of PDGF-BB in Transgenic Silkworms by RT-PCR
To detect and compare the expression levels of the exogenous
PDGF-B and endogenous
sericin-1, the total RNAs from the middle silk glands of WT and transgenic silkworms on day 6 of 5th instar were extracted using a Total RNA Kit (Omega, Biel/Bienne, Switzerland). 2 μg of each was used for reverse transcription to obtain the corresponding cDNA by a GoScript Reverse Transcription System (Promega, Madison, WI, USA). Subsequently, an equal amount of the cDNA with a concentration of 100 ng/μL was subjected for qRT-PCR assay using the SYBR Premix Ex TaqTM II kit (TaKaRa & Clontech, Dalian, China) on the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with a program consisting of an initial denaturing step of 30 s at 95 °C and 40 amplification cycles consisting of 5 s at 95 °C followed by 30 s at 60 °C. The transcriptional expression levels of the exogenous
PDGF-B or the endogenous
sericin-1 in the WT silkworm and the PDGF-21 transgenic silkworm were firstly normalized to the corresponding internal control SW22934 and then compared with each other. The used primers were shown in
Table 2.
4.7. Protein Analysis
Cocoons were frozen in liquid nitrogen for one minute and then shattered into powder. The powder with a concentration of 30 mg/mL was suspended into buffer containing 50 mM Tris-HCl and 8 M urea at pH 7.0 at 80 °C for 30 min which could extract the major proteins of the sericin layer from the cocoons. Then, the extracts were centrifuged at 20,000×
g for 5 min to collect the supernatant samples. The samples, with equal volumes of 20 μL, were separated by the reduced or nonreduced SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) with a gel concentration of 12% (
w/
v). After electrophoresis, the gels were stained with coomassie brilliant blue (CBB) R250 or transferred to the Western blot analysis. For the Western blot analysis, 10 μL of the protein samples, together with 150 ng and 200 ng of PDGF-BB standard (Abcam, Cambridge, UK) were separated on the polyacrylamide gel and electronically transferred to the polyvinylidene fluoride (PVDF) membrane, followed by the immunoreaction with anti-PDGF antibody (Abcam, Cambridge, UK) at a dilution of 1:10,000 and secondary horse radish peroxidase (HRP)-labeled goat anti-rabbit IgG (Beyotime) at a dilution of 1:20,000. The immunoreacted PVDF membrane was finally visualized using ECL plus (Amersham Biosciences, Little Chalfont, UK), and the images were recorded using a Chemiscope Series (Clinx Science Instruments, Shanghai, China). The content of PDGF-BB in the cocoon shell weight was quantified by densitometric analyzing the intensity of the extracted PDGF-BB with PDFG-BB standard on the Western blots using Image J software (
http://rsb.info.nih.gov/ij/).
4.8. Extraction and Purification of the Recombinant PDGF-BB from the Cocoon Silk
The cocoons spun by the PDGF-BB transgenic silkworm were ground and extracted with a concentration of 30 mg/mL using the extraction buffer (50 mM Tris-HCl, 8 M urea, pH 7.0) at 4 °C for 2 days. The crude extract was then centrifuged at 20,000×
g for 20 min, and the supernatant was filtrated with 0.45 μm Durapore
®PVDF Membrane (Millex
®-HV). The concentration of the total extracted proteins from the cocoons was calculated with a BCA Protein Quantification Kit (Beyotime, Shanghai, China). The content of the recombinant PDGF-BB protein in the total extracted cocoon proteins was calculated by densitometric analysis of the band intensities of the CBB-stained gel using BandScan5.0 software. Subsequently, the filtered sample was applied to HiTrapTM Heparin HP (GE Healthcare, Chicago, IL, USA) column, and washing steps were conducted using wash buffer (50 mM Tris-HCl, 8 M urea, pH 7.0). The recombinant PDGF-BB was then eluted with elution buffer (50 mM Tris-HCl, 8 M urea, pH 6.0, NaCl with gradient concentrations of 100 mM, 150 mM NaCl, and 200 mM NaCl, respectively). Next, the elution products were applied to a HiTrapTMSP HP (GE Healthcare, Chicago, IL, USA) column, followed by the gradient washing steps using wash buffer (50 mM Tris-HCl, 8 M urea, pH 6.0). The recombinant PDGF was then eluted with elution buffer (50 mM Tris-HCl, 8 M urea, pH 6.0 and NaCl at gradient concentrations of 100 mM and 200 mM, respectively). Finally, the elution products were applied to a HiTrapTM Q HP (GE Healthcare, Chicago, IL, USA) column, and the production flow through was collected and concentrated for SDS-PAGE analysis and concentration calculation by BCA Protein Quantification Kit (Beyotime, Shanghai, China), as described above. The purity of the recombinant PDGF-BB was calculated by densitometric analysis of the bands of purified PDGF-BB on the SDS-PAGE gel using Image J software (
http://rsb.info.nih.gov/ij/).
4.9. Cell Culture with Purified PDGF-BB
NIH/3T3 cells of 90% confluence were seeded into 96-well plates at a density of 2000 cells per well and starved in 100 µL of DMEM medium containing 0.5% (w/v) FBS overnight to inactivate cell proliferation. Then, the purified PDGF (100 ng/mL) and an equal amount of hPDGF-BB standard (Abcam, Cambridge, UK) were added to the medium for an additional 24 h.
4.10. Cell Proliferation Assays
The NIH/3T3 cells were observed and photographed using a microscope (Nikon, Tokyo, Japan) after being incubated with purified PDGF-BB and hPDGF-BB standard at a dosage of 100 ng/mL for 24 h. Cell proliferation of NIH/3T3 was measured using the Cell Counting Kit-8 (Beyotime, Shanghai, China) and the Click-iT®EdU Imaging Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturers’ directions. For the CCK-8 assays, CCK-8 solution (10 µL per well of a 96-well plate) was added to each well of NIH/3T3 cells treated by different groups. The cells were incubated at 37 °C for 1 h, and then the absorbance was measured at 450 nm using a Glomax Multi Detection System (Promega, Madison, WI, USA). Measurements were performed in triplicate and repeated independently three times. For EdU dyeing, after treatment, NIH/3T3 cells were labeled by EdU at a concentration of 2 µM at 37 °C for 2 h. Then, labeled cells were immediately fixed with 3.7% formaldehyde in PBS and permeabilized by 0.5% Triton® X-100, followed by EdU detection using 10 µM Alexa Fluor® 555 azide and imaged by fluorescence microscopy at 555 nm excitation and 565 nm emission maxima. The proliferated cell was photographed under a fluorescence microscope (Olympus).
4.11. In Vitro Wound Healing Assay
NIH/3T3 cells were grown in 24-well plates to 100% confluence. Cells were serum-starved overnight and scratched with a sterile pipet tip. Cellular debris was removed by washing with PBS gently. Cells were then stimulated with purified PDGF-BB and the hPDGF-BB standard at a dosage of 100 ng/mL for 24 h. Treatment by an equal amount of PBS was used as control. Initial wounding and the migration of the cells in the scratched area after 24 h were photographically monitored using a microscope (Nikon, Tokyo, Japan). The cell number in the scratched area was calculated using Image J software. Results are representative of three independent experiments. The cells were collected after the same processes, and the total PDGF receptor and phosphorylated PDGF receptor were detected by Western blot analyses using the corresponding antibody (Abcam, Cambridge, UK).
4.12. Statistical Data Analysis
Data are means ± standard deviations (SD) for n = 3. Statistical analyses were calculated using Student’s t-test; * p < 0.05, ** p < 0.01, and *** p < 0.001 was considered statistically significant.