1. Introduction
Gold nanoparticles (AuNPs) have attracted increasing interest as a promising new vehicle for gene therapy, stimulated by their basic physical, chemical and optical properties [
1]. First of all, the gold core is essentially inert and non-toxic [
2]. Secondly, synthesis and surface modifications are fairly straightforward and allow the preparation of a variety of AuNPs that are able to bind macromolecular therapeutics (e.g., proteins, siRNA, pDNA, etc.) [
3]. Finally, their optical properties make it interesting to investigate the use of AuNPs for spatio-temporal controlled delivery of the cargo. AuNPs and other metal NPs (e.g., Ag [
4,
5], Cu [
6] and Al [
7]) are known to have an enhanced optical absorption via Localized Surface Plasmon Resonance (LSPR). LSPR is a consequence of the interaction between the free electrons of the conduction band of a metal NP and an external oscillating electromagnetic field, as shown in
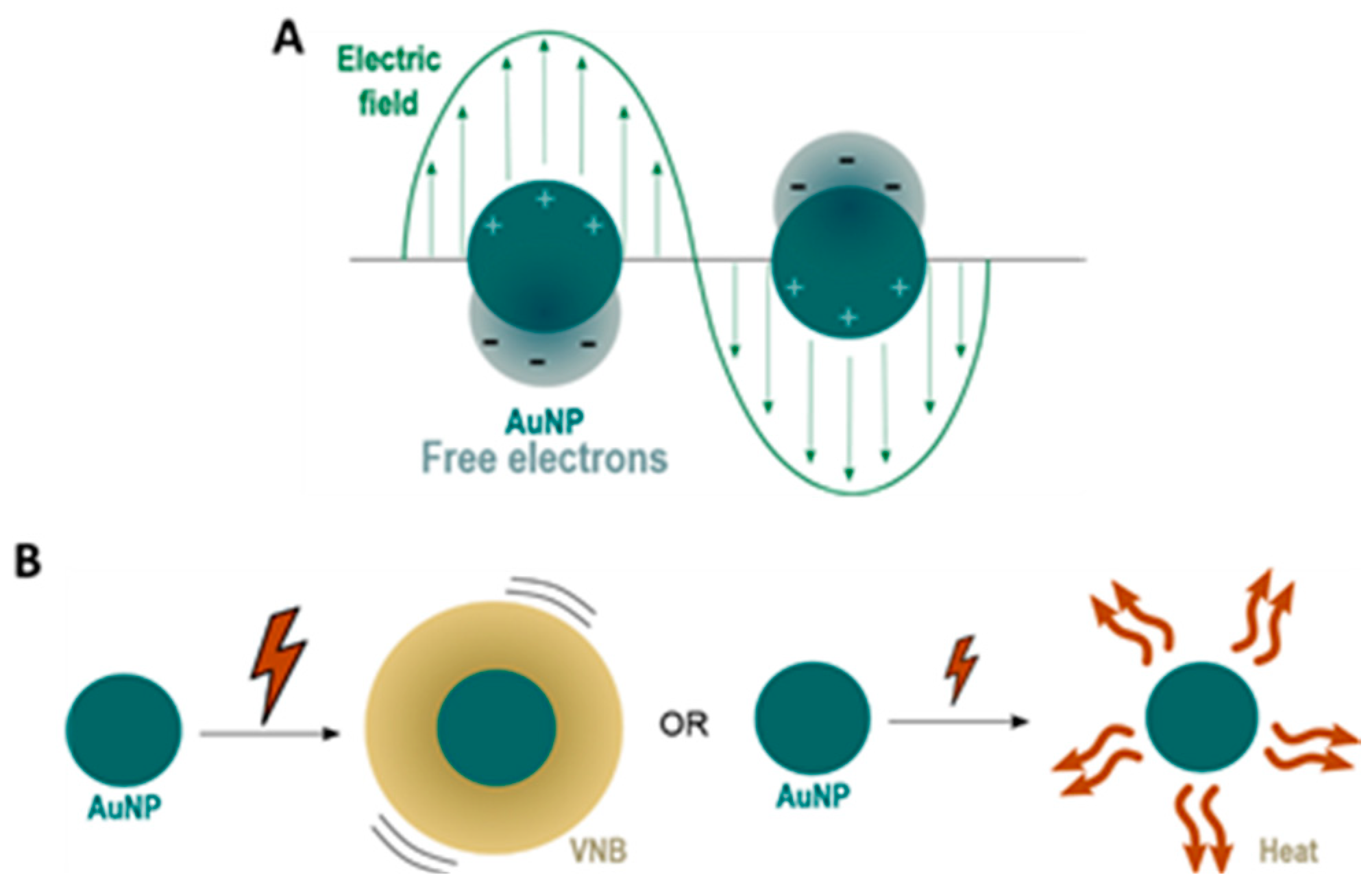
Figure 1A [
8,
9,
10].
Due to the oscillations of the localized surface plasmons, a series of sequential energy transfer processes occur within the NP, which results in an increase of the NP temperature, which is followed by heat transfer from the particle to the environment [
11]. By using continuous wave laser irradiation or low intensity laser pulses, direct heat transfer can lead to an increase in temperature of the local surroundings by ten to several hundreds of degrees. On the other hand, the use of intense short laser pulses (<10 ns) can cause an extremely rapid increase in the NP temperature of several hundreds to even thousand degrees. Due to these very high AuNP temperatures, the water surrounding the NP quickly evaporates, leading to the formation of (laser-induced) water vapour nanobubbles (VNB). The expansion and collapse of VNB creates high pressure shockwaves without transferring heat to the environment since nearly all incident laser energy is essentially converted into mechanical energy of the expanding VNB. The size of VNBs can be tuned from tens to several hundreds of nm by varying the laser intensity and the size of NPs [
8]. Both heat transfer and VNB formation are depicted in
Figure 1B.
LSPR-induced photothermal heating can be used in photothermal therapies. Photothermal cancer therapy is one of the earliest studied applications, where plasmonic NPs act as a localized source of heat to damage and destroy cancer cells [
1,
9,
11]. Later on, these light-triggered properties were employed for drug delivery purposes. Besides the delivery of chemical payloads [
12,
13,
14], currently there is an increasing interest in the use of light-triggered delivery of nucleic acids (NAs) for gene therapy where plasmonic NPs are used as NA nanocarriers. Plasmonic effects can be used for two purposes: (1) light-triggered release of the NA payload at the desired time and place, and (2) overcoming intracellular (IC) barriers. For photothermal release of NAs from plasmonic carriers, different strategies have been investigated to couple NAs to the surface of the NP, which can be subdivided into covalent vs. non-covalent approaches [
15]. When the NA is covalently attached to the gold surface, femtosecond laser pulses that break the covalent bond can be employed in order to release the intact NA [
16,
17]. The second strategy consists of loading the NA onto a carrier molecule using weaker, non-covalent bonds that can be disrupted more easily using lower laser power densities. The carrier molecule should be covalently attached to the gold surface before loading the NA [
15,
18,
19]. Photothermal effects can also aid in tackling IC barriers that obstruct the way for efficient gene delivery. In one approach, especially for in vitro or ex vivo cell transfections, NP-sensitized photoporation of the plasma membrane has been investigated to gain direct access to the cytosol (i.e., without the need to escape endosomes) [
20].
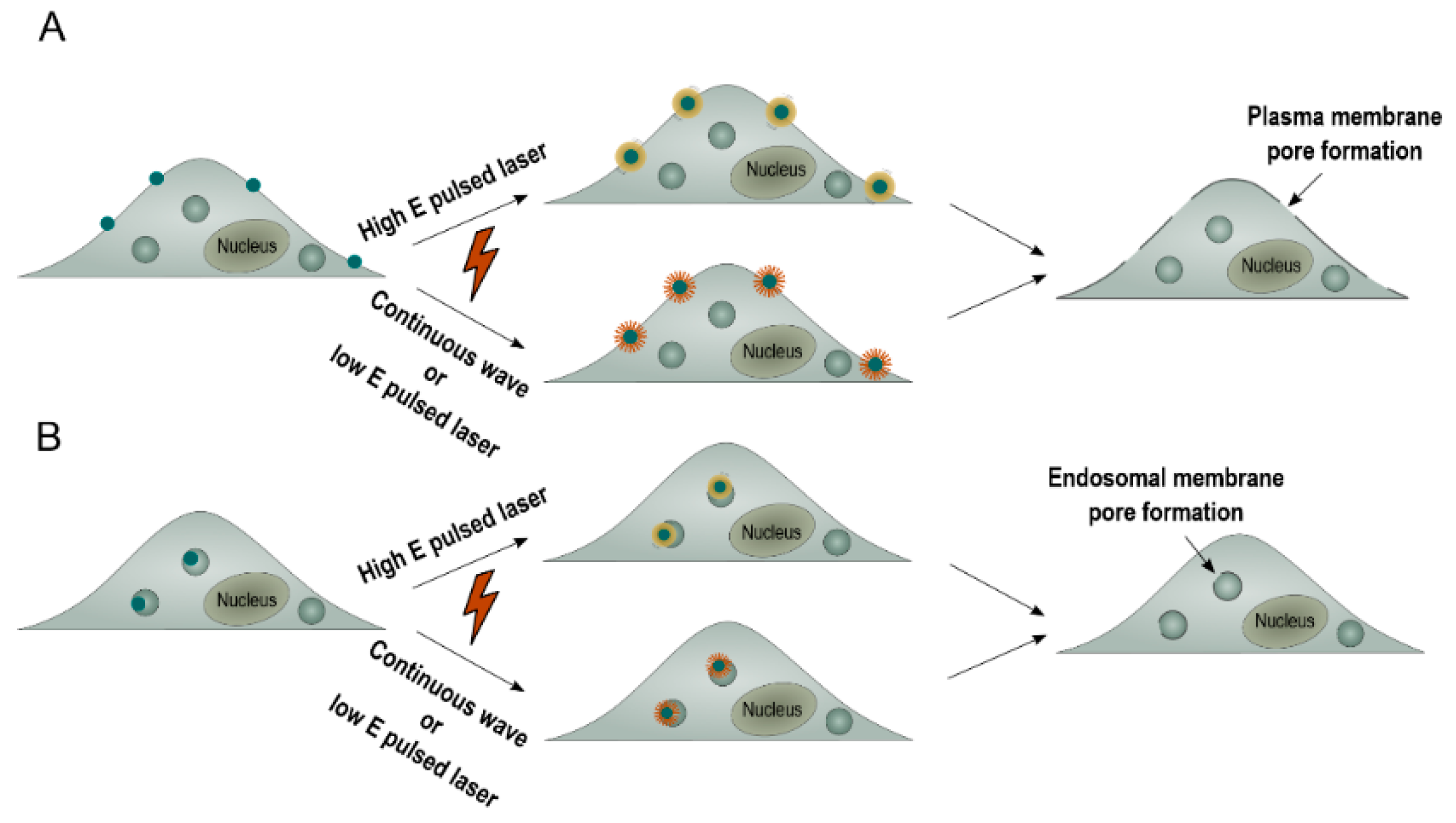
This so-called photoporation of the plasma membrane can be achieved by thermal membrane permeabilization through a local phase transition of the lipid bilayer in response to the heat transfer of the irradiated plasmonic NP to the environment. Alternatively, the generation of a VNB can cause mechanical membrane poration. In either of both cases, after laser irradiation, exogenous compounds can diffuse through the pores into the cytoplasm (
Figure 2A) [
8,
11]. While this has proven to work very well for compounds up to about a few hundred kDa, delivering larger (charged) molecules such as mRNA or pDNA through the plasma membrane has proven to be more difficult likely due to the pores not being large enough [
20,
21,
22]. An alternative strategy is to allow cellular uptake of NA-loaded plasmonic NPs via endocytosis, followed by light-triggered endosomal escape, as depicted in
Figure 2B [
23,
24]. Plasmonic NPs have already been used successfully to induce light-triggered endosomal rupture and cytosolic delivery of several macromolecules such as proteins [
25,
26,
27], siRNA [
15,
28,
29] and ONs (oligonucleotides) [
15]. However, to date, there has been no record of successful delivery of the larger range of NAs, such as pDNA, via light-triggered endosomal escape. In this paper, we report on a first trial to do so, by making use of nanocomplexes composed of the cationic polymer JetPEI, pDNA and anionic 10 nm AuNP. In HeLa cells, we systematically evaluated the effect of heating vs. VNB formation on endosomal escape efficiency, pDNA integrity and final transfection efficiency. We demonstrate that laser-induced VNB are clearly more efficient in disrupting the endosomal membrane than mere heating. However, in the current JetPEI/pDNA/AuNPNP nanocomplex, the pDNA does not survive the highly intense physical forces when VNB are formed. On the other hand, while the generation of heat is less damaging to pDNA, endosomal escape is much less efficient. We conclude that a different design of AuNP containing nanocomplexes is needed that is still capable of forming VNB but that poses less stress on the pDNA.
3. Discussion
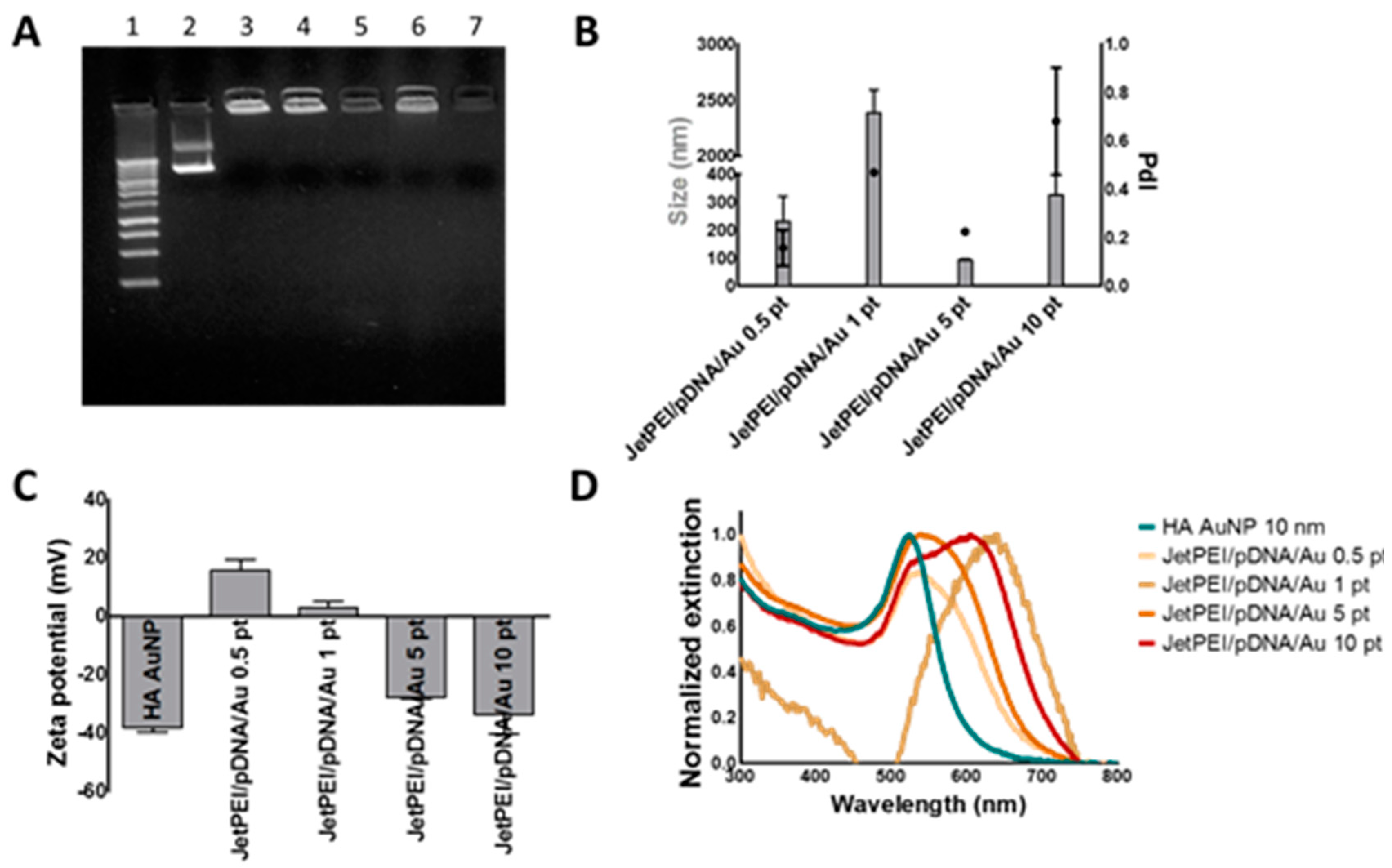
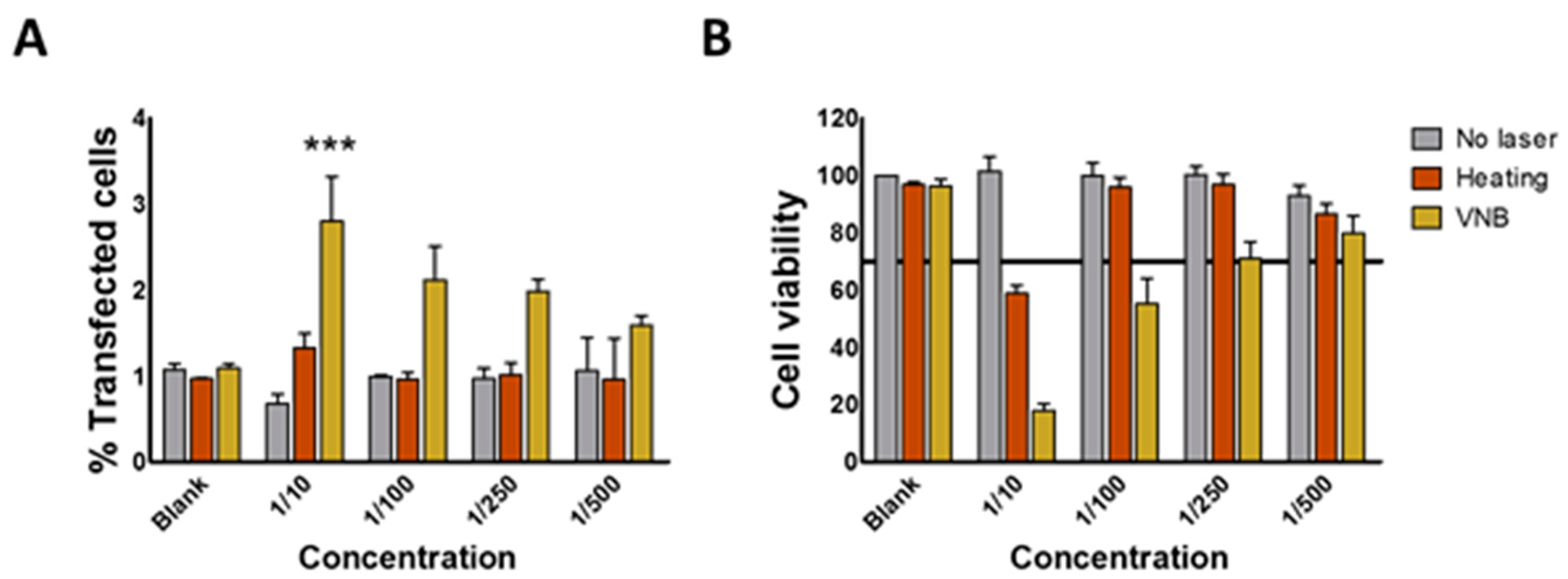
In recent years, the interest in AuNPs for drug delivery purposes has increased tremendously, not in the least because their optical properties could be used in order to obtain spatio-temporally controlled delivery of cargo. In this article, we aimed to use the plasmonic properties of AuNPs to induce light triggered rupture of the endosomal membrane after incubation of HeLa cells with JetPEI/pDNA/AuNP complexes. We examined if photothermal effects such as heating and VNB formation were able to induce endosomal release and transfect HeLa cells. After evaluation of JetPEI/pDNA/AuNP complexes prepared with different amounts of AuNPs, JetPEI/pDNA/AuNP 5 pt complexes were selected for further experiments since they showed a good size, zeta potential and UV/VIS extinction spectrum. Even though the complexes were taken up by cells, unfortunately, we observed that neither heat transfer nor VNB formation were able to induce any meaningful transfection.
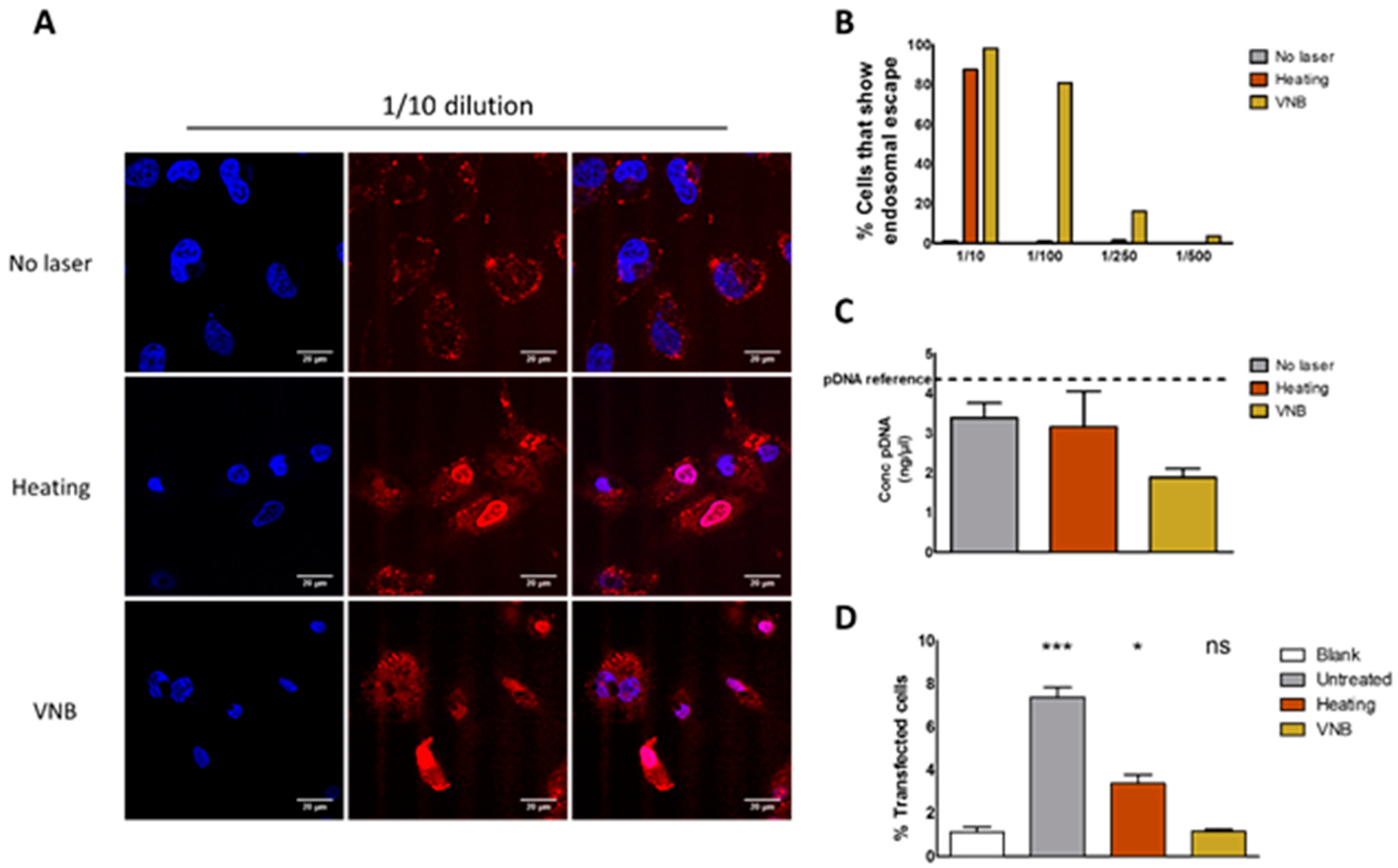
In order to find out what the cause of this inefficient transfection is, we first studied endosomal escape through the use of an ON dequenching assay. Results showed that both heat transfer and VNB formation were able to induce endosomal escape, with VNB being by far the most efficient of the two. We hypothesize that, in order to induce endosomal escape via heating, a relatively high concentration of AuNPs is needed per endosome in order to heat up the endosomal lumen to a temperature that is high enough to induce thermal membrane destabilization. On the other hand, the mechanical force arising from the formation of already one VNB is likely sufficient to induce mechanical disruption of the endosomal membrane and thus release endosomal content. Two side notes must be made on the use of the ON dequenching assay to evaluate endosomal escape. First of all, it should be kept in mind that the endosomal release of the small ONs might not be fully representative to evaluate endosomal escape of a much larger construct such as pDNA. While it shows that the endosomal membrane was permeabilized, it remains uncertain if larger pDNA molecules can escape from the endosomes—especially in the case of heat-induced permeabilization pores possibly being on the small side, as already known from plasma membrane photoporation [
20]. Secondly, low concentrations of complexes had to be used to keep toxicity at an acceptable level. When using these low concentrations, visual confirmation of endosomal escape based on the ON dequenching assay after VNB formation became difficult. If the amount of AF647 ONs per endosome is not high enough to be detected, endosomal escape could remain invisible using this method, despite the fact that it may actually have happened. In order to confirm our hypothesis that one VNB is capable of disrupting the endosomal membrane, it would be useful to use a more sensitive assay to detect endosomal escape. Such an assay was proposed by Wittrup et al. where they used two different exposure settings in order to extend the dynamic range. Using long exposure times, weakly fluorescent signals that may remain unnoticed in the dequenching assay should become detectable [
32]. Nevertheless, since, in the 1/10 dilution, the majority of cells show endosomal escape after VNB formation and after heat transfer, we conclude that the endosomal rupture is not the limiting factor for photothermally triggered transfection.
We next went on to evaluate the integrity of pDNA after inducing the photothermal effects. Mechanical or thermal stimuli may damage nucleic acids, rendering the pDNA ineffective even when it is able to reach the cytosol. Although photothermally triggered endosomal escape of siRNA has been performed successfully [
15,
28,
29], pDNA offers an extra challenge because of its much longer sequence. Therefore, we examined the pDNA content after VNB formation and heating regime via PicoGreen assay. We found that a large part of the cargo was degraded after VNB formation as PicoGreen was no longer able to intercalate. However, there was still a considerable amount of structural pDNA detected after heating and VNB formation. Since a PicoGreen assay only indicates the presence of pDNA structure, it does not provide any information regarding the integrity (and thus transfection potential) of the remaining pDNA. Therefore, we evaluated the functionality of pDNA after release from the (laser treated) complexes by dextran sulphate. HeLa cells were transfected with this released pDNA by electroporation. We found that the formation of VNBs damages the pDNA completely in JetPEI/pDNA/AuNP complexes, thereby abolishing the potential for transfection. In the case of heating, part of the pDNA survived. In spite of that, no transfection was observed, which points to the fact that heat-induced pores in the endosomal membrane are likely not large enough for pDNA to pass through, as already mentioned above.
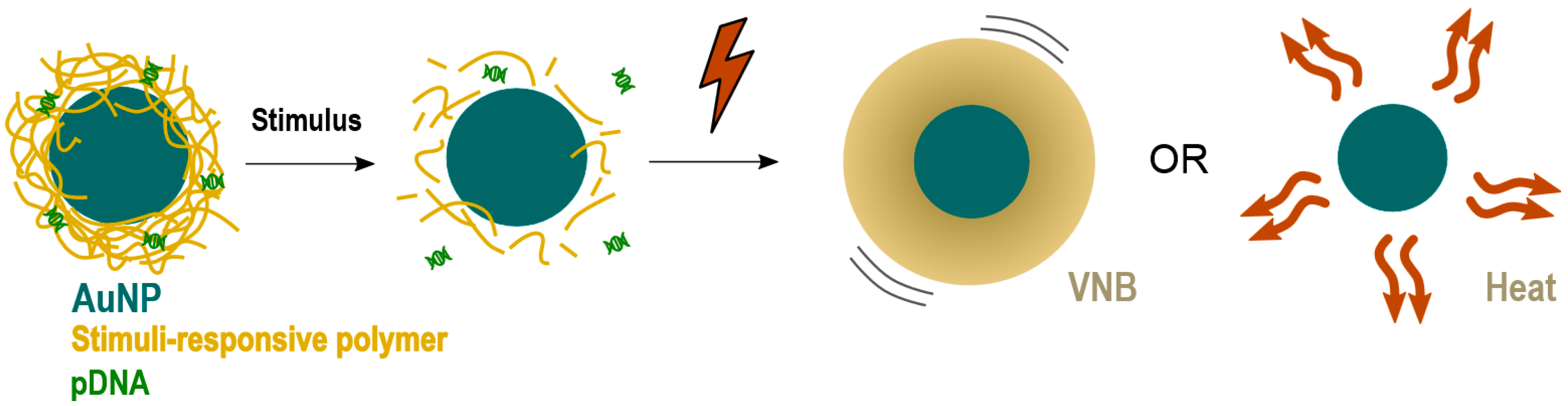
The results presented here demonstrate that VNBs can rupture endosomes. Nevertheless, the current design must be improved in order to be able to generate transfections’ efficiencies comparable to benchmarks techniques like lipofectamine, which show a transfection efficiency of 15% for an equal amount of pDNA as used for JetPEI/pDNA/AuNP complexes in the 1/10 condition (
Figure S1). To reach this goal, an NP has to be designed that better protects the pDNA against these photothermal effects. One option would be to design an NP that has a gold core, surrounded by a stimuli-responsive polymer that allows the release of pDNA prior to photothermal laser treatment, as shown in
Figure 9. Such stimuli-responsive polymers could be pH-responsive polymers that degrade upon endosomal acidification or thermo-sensitive polymers that degrade after an initial soft heating step. By making sure that the pDNA is dissociated from the carrier before laser treatment, pDNA is expected to better survive the subsequent irradiation step to induce endosomal escape.
4. Materials and Methods
4.1. Materials
DMEM (Dulbecco’s Modified Eagle Medium)/F-12, l-Glutamine, Penicillin-Streptomycin solution (5000 IU/mL penicillin and 5000 μg/mL streptomycin) (P/S), Fetal Bovine Serum (FBS), Opti-MEM, Trypan Blue, 0.25% Trypsin-EDTA (ethylenediaminetetraacetic acid) and Dulbecco’s phosphate-buffered saline 1× without Ca2+/Mg2+ (DPBS-) were provided by GibcoBRL (Merelbeke, Belgium). YOYO-1 iodide, Hoechst 33342 and Quant-IT PicoGreen dsDNA Assay Kit were supplied by Molecular Probes (Erembodegem, Belgium). Other reagents were purchased from Sigma-Aldrich (Bornem, Belgium) unless otherwise specified.
4.2. Synthesis and Characterization of Hyaluronic Acid Coated 10 nm Gold Nanoparticles
4.2.1. Synthesis of 10 nm Gold Nanoparticles
The synthesis of 10 nm gold nanoparticles (AuNPs) was performed using ascorbate as reducing agent. A typical synthesis consists of adding Au to give a final concentration of 0.2 mM HAuCl4 with the addition of equimolar quantities of sodium ascorbate (final volume = 100 mL) under rapid stirring and let react for 30 min.
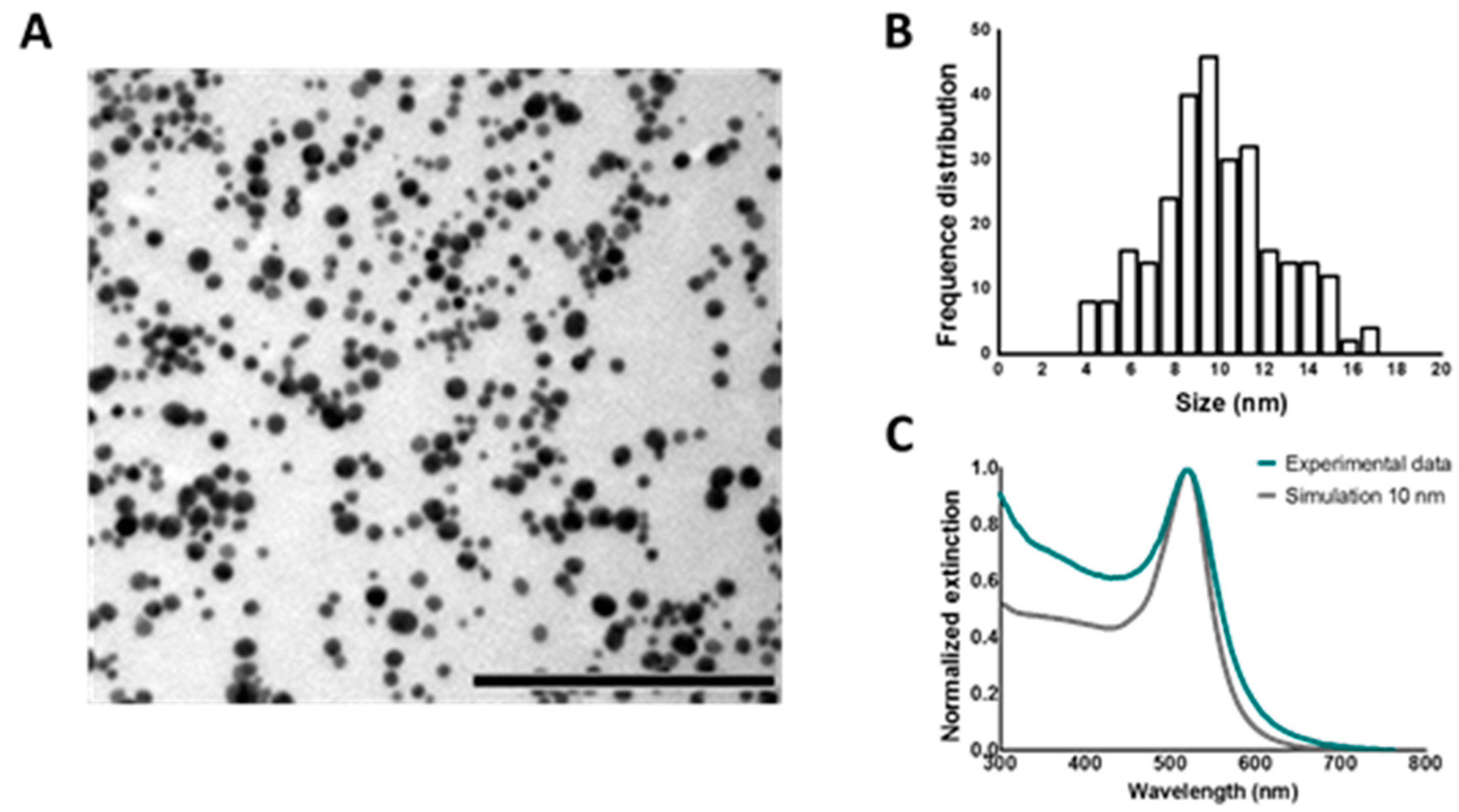
The characterization of AuNPs was performed combining UV/VIS spectroscopy, dynamic light scattering (DLS), transmission electron microscopy (TEM), and electrodynamic modeling using Mie theory. UV/VIS spectroscopy was performed on a NanoDrop 2000c spectrophotometer (Thermo Scientific, Rockford, IL, USA). DLS measurements were carried out using a Zetasizer Nano (Malvern, Worcestershire, UK) and disposable folded capillary cells (Malvern, Worcestershire, UK) to determine hydrodynamic diameter, polydispersity index and zeta potential. TEM images were obtained at the VIB-UGent Transmission Electron Microscopy-Core facility using a JEM 1400 plus transmission electron microscope (JEOL, Tokyo, Japan) operating at 60 kV. Samples were prepared by adding one drop (~50 μL) of the samples colloidal solution onto formvar/C-coated hexagonal copper grids (EMS G200H-Cu) for 20 min and washed 5 times in double distilled water (ddiH
2O). Finally, the size and concentration of AuNPs was estimated using the experimental extinction intensities at the maximum wavelength (λ
max = 520 nm), and Mie theory calculations [
33,
34,
35] of the extinction cross section for spherical particles (σ
ext(520 nm) = 5.2 × 10
−13 cm
2/NP).
4.2.2. Functionalization with HA to Form HA AuNP 10 nm
The synthetized NPs were immediately functionalized with hyaluronic acid (HA) to install a negative Z potential, required for complexation with positively charged JetPEI/pDNA complexes. Typically, functionalization with HA was performed by adding 6–10 mg of the polymer (all stock solutions of the synthetized AuNPs in pM concentration). Successful functionalization was confirmed by DLS Z potential measurements, performed after centrifugation of the AuNPs to remove unbound HA.
4.3. Preparation of Plasmids
gWIZ GFP (Promega, Leiden, The Netherlands) was amplified in transformed E. coli bacteria and isolated from this bacteria suspension using a Qiafilter Plasmid Giga Kit (Qiagen, Venlo, The Netherlands). Concentration was determined on a NanoDrop 2000c (Thermo Fisher Scientific, Rockford, IL, USA) by UV absorption at 260 and 280 nm and adjusted to a final concentration of 1 μg/μL with HEPES buffer (20 mM, pH 7.2).
4.4. Preparation of Au Functionalized JetPEI/pDNA Nanoparticles
JetPEI/pDNA polyplexes were prepared using commercially available JetPEI (Polyplus transfection, Leusden, The Netherlands). JetPEI/pDNA complexes were obtained by mixing the polymer solution with an equal volume of pDNA solution. N/P ratio of the polyplexes was calculated using the formula provided by the manufacturer (Equation (1)). Next, the mixture was vortexed for 10 s at 2200 rpm and polyplexes were allowed to stabilize for 15 min:
Next, the required amount of HA coated 10 nm AuNPs was centrifuged at 12,000× g for 10 min. The supernatant was removed and the pellet was resuspended in ddiH2O before mixing with JetPEI/pDNA complexes in equal volumes. The resultant mixture was allowed to stabilize for 30 min followed by final dilution with ddiH2O.
4.5. Physicochemical Characterization of JetPEI/pDNA/AuNP Complexes
To evaluate complexation of pDNA to the JetPEI/pDNA/AuNP complexes, gel electrophoresis was performed. The complexes were prepared as described above. A 1% agarose gel was prepared by dissolving 1 g of agarose (UltraPure Agarose, Invitrogen, Erembodegem, Belgium) in 100 mL of 1× Tris/Borate/EDTA (TBE) buffer after which GelRed (Biotium, Hayward, CA, USA) was added in order to detect pDNA. In addition, 5 μL of Gel Loading Buffer (Ambion, Merelbeke, Belgium) was added to 20 μL of complexes and a total volume of 25 μL was pipetted in every lane. A 1 kb ladder (Bioron GmbH, Ludwigshafen, Germany) and uncomplexed pDNA were taken along as controls. Gel electrophoresis was performed at 100 V for 30 min and a PhotoDoc-It Imaging system (Upland, CA, USA) was used to acquire an image of the gel under UV light (Bio-Rad UV transilluminator 2000, Hercules, CA, USA).
Next, Dynamic Light Scattering measurements were performed on the NanoZS Zetasizer. The complexes were prepared as described above and were transferred to disposable folded capillary cells to determine hydrodynamic diameter, polydispersity index and zeta potential. The same complexes were used to measure the UV/VIS spectrum on a NanoDrop 2000c. Finally, the concentration of JetPEI/pDNA/AuNP complexes was measured via Nanoparticle Tracking Analysis using the NanoSight LM10 (Malvern, Worcestershire, UK). The measurements were performed in quintuplet.
4.6. Cell Culture
HeLa cells (cervical adenocarcinoma cells, ATCC CCL-2) were cultured in Dulbecco’s modified Eagle’s medium supplemented with growth factor F12 (DMEM/F-12) and enriched with 10% FBS, 2 mM l-Glutamine and 100 μg/mL P/S. Cells were cultured in a humidified atmosphere at 37 °C and 5% CO2. Experiments were performed on cells with a passage number below 25.
4.7. Generation and Detection of JetPEI/pDNA/AuNP Complex Heating and Vapour Nanobubble (VNB) Formation
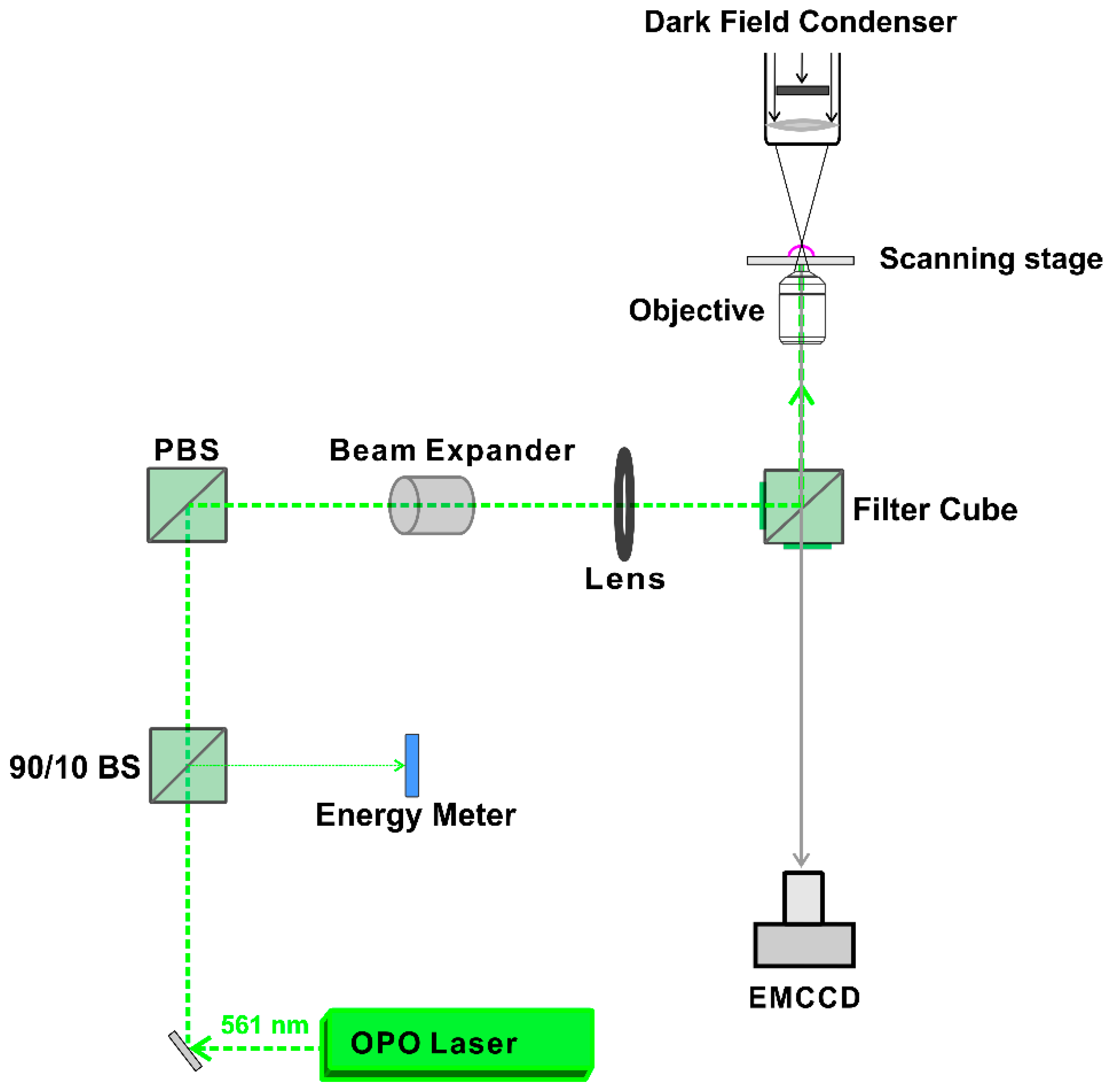
A homemade setup, according to the optical design in
Figure 10, was used to generate and detect heating or VNB formation. The setup is built around an inverted TE2000 epi-fluorescence microscope (Nikon, Nikon BeLux, Brussels, Belgium) equipped with a Plan Fluor 10 × 0.3 NA lens (Nikon, Tokyo, Japan). A pulsed laser with a pulse duration of ~7 ns was tuned at a wavelength of 561 nm with an Optical Parametric Oscillator (OPO) laser (Opolette™ HE 355 LD, OPOTEK Inc., Carlsbad, CA, USA) and used to excite the localized surface plasmon resonance of the JetPEI/pDNA/AuNP complexes. The energy of the laser pulses was measured with an energy meter (J-25MB-HE&LE, Energy Max-USB/RS sensors, Coherent, Santa Clara, CA, USA).
Detection of VNB formation was performed using dark-field microscopy as VNBs efficiently scatter light. Since VNBs typically have a very short lifetime (<1 μs), the camera (EMCCD camera, Cascade II: 512, Photometrics, Tucson, AZ, USA) was synchronized with the pulsed laser using an electronic pulse generator (BNC575, Berkely Nucleonics Corporation, San Rafael, CA, USA). The pulse laser sends a Q-switch signal to trigger the pulse generator and the camera at a certain delay. In this way, dark-field images were taken before, during and after illumination. Dark-field microscopy was used to determine the fluence threshold for VNB formation and heating of JetPEI/pDNA/AuNP complexes. To determine the thresholds in HeLa cells, cells were first seeded in 50 mm γ-irradiated glass bottom dishes (MatTek Corporation, Ashland, MA, USA) at a density of 600,000 cells. Cells were allowed to attach overnight and the next day, complexes were added to the cells in Opti-MEM. After incubation at 37 °C for 1 h, the cells were washed, full culture medium was added and dark-field microscopy was performed. In order to calculate the threshold for heating and VNB formation, dark-field images were analyzed using ImageJ (
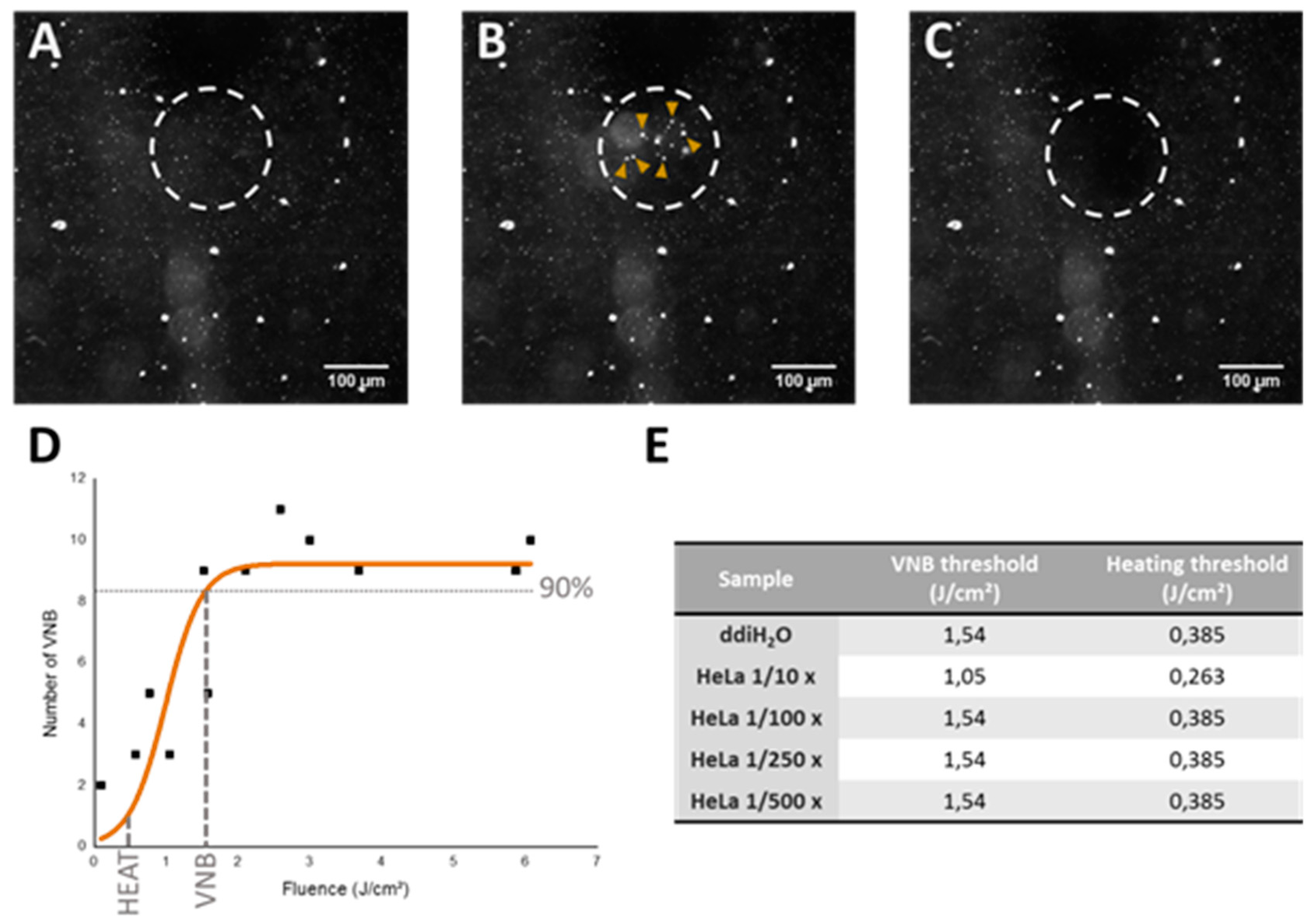
https://fiji.sc/) and the number of VNBs was plotted in function of the laser fluence that was used.
For scanning larger areas such as wells of a 96-well plate, we used an automatic Prior Proscan III stage (Prior Scientific Ltd., Cambridge, UK) to scan the sample line by line with a scanning speed of 2.2 mm/s and a 150 μm diameter laser beam with 20 Hz pulse frequency. The distance between subsequent lines was set to 110 μm to ensure the illumination of all complexes present in the sample.
4.8. Evaluation of Transfection Efficiency and Cytotoxicity
HeLa cells were seeded in 96-well plates at 10,000 cells per well and were allowed to attach overnight. The next day, JetPEI/pDNA/AuNP complexes containing gWIZ GFP were prepared as described above. Cells were incubated with JetPEI/pDNA/AuNP complexes in Opti-MEM for 1 h at 37 °C. Afterwards, they were washed with Opti-MEM and full cell culture medium was added before laser treatment, as described above. After laser treatment, the cells were cultured for another 24 h before they were prepared for flow cytometry analysis. To examine transfection efficiency, expression of gWIZ GFP was measured in the green channel. For cytotoxicity evaluation, DAPI (Thermofischer, Waltham, MA, USA) was added to the flow buffer according to the manufacturer’s instructions.
Additionally, the results obtained with JetPEI/pDNA/AuNP complexes were compared with a positive control using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions and using the same final concentrations as for the JetPEI/DNA complexes.
4.9. Flow Cytometry
To perform analysis by flow cytometry, cells were detached using trypsin and transferred to flow cytometry tubes (BD Falcon, Radnor, PA, USA). Next, the cell suspensions were centrifuged at 300× g for 5 min (Bio-Rad DiaCent-12, DieMed GmbH, Cressier, Switzerland) and resuspended in flow buffer (DPBS-, 0.1% Sodium Azide, 1% Bovine Serum Albumine). Finally, samples were vortexed at 2200 rpm (YellowLine TTS2, IKA works, Wilmington, DC, USA). Flow cytometry was performed on 10,000 events per sample (CytoFLEXTM Flow Cytometer, Beckman Coulter, Krefeld, Germany) or for a total duration of 120 s. gWIZ GFP fluorescence was detected with 525/40 nm bandpass filter after 488 nm excitation. DAPI fluorescence was detected with a 450/45 nm bandpass filter after 405 nm excitation. FlowJo software (version, Treestar Inc., Ashland, OR, USA) was used to perform the analysis.
4.10. Evaluation of Uptake Efficiency
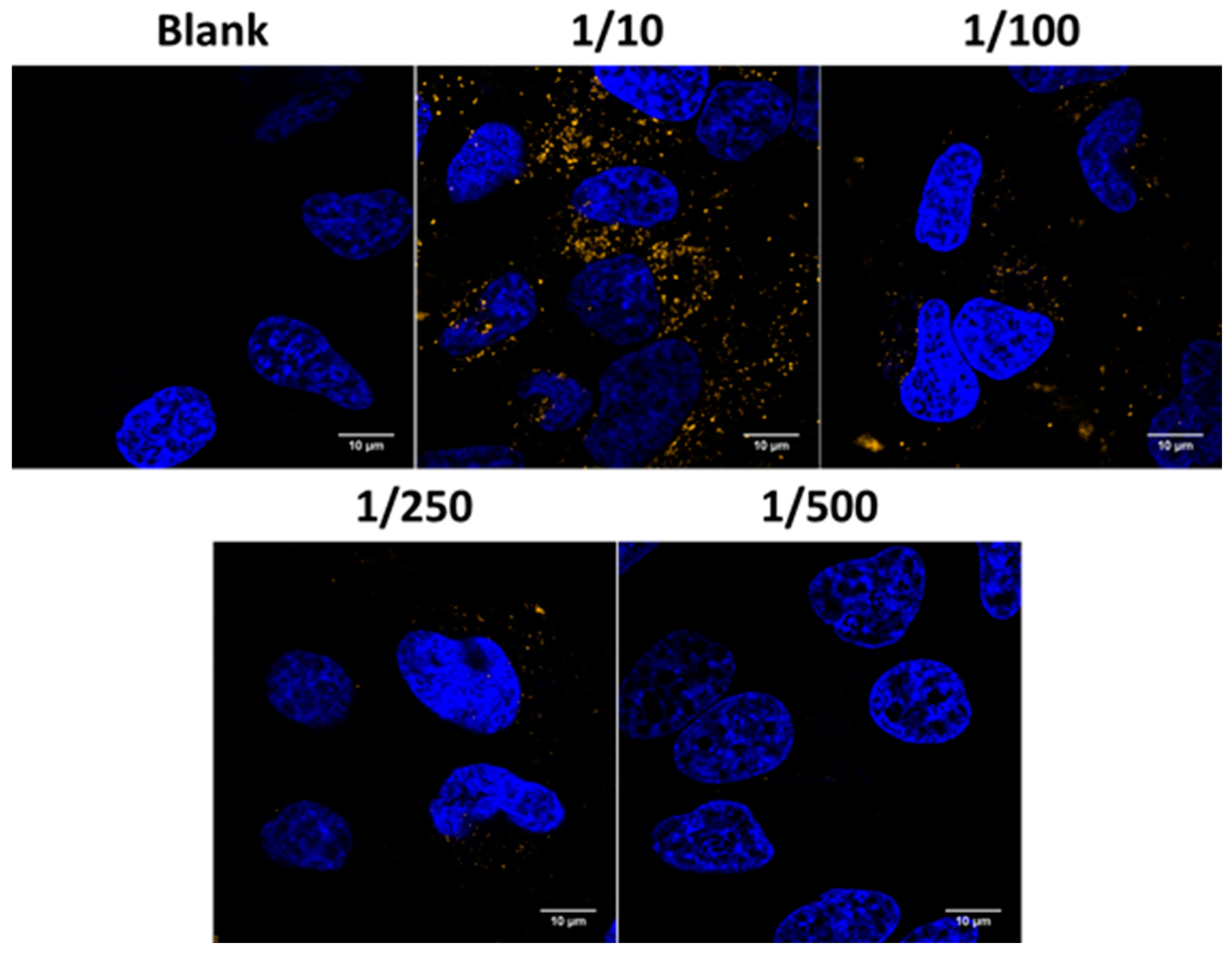
Cells were seeded in 96-well plates with glass bottom (Greiner Bio-One, Frickenhausen, Germany) at a density of 10,000 cells per well and were allowed to attach overnight. The next day, cell nuclei were stained with Hoechst 33342 staining (1 mg/mL in H2O; 1000× diluted). JetPEI/pDNA/AuNP 5 pt complexes were prepared as described above. Cells were incubated with JetPEI/pDNA/AuNP 5 pt complexes in Opti-MEM for 1 h at 37 °C. After washing the particles off, the cells were provided with full cell culture medium and live-cell imaging was performed using a confocal laser scanning microscope (C1si, Nikon, Tokyo, Japan). A Plan Apo VS 60× 1.4 NA oil immersion objective lens (Nikon, Tokyo, Japan) was used to obtain a pixel size of 70 nm and AuNP were detected by the reflected laser light of the 561 nm laser. Image processing was performed using ImageJ (FIJI) software.
4.11. Visualization and Quantification of Endosomal Escape
Visualization and quantification of endosomal escape was performed based on the dequenching assay that was first published by Rehman et al. [
30] Therefore, red-fluorescent oligonucleotides (AF647 ONs) were co-incorporated into the complexes. Cells were seeded in 96-well plates with glass bottom at a density of 10,000 cells per well and were allowed to attach overnight. Cell nuclei were stained with Hoechst 33342 staining (1 mg/mL in H
2O; 1000× diluted). Next, AF647 ON-containing complexes were added to the cells in Opti-MEM and incubated for 1 h at 37 °C. After washing off the complexes, the cells were provided with full cell culture medium and laser treatment was performed, as described above. After laser treatment, the cells were imaged using a spinning disk confocal (SDC) microscope (Nikon Eclipse Ti, Tokyo, Japan) equipped with an MLC 400 B laser box (Agilent Technologies, Santa Clara, CA, USA), a Yokogawa CSU-X confocal spinning disk device (Andor, Belfast, UK), an iXon ultra EMCCD camera (Andor Technology, Belfast, UK) and NIS Elements software (Nikon, Japan). A Plan Apo VC 60× 1.4 NA oil immersion objective lens (Nikon, City, Japan) was used to yield an image pixel size of 234 nm. Exposure time was set to 20 ms and the images were processed using ImageJ (FIJI).
4.12. Determination of pDNA Integrity
4.12.1. PicoGreen Assay to Evaluate pDNA Structure
JetPEI/pDNA/AuNP 5 pt complexes were prepared and underwent laser treatment, as described above. To the resulting samples (untreated, heat and VNB treated), dextran sulphate (50 mg/mL in PBS-) was added in order to release the complexed pDNA. Finally, the amount of pDNA in the samples was quantified using Quant-IT PicoGreen dsDNA Assay Kit according to the manufacturer’s protocol. Fluorescent measurements of the assay were performed on a fluorescence microplate reader (Tecan, Mechelen, Belgium).
4.12.2. Electroporation to Evaluate pDNA Functionality
JetPEI/pDNA/AuNP 5 pt complexes were prepared and underwent laser treatment, as described above. To the resulting samples (untreated, heat and VNB treated), dextran sulphate (50 mg/mL in PBS-) was added in order to release the complexed pDNA. In order to evaluate the functionality, HeLa cells were electroporated in the presence of this released pDNA. In addition, 500,000 HeLa cells were suspended in 90 μL SE cell line solution (Lonza Cologne GmbH, Cologne, Germany) and 10 μL of the corresponding samples (containing an originally complexed amount of 17 ng gWIZ GFP pDNA) was added to the cells. Electroporation was performed using an Amaxa 4D-Nucleofector in HeLa cells, according to the manufacturer’s protocol (Lonza Cologne GmbH, Cologne, Germany). Next, samples were diluted with cell culture medium and seeded in 96-well plates at a density of 15,000 cells. The cells were incubated for 24 h and were then prepared for analysis via flow cytometry to evaluate transfection efficiency.