Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies
Abstract
1. Introduction
2. Chronic Niche Inflammation in Endometriosis Development
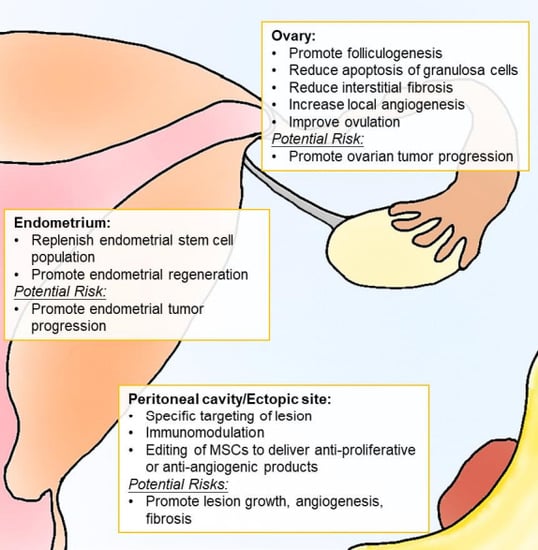
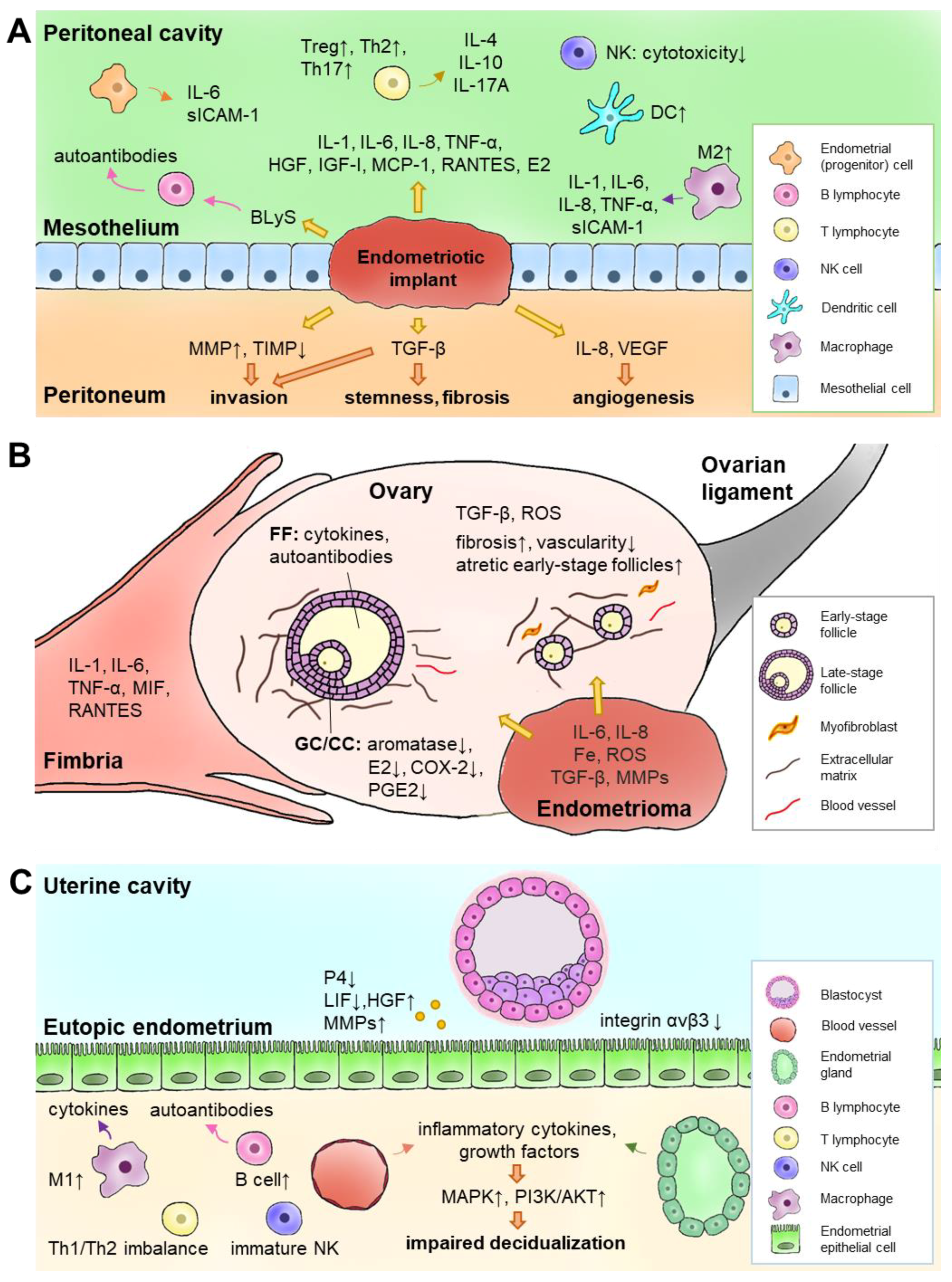
2.1. Peritoneal Cavity
2.2. Ovaries
2.3. Uterus
3. Targeting Inflammation for Treatment of Endometriosis-Associated Infertility
3.1. Immunomodulators
3.2. Anticytokine Treatment
3.3. Statins
3.4. Tyrosine Kinase Inhibitors
3.5. Prostaglandin E2 (PGE2) Inhibitors
3.6. Antioxidants
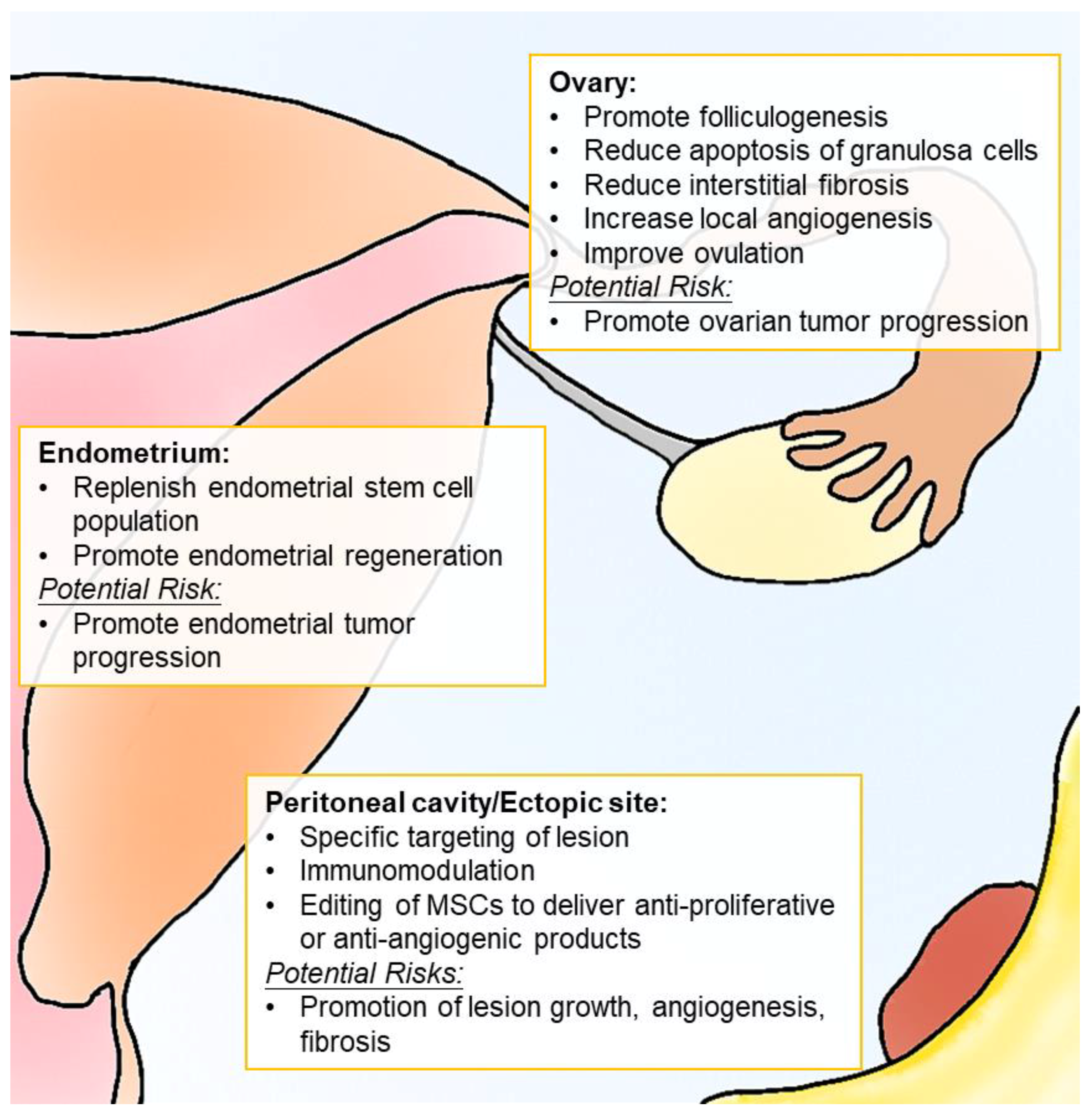
4. Cell-Based Therapy for Endometriosis Treatment
4.1. Stem Cells in Endometriosis Pathogenesis
4.2. Inflammatory Niche–Induced Stemness Re-Expression in Endometriosis Pathogenesis
4.3. Immunomodulation of MSCs
4.4. Safety and Efficacy of Stem Cell Therapy in Endometriosis-Associated Infertility
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.E. Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J. Am. Assoc. Gynecol. Laparosc. 1994, 2, 43–47. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, diagnosis and clinical management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kok, V.C.; Tsai, H.J.; Su, C.F.; Lee, C.K. The risks for ovarian, endometrial, breast, colorectal, and other cancers in women with newly diagnosed endometriosis or adenomyosis a population-based study. Int. J. Gynecol. Cancer 2015, 25, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Králíčková, M.V.; Vetvicka, V. Endometriosis and ovarian cancer. World J. Clin. Oncol. 2014, 5, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Forman, J.P.; Missmer, S.A. Association between endometriosis and hypercholesterolemia or hypertension. Hypertension 2017, 70, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Jørgensen, K.T.; Pedersen, B.V.; Rostgaard, K.; Frisch, M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and sjögren syndrome. Hum. Reprod. 2011, 26, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Bungum, H.F.; Vestergaard, C.; Knudsen, U.B. Endometriosis and type 1 allergies/immediate type hypersensitivity: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Hummelshoj, L.; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis: A committee opinion. Fertil. Steril. 2014, 101, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Allaire, C.; Yong, P.; Alfaraj, S. Medical management of endometriosis in patients with chronic pelvic pain. Semin. Reprod. Med. 2017, 35, 38–53. [Google Scholar] [PubMed]
- Hughes, E.; Brown, J.; Collins, J.J.; Farquhar, C.; Fedorkow, D.M.; Vanderkerchove, P. Ovulation suppression for endometriosis for women with subfertility. Cochrane Database Syst. Rev. 2007, 3, CD000155. [Google Scholar] [CrossRef] [PubMed]
- Sallam, H.N.; Garcia-Velasco, J.A.; Dias, S.; Arici, A.; Abou-Setta, A.M. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst. Rev. 2006, 1, CD004635. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Kim, M.R.; Lee, J.H.; Kim, J.Y.; Hwang, K.J.; Kim, H.S.; Lee, E.S. Gonadotropin-releasing hormone agonist reduces aromatase cytochrome P450 and cyclooxygenase-2 in ovarian endometrioma and eutopic endometrium of patients with endometriosis. Gynecol. Obstet. Investig. 2009, 68, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Van der Houwen, L.E.E.; Mijatovic, V.; Leemhuis, E.; Schats, R.; Heymans, M.W.; Lambalk, C.B.; Hompes, P.G.A. Efficacy and safety of IVF/ICSI in patients with severe endometriosis after long-term pituitary down-regulation. Reprod. Biomed. Online 2014, 28, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S.; Katz-Jaffe, M.; Kondapalli, L.V.; Gustofson, R.L.; Schoolcraft, W.B. GnRH agonist administration prior to embryo transfer in freeze-all cycles of patients with endometriosis or aberrant endometrial integrin expression. Reprod. Biomed. Online 2017, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.B.; Parnell, B.A.; Bushnell, G.; Tallman, N.; Forstein, D.A.; Higdon, I.I.I.H.L.; Kitawaki, J.; Lessey, B.A. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum. Reprod. 2012, 27, 881–888. [Google Scholar] [CrossRef] [PubMed]
- De Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Kennedy, S.H.; Barlow, D.H. Endometriotic disease: The role of peritoneal fluid. Hum. Reprod. Update 1998, 4, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of cytokines in endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef]
- Jeung, I.; Cheon, K.; Kim, M.R. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. Biomed Res. Int. 2016, 2016, 2916070. [Google Scholar] [CrossRef] [PubMed]
- Králíčková, M.; Vetvicka, V. Immunological aspects of endometriosis: A review. Ann. Transl. Med. 2015, 3, 153. [Google Scholar] [PubMed]
- Santanam, N.; Murphy, A.A.; Parthasarathy, S. Macrophages, oxidation, and endometriosis. Ann. N. Y. Acad. Sci. 2002, 955, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Rakhila, H.; Al-Akoum, M.; Bergeron, M.E.; Leboeuf, M.; Lemyre, M.; Akoum, A.; Pouliot, M. Promotion of angiogenesis and proliferation cytokines patterns in peritoneal fluid from women with endometriosis. J. Reprod. Immunol. 2016, 116, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cosin, R.; Gilabert-Estelles, J.; Ramon, L.A.; Gomez-Lechon, M.J.; Gilabert, J.; Chirivella, M.; Braza-Boils, A.; Espana, F.; Estelles, A. Influence of peritoneal fluid on the expression of angiogenic and proteolytic factors in cultures of endometrial cells from women with endometriosis. Hum. Reprod. 2010, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Harada, T.; Iwabe, T.; Taniguchi, F.; Mitsunari, M.; Yamauchi, N.; Deura, I.; Horie, S.; Terakawa, N. A combination of interleukin-6 and its soluble receptor impairs sperm motility: Implications in infertility associated with endometriosis. Hum. Reprod. 2004, 19, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Carli, C.; Leclerc, P.; Metz, C.N.; Akoum, A. Direct effect of macrophage migration inhibitory factor on sperm function: Possible involvement in endometriosis-associated infertility. Fertil. Steril. 2007, 88, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Mansour, G.; Aziz, N.; Sharma, R.; Falcone, T.; Goldberg, J.; Agarwal, A. The impact of peritoneal fluid from healthy women and from women with endometriosis on sperm DNA and its relationship to the sperm deformity index. Fertil. Steril. 2009, 92, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Faber, B.M.; Chegini, N.; Mahony, M.C.; Coddington, C.C. Macrophage secretory products and sperm zona pellucida binding. Obstet. Gynecol. 2001, 98, 668–673. [Google Scholar] [PubMed]
- Zhao, Y.; Gong, P.; Chen, Y.; Nwachukwu, J.C.; Srinivasan, S.; Ko, C.; Bagchi, M.K.; Taylor, R.N.; Korach, K.S.; Nettles, K.W.; et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci. Transl. Med. 2015, 7, 271ra279. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Thomas, S.Y.; Hamilton, K.J.; Young, S.L.; Cook, D.N.; Korach, K.S. Early endometriosis in females is directed by immune-mediated estrogen receptor alpha and IL-6 cross-talk. Endocrinology 2018, 159, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Riccio, L.G.C.; Baracat, E.C.; Chapron, C.; Batteux, F.; Abrão, M.S. The role of the B lymphocytes in endometriosis: A systematic review. J. Reprod. Immunol. 2017, 123, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hever, A.; Roth, R.B.; Hevezi, P.; Marin, M.E.; Acosta, J.A.; Acosta, H.; Rojas, J.; Herrera, R.; Grigoriadis, D.; White, E.; et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc. Natl. Acad. Sci. USA. 2007, 104, 12451–12456. [Google Scholar] [CrossRef] [PubMed]
- Cancro, M.P.; D’Cruz, D.P.; Khamashta, M.A. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J. Clin. Investig. 2009, 119, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Gorai, I.; Ishikawa, M.; Onose, R.; Hirahara, F.; Minaguchi, H. Antiendometrial autoantibodies are generated in patients with endometriosis. Am. J. Reprod. Immunol. 1993, 29, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.P. Autoimmunity in endometriosis: Relevance to infertility. Am. J. Reprod. Immunol. 2000, 44, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, K.; Chee Yip, Y. De novo formation of adhesions in endometriosis: The role of iron and free radical reactions. Fertil. Steril. 1995, 64, 62–64. [Google Scholar] [PubMed]
- Pillai, S.; Rust, P.F.; Howard, L. Effects of antibodies to transferrin and alpha 2-HS glycoprotein on in vitro sperm motion: Implications in infertility associated with endometriosis. Am. J. Reprod. Immunol. 1998, 39, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Prentice, L.; Bossuyt, P.M.; Farquhar, C.; Hull, M.L.; Johnson, N. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 7, CD012281. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Tapmeier, T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.; Becker, C. The miRNA mirage: How close are we to finding a non-invasive diagnostic biomarker in endometriosis? A systematic review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Suh, C.S.; Kim, S.H.; Choi, Y.M.; Moon, S.Y.; Lee, J.Y. Insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP-3 protease activity in the peritoneal fluid of patients with and without endometriosis. Fertil. Steril. 2000, 73, 996–1000. [Google Scholar] [CrossRef]
- Gurgan, T.; Bukulmez, O.; Yarali, H.; Tanir, M.; Akyildiz, S. Serum and peritoneal fluid levels of IGF I and II and insulinlike growth binding protein-3 in endometriosis. J. Reprod. Med. 1999, 44, 450–454. [Google Scholar] [PubMed]
- Giudice, L.C.; Dsupin, B.A.; Gargosky, S.E.; Rosenfeld, R.G.; Irwin, J.C. The insulin-like growth factor system in human peritoneal fluid: Its effects on endometrial stromal cells and its potential relevance to endometriosis. J. Clin. Endocrinol. Metab. 1994, 79, 1284–1293. [Google Scholar] [PubMed]
- Koutsilieris, M.; Akoum, A.; Lazure, C.; Frenette, G.; Lemay, A. N-terminal truncated forms of insulin-like growth factor binding protein-3 in the peritoneal fluid of women without laparoscopic evidence of endometriosis. Fertil. Steril. 1995, 63, 314–321. [Google Scholar] [CrossRef]
- Beste, M.T.; Pfaffle-Doyle, N.; Prentice, E.A.; Morris, S.N.; Lauffenburger, D.A.; Isaacson, K.B.; Griffith, L.G. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci. Transl. Med. 2014, 6, 222ra216. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.; Hill, A.S.; Beste, M.T.; Kumar, M.P.; Chiswick, E.; Fedorcsak, P.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Qvigstad, E. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil. Steril. 2017, 107, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Vigano, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Defrère, S.; Dolmans, M.-M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Dolmans, M.-M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Navarro, P.A. Oxidative stress and oocyte quality: Ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016, 364, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-beta and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Altun, T.; Jindal, S.; Greenseid, K.; Shu, J.; Pal, L. Low follicular fluid IL-6 levels in IVF patients are associated with increased likelihood of clinical pregnancy. J. Assist. Reprod. Genet. 2011, 28, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zollner, K.P.; Hofmann, T.; Zollner, U. Good fertilization results associated with high IL-1beta concentrations in follicular fluid of IVF patients. J. Reprod. Med. 2013, 58, 485–490. [Google Scholar] [PubMed]
- Singh, A.K.; Dutta, M.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J. Assist. Reprod. Genet. 2016, 33, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bersinger, N.A.; Mueller, M.D.; von Wolff, M. Intrafollicular inflammatory cytokines but not steroid hormone concentrations are increased in naturally matured follicles of women with proven endometriosis. J. Assist. Reprod. Genet. 2017, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Heublein, S.; Vrekoussis, T.; Kuhn, C.; Friese, K.; Makrigiannakis, A.; Mayr, D.; Lenhard, M.; Jeschke, U. Inducers of G-protein coupled estrogen receptor (GPER) in endometriosis: Potential implications for macrophages and follicle maturation. J. Reprod. Immunol. 2013, 97, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Plante, B.J.; Lessey, B.A.; Taylor, R.N.; Wang, W.; Bagchi, M.K.; Yuan, L.; Scotchie, J.; Fritz, M.A.; Young, S.L. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod. Sci. 2012, 19, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Heublein, S.; Lenhard, M.; Vrekoussis, T.; Schoepfer, J.; Kuhn, C.; Friese, K.; Makrigiannakis, A.; Mayr, D.; Jeschke, U. The G-protein-coupled estrogen receptor (GPER) is expressed in normal human ovaries and is upregulated in ovarian endometriosis and pelvic inflammatory disease involving the ovary. Reprod. Sci. 2012, 19, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Santanam, N.; Zoneraich, N.; Parthasarathy, S. Myeloperoxidase as a potential target in women with endometriosis undergoing IVF. Reprod. Sci. 2017, 24, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, A.; Albert, C.; Mercader, A.; Bonilla-Musoles, F.; Remohi, J.; Simon, C. The follicular and endocrine environment in women with endometriosis: Local and systemic cytokine production. Fertil. Steril. 1998, 70, 425–431. [Google Scholar] [CrossRef]
- Nieweglowska, D.; Hajdyla-Banas, I.; Pitynski, K.; Banas, T.; Grabowska, O.; Juszczyk, G.; Ludwin, A.; Jach, R. Age-related trends in anti-mullerian hormone serum level in women with unilateral and bilateral ovarian endometriomas prior to surgery. Reprod. Biol. Endocrinol. 2015, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Wahd, S.A.; Alalaf, S.K.; Al-Shawaf, T.; Al-Tawil, N.G. Ovarian reserve markers and assisted reproductive technique (ART) outcomes in women with advanced endometriosis. Reprod. Biol. Endocrinol. 2014, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Geng, Y.; Li, Y.; Chen, C.; Gao, Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 31, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Scala, C.; Tafi, E.; Racca, A.; Venturini, P.L.; Leone Roberti Maggiore, U. Impact of large ovarian endometriomas on the response to superovulation for in vitro fertilization: A retrospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Lamau, M.C.; Marcellin, L.; Gayet, V.; Marzouk, P.; Borghese, B.; Lafay Pillet, M.-C.; Chapron, C. Endometriosis-related infertility: Ovarian endometrioma per se is not associated with presentation for infertility. Hum. Reprod. 2016, 31, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, E.; Ottolina, J.; Vigano, P.; Brigante, C.; Marsiglio, E.; de Michele, F.; Candiani, M. Deep pelvic endometriosis negatively affects ovarian reserve and the number of oocytes retrieved for in vitro fertilization. Acta Obstet. Gynecol. Scand. 2011, 90, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Kim, J.J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil. Steril. 2017, 108, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Miravet-Valenciano, J.; Ruiz-Alonso, M.; Gómez, E.; Garcia-Velasco, J.A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: It is not affected, and let me show you why. Fertil. Steril. 2017, 108, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Sammel, M.D.; Morse, C.; Barnhart, K.T. Impact of endometriosis on IVF outcomes: An evaluation of the society for assisted reproductive technologies database. Fertil. Steril. 2016, 106, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef]
- Arici, A.; Oral, E.; Bukulmez, O.; Duleba, A.; Olive, D.L.; Jones, E.E. The effect of endometriosis on implantation: Results from the yale university in vitro fertilization and embryo transfer program. Fertil. Steril. 1996, 65, 603–607. [Google Scholar] [CrossRef]
- Harb, H.M.; Gallos, I.D.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 2013, 120, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Prapas, Y.; Goudakou, M.; Matalliotakis, I.; Kalogeraki, A.; Matalliotaki, C.; Panagiotidis, Y.; Ravanos, K.; Prapas, N. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod. Biomed. Online 2012, 25, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Gutierrez, A.; Vidal, A.; de los Santos, M.J.; Tarin, J.J.; Remohi, J.; Pellicer, A. Outcome of patients with endometriosis in assisted reproduction: Results from in-vitro fertilization and oocyte donation. Hum. Reprod. 1994, 9, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Sung, L.; Mukherjee, T.; Takeshige, T.; Bustillo, M.; Copperman, A.B. Endometriosis is not detrimental to embryo implantation in oocyte recipients. J. Assist. Reprod. Genet. 1997, 14, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.; Navarro, J.; Blasco, L.; Simon, C.; Pellicer, A.; Remohi, J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: Matched case-control study. Fertil. Steril. 2000, 74, 31–34. [Google Scholar] [CrossRef]
- González-Comadran, M.; Schwarze, J.E.; Zegers-Hochschild, F.; Souza, M.D.; Carreras, R.; Checa, M.A. The impact of endometriosis on the outcome of assisted reproductive technology. Reprod. Biol. Endocrinol. 2017, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, R.; Defrère, S.; Devoto, L. Nuclear factor-kappaB: A main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil. Steril. 2012, 98, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Khalaj, K.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Immune-inflammation gene signatures in endometriosis patients. Fertil. Steril. 2016, 106, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Berbic, M.; Hey-Cunningham, A.J.; Ng, C.; Tokushige, N.; Ganewatta, S.; Markham, R.; Russell, P.; Fraser, I.S. The role of Foxp3+ regulatory t-cells in endometriosis: A potential controlling mechanism for a complex, chronic immunological condition. Hum. Reprod. 2010, 25, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Koval, H.D.; Chopyak, V.V.; Kamyshnyi, O.M.; Kurpisz, M.K. Transcription regulatory factor expression in T-helper cell differentiation pathway in eutopic endometrial tissue samples of women with endometriosis associated with infertility. Cent. Eur. J. Immunol. 2018, 43, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Increased uNK progenitor cells in women with endometriosis and infertility are associated with low levels of endometrial stem cell factor. Am. J. Reprod. Immunol. 2016, 75, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.-W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Palomino, W.A.; Apparao, K.B.; Young, S.L.; Lininger, R.A. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod. Biol. Endocrinol. 2006, 4 (Suppl. 1), S9. [Google Scholar] [CrossRef]
- Bulun, S.E.; Utsunomiya, H.; Lin, Z.; Yin, P.; Cheng, Y.-H.; Pavone, M.E.; Tokunaga, H.; Trukhacheva, E.; Attar, E.; Gurates, B.; et al. Steroidogenic factor-1 and endometriosis. Mol. Cell. Endocrinol. 2009, 300, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, P.A.; Carver, J.G.; Kennedy, S.H.; Koninckx, P.R.; Mardon, H.J. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil. Steril. 2006, 85, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kanzaki, H.; Iwai, M.; Imai, K.; Narukawa, S.; Higuchi, T.; Katsuragawa, H.; Mori, T. Tumour necrosis factor alpha inhibits in-vitro decidualization of human endometrial stromal cells. Hum. Reprod. 1994, 9, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Illera, M.J.; Juan, L.; Stewart, C.L.; Cullinan, E.; Ruman, J.; Lessey, B.A. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil. Steril. 2000, 74, 41–48. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The role of Hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb. Perspect. Med. 2016, 6, a023002. [Google Scholar] [CrossRef] [PubMed]
- Pina Carvalho, L.F.; Hui, C.Y.Y.; Agarwal, A. Endometriosis and infertility: Biomarkers affecting implantation rate. Expert Rev. Obstet. Gynecol. 2013, 8, 467–473. [Google Scholar] [CrossRef]
- Troy, P.J.; Daftary, G.S.; Bagot, C.N.; Taylor, H.S. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol. Cell. Biol. 2003, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miravet-Valenciano, J.A.; Rincon-Bertolin, A.; Vilella, F.; Simon, C. Understanding and improving endometrial receptivity. Curr. Opin. Obstet. Gynecol. 2015, 27, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Koizumi, M.; Doshida, M.; Toya, M.; Sagara, E.; Oka, N.; Nakajo, Y.; Aono, N.; Igarashi, H.; Kyono, K. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: A retrospective, two-centers study. Reprod. Med. Biol. 2017, 16, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gimeno, P.; Ruiz-Alonso, M.; Blesa, D.; Bosch, N.; Martinez-Conejero, J.A.; Alama, P.; Garrido, N.; Pellicer, A.; Simon, C. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil. Steril. 2013, 99, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Fassbender, A.; Ruiz-Alonso, M.; Blesa, D.; D’Hooghe, T.; Simon, C. Is endometrial receptivity transcriptomics affected in women with endometriosis? A pilot study. Reprod. Biomed. Online 2015, 31, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P. Review of lipiodol treatment for infertility—An innovative treatment for endometriosis-related infertility? Aust. N. Z. J. Obstet. Gynaecol. 2014, 54, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, K.; van Rijswijk, J.; Mijatovic, V.; Goddijn, M.; Verhoeve, H.R.; van Rooij, I.A.J.; Hoek, A.; Bourdrez, P.; Nap, A.W.; Rijnsaardt-Lukassen, H.G.M.; et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N. Engl. J. Med. 2017, 376, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Onalan, G.; Tohma, Y.A.; Zeyneloglu, H.B. Effect of etanercept on the success of assisted reproductive technology in patients with endometrioma. Gynecol. Obstet. Investig. 2018, 83, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Craessaerts, M.; Timmerman, D.; Cornillie, F.; Kennedy, S. Anti-TNF-α treatment for deep endometriosis-associated pain: A randomized placebo-controlled trial. Hum. Reprod. 2008, 23, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Acien, P.; Quereda, F.J.; Gomez-Torres, M.J.; Bermejo, R.; Gutierrez, M. GnRH analogues, transvaginal ultrasound-guided drainage and intracystic injection of recombinant interleukin-2 in the treatment of endometriosis. Gynecol. Obstet. Investig. 2003, 55, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Acien, P.; Quereda, F.; Campos, A.; Gomez-Torres, M.J.; Velasco, I.; Gutierrez, M. Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: A randomized clinical trial. Fertil. Steril. 2002, 78, 705–711. [Google Scholar] [CrossRef]
- Almassinokiani, F.; Mehdizadeh, A.; Sariri, E.; Rezaei, M.; Almasi, A.; Akbari, H.; Pazouki, A.; Solaymani-Dodaran, M.; Asadollah, S.; Amirkhani, J.; et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med. Sci. Monit. 2013, 19, 534–539. [Google Scholar] [PubMed]
- Gomez, R.; Abad, A.; Delgado, F.; Tamarit, S.; Simon, C.; Pellicer, A. Effects of hyperprolactinemia treatment with the dopamine agonist quinagolide on endometriotic lesions in patients with endometriosis-associated hyperprolactinemia. Fertil. Steril. 2011, 95, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Creus, M.; Fábregues, F.; Carmona, F.; del Pino, M.; Manau, D.; Balasch, J. Combined laparoscopic surgery and pentoxifylline therapy for treatment of endometriosis-associated infertility: A preliminary trial. Hum. Reprod. 2008, 23, 1910–1916. [Google Scholar] [CrossRef] [PubMed]
- Alborzi, S.; Ghotbi, S.; Parsanezhad, M.E.; Dehbashi, S.; Alborzi, S.; Alborzi, M. Pentoxifylline therapy after laparoscopic surgery for different stages of endometriosis: A prospective, double-blind, randomized, placebo-controlled study. J. Minim. Invasive Gynecol. 2007, 14, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Kamencic, H.; Thiel, J.A. Pentoxifylline after conservative surgery for endometriosis: A randomized, controlled trial. J. Minim. Invasive Gynecol. 2008, 15, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, Y.J.; Kim, J.B.; Lee, K.H.; Kwon, S.K.; Ahn, J.W.; Kim, S.H.; Chae, H.D.; Kang, B.M. Effect of pioglitazone on production of regulated upon activation normal T-cell expressed and secreted (RANTES) and IVF outcomes in infertile women with endometriosis. Dev. Reprod. 2013, 17, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Foda, A.A.; Aal, I.A.A. Metformin as a new therapy for endometriosis, its effects on both clinical picture and cytokines profile. Middle East Fertil. Soc. J. 2012, 17, 262–267. [Google Scholar] [CrossRef]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl. Res. 2013, 161, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, A.; Conceicao Dos Santos, C.C.; Costa, G.D.; Deitos, A.; de Souza, A.; de Souza, I.C.; Torres, I.L.; da Cunha Filho, J.S.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B.; Curry, T.E.; Vernon, M.W. Immunomodulation of rat endometriotic implant growth and protein production. Am. J. Reprod. Immunol. 1994, 31, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Steinleitner, A.; Lambert, H.; Suarez, M.; Serpa, N.; Roy, S. Immunomodulation in the treatment of endometriosis-associated subfertility: Use of pentoxifylline to reverse the inhibition of fertilization by surgically induced endometriosis in a rodent model. Fertil. Steril. 1991, 56, 975–979. [Google Scholar] [CrossRef]
- Lu, D.; Song, H.; Li, Y.; Clarke, J.; Shi, G. Pentoxifylline for endometriosis. Cochrane Database Syst. Rev. 2012, 1, CD007677. [Google Scholar] [CrossRef] [PubMed]
- Uygur, D.; Aytan, H.; Zergeroglu, S.; Batioglu, S. Leflunomide—An immunomodulator—Induces regression of endometrial explants in a rat model of endometriosis. J. Soc. Gynecol. Investig. 2006, 13, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Keenan, J.A.; Williams-Boyce, P.K.; Massey, P.J.; Chen, T.T.; Caudle, M.R.; Bukovsky, A. Regression of endometrial explants in a rat model of endometriosis treated with the immune modulators loxoribine and levamisole. Fertil. Steril. 1999, 72, 135–141. [Google Scholar] [CrossRef]
- Olivares, C.; Ricci, A.; Bilotas, M.; Baranao, R.I.; Meresman, G. The inhibitory effect of celecoxib and rosiglitazone on experimental endometriosis. Fertil. Steril. 2011, 96, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Della-Morte, D.; Palmirotta, R.; Rehni, A.K.; Pastore, D.; Capuani, B.; Pacifici, F.; De Marchis, M.L.; Dave, K.R.; Bellia, A.; Fogliame, G.; et al. Pharmacogenomics and pharmacogenetics of thiazolidinediones: Role in diabetes and cardiovascular risk factors. Pharmacogenomics 2014, 15, 2063–2082. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Arya, A.; Malecek, M.K.; Shin, D.S.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Altman, J.K.; Platanias, L.; et al. Repurposing metformin for cancer treatment: Current clinical studies. Oncotarget 2016, 7, 40767–40780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, J.N.; Zeng, C.; Li, X.; Zhou, Y.F.; Qi, Y.; Xue, Q. Metformin suppresses prostaglandin E2-induced cytochrome P450 aromatase gene expression and activity via stimulation of AMP-activated protein kinase in human endometriotic stromal cells. Reprod. Sci. 2015, 22, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.N.; Zeng, C.; Zhou, Y.; Peng, C.; Zhou, Y.F.; Xue, Q. Metformin inhibits StAR expression in human endometriotic stromal cells via AMPK-mediated disruption of CREB-CRTC2 complex formation. J. Clin. Endocrionol. Metab. 2014, 99, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Ozcan Cenksoy, P.; Oktem, M.; Erdem, O.; Karakaya, C.; Cenksoy, C.; Erdem, A.; Guner, H.; Karabacak, O. A potential novel treatment strategy: Inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol. Endocrinol. 2015, 31, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Aydin, N.E.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Chen, T.S.; Liou, S.Y.; Hsieh, C.C. Immunomodulatory effects of EGCg fraction of green tea extract in innate and adaptive immunity via T regulatory cells in murine model. Immunopharmacol. Immunotoxicol. 2014, 36, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Grund, E.M.; Kagan, D.; Tran, C.A.; Zeitvogel, A.; Starzinski-Powitz, A.; Nataraja, S.; Palmer, S.S. Tumor necrosis factor-α regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor κB in human endometriotic epithelial cells. Mol. Pharmacol. 2008, 73, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Song, H.; Shi, G. Anti-TNF-α treatment for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2013, 3, CD008088. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.I.; Gungor, A.C.; Adali, E.; Yay, A.; Onder, G.O.; Inceboz, U. A humanized anti-interleukin 6 receptor monoclonal antibody, tocilizumab, for the treatment of endometriosis in a rat model. Reprod. Sci. 2016, 23, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Quattrone, F.; Sanchez, A.M.; Pannese, M.; Hemmerle, T.; Vigano, P.; Candiani, M.; Petraglia, F.; Neri, D.; Panina-Bordignon, P. The targeted delivery of interleukin 4 inhibits development of endometriotic lesions in a mouse model. Reprod. Sci. 2015, 22, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Awonuga, A.O.; Saed, G.M.; Diamond, M.P. Laparoscopy in gynecologic surgery: Adhesion development, prevention, and use of adjunctive therapies. Clin. Obstet. Gynecol. 2009, 52, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.N.; Saed, G.M.; Diamond, M.P. Predisposing factors to post-operative adhesion development. Hum. Reprod. Update 2015, 21, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Shen, F.; Lu, Y.; Liu, X.; Guo, S.-W. Cyclooxygenase-2 overexpression in ovarian endometriomas is associated with higher risk of recurrence. Fertil. Steril. 2009, 91, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, B.W.; Emeis, J.J.; Kooistra, T.; Trimbos, J.B.; Moore, N.R.; Zwinderman, K.H.; Trimbos-Kemper, T.C. A role for the fibrinolytic system in postsurgical adhesion formation. Fertil. Steril. 2005, 83, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.C.; Shelton, J.B.; Laird, S.M.; Richmond, M.; Kudesia, G.; Li, T.C.; Ledger, W.L. IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Hum. Reprod. 2002, 17, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Barcz, E.; Milewski, Ł.; Dziunycz, P.; Kamiński, P.; Płoski, R.; Malejczyk, J. Peritoneal cytokines and adhesion formation in endometriosis: An inverse association with vascular endothelial growth factor concentration. Fertil. Steril. 2012, 97, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Au, H.K.; Chang, J.H.; Wu, Y.C.; Kuo, Y.C.; Chen, Y.H.; Lee, W.C.; Chang, T.S.; Lan, P.C.; Kuo, H.C.; Lee, K.L.; et al. TGF-betaI regulates cell migration through pluripotent transcription factor OCT4 in endometriosis. PLoS ONE 2015, 10, e0145256. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Au, H.K.; Lee, W.C.; Chi, C.C.; Ling, T.Y.; Wang, L.M.; Kao, S.H.; Huang, Y.H.; Tzeng, C.R. Expression of the pluripotent transcription factor OCT4 promotes cell migration in endometriosis. Fertil. Steril. 2013, 99, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Duan, J.; Liu, X.; Guo, S.W. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial–mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol. Cell. Endocrinol. 2016, 428, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cen, B.; Chen, S.; He, Y. MicroRNA-29b inhibits TGF-beta1-induced fibrosis via regulation of the TGF-beta1/Smad pathway in primary human endometrial stromal cells. Mol. Med. Rep. 2016, 13, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Stocks, M.M.; Crispens, M.A.; Ding, T.; Mokshagundam, S.; Bruner-Tran, K.L.; Osteen, K.G. Therapeutically targeting the inflammasome product in a chimeric model of endometriosis-related surgical adhesions. Reprod. Sci. 2017, 24, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R. Immune modulatory effects of statins. Immunology 2018, 154, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Ridker, P.M. Anti-inflammatory effects of statins: Clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 2005, 4, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Noventa, M.; Quaranta, M.; Gizzo, S. Statins as targeted “magical pills” for the conservative treatment of endometriosis: May potential adverse effects on female fertility represent the “dark side of the same coin”? A systematic review of literature. Reprod. Sci. 2015, 23, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.; Basar, M.; Seval-Celik, Y.; Osteen, K.G.; Duleba, A.J.; Taylor, H.S.; Lockwood, C.J.; Arici, A. Statins inhibit monocyte chemotactic protein 1 expression in endometriosis. Reprod. Sci. 2012, 19, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Dhawan, V.; Mahajan, N.; Saha, S.C.; Dhaliwal, L.K. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil. Steril. 2010, 94, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Rung, E.; Friberg, P.A.; Bergh, C.; Billig, H. Depletion of substrates for protein prenylation increases apoptosis in human periovulatory granulosa cells. Mol. Reprod. Dev. 2006, 73, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Cress, A.B.; Wong, D.H.; Villanueva, J.A.; Sokalska, A.; Moeller, B.C.; Stanley, S.D.; Duleba, A.J. Simvastatin reduces steroidogenesis by inhibiting Cyp17a1 gene expression in rat ovarian theca-interstitial cells. Biol. Reprod. 2012, 86, 20. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, S.; Capuzzo, D.; Zicchina, C.; Di Gangi, S.; Coronella, M.L.; Andrisani, A.; Gangemi, M.; Nardelli, G.B. Could empirical low-dose-aspirin administration during IVF cycle affect both the oocytes and embryos quality via COX 1-2 activity inhibition? J. Assist. Reprod. Genet. 2014, 31, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Uimari, O.; Rahmioglu, N.; Nyholt, D.R.; Vincent, K.; Missmer, S.A.; Becker, C.; Morris, A.P.; Montgomery, G.W.; Zondervan, K.T. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum. Reprod. 2017, 32, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yu, Y.; Luo, L.; Lydon, J.P.; Jeong, J.W.; Kim, J.J. Activated AKT pathway promotes establishment of endometriosis. Endocrinology 2014, 155, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum. Reprod. 2015, 30, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Marcellin, L.; Tosti, C.; Chouzenoux, S.; Cerles, O.; Borghese, B.; Batteux, F.; Chapron, C. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert Opin. Ther. Targets 2015, 19, 1465–1483. [Google Scholar] [CrossRef] [PubMed]
- Leconte, M.; Nicco, C.; Ngo, C.; Arkwright, S.; Chereau, C.; Guibourdenche, J.; Weill, B.; Chapron, C.; Dousset, B.; Batteux, F. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am. J. Pathol. 2010, 177, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Nicco, C.; Leconte, M.; Chereau, C.; Arkwright, S.; Vacher-Lavenu, M.C.; Weill, B.; Chapron, C.; Batteux, F. Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo. J. Pathol. 2010, 222, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Pala, H.G.; Erbas, O.; Pala, E.E.; Artunc Ulkumen, B.; Akman, L.; Akman, T.; Oltulu, F.; Yavasoglu, A. The effects of sunitinib on endometriosis. J. Obstet. Gynaecol. 2015, 35, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Montor, W.R.; Salas, A.; Melo, F.H.M. Receptor tyrosine kinases and downstream pathways as druggable targets for cancer treatment: The current arsenal of inhibitors. Mol. Cancer 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Lam, W.; Guo, W.; Feng, Y.; Cheng, Y.C. Targeting tumour microenvironment by tyrosine kinase inhibitor. Mol. Cancer 2018, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Marcellin, L.; Chouzenoux, S.; Boulard, V.; Just, P.A.; Nicco, C.; Chereau, C.; Tosti, C.; Chapron, C.; Batteux, F. Role of the protein kinase BRAF in the pathogenesis of endometriosis. Expert Opin. Ther. Targets 2016, 20, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Disi, A.M.; Taha, M.O. Sunitinib as an anti-endometriotic agent. Eur. J. Pharm. Sci. 2013, 49, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, C.; Kacan, T.; Akkar, O.B.; Karakus, S.; Kacan, S.B.; Ozer, H.; Cetin, A. Effects of pazopanib, sunitinib, and sorafenib, anti-VEGF agents, on the growth of experimental endometriosis in rats. Reprod. Sci. 2015, 22, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xin, X.; Hua, T.; Shi, R.; Chi, S.; Jin, Z.; Wang, H. Efficacy of anti-VEGF/VEGFR agents on animal models of endometriosis: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0166658. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.; Portelli, M.; Pollacco, J.; Schembri-Wismayer, P.; Calleja-Agius, J. The role of prostaglandin E2 in endometriosis. Gynecol. Endocrinol. 2012, 28, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Rakhila, H.; Carli, C.; Daris, M.; Lemyre, M.; Leboeuf, M.; Akoum, A. Identification of multiple and distinct defects in prostaglandin biosynthetic pathways in eutopic and ectopic endometrium of women with endometriosis. Fertil. Steril. 2013, 100, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Rakhila, H.; Bourcier, N.; Akoum, A.; Pouliot, M. Abnormal expression of prostaglandins E2 and F2alpha receptors and transporters in patients with endometriosis. Biomed Res. Int. 2015, 2015, 808146. [Google Scholar]
- Banu, S.K.; Lee, J.; Speights, V.O., Jr.; Starzinski-Powitz, A.; Arosh, J.A. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol. Endocrinol. 2009, 23, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Lee, J.; Balasubbramanian, D.; Stanley, J.A.; Long, C.R.; Meagher, M.W.; Osteen, K.G.; Bruner-Tran, K.L.; Burghardt, R.C.; Starzinski-Powitz, A.; et al. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc. Natl. Acad. Sci. USA 2015, 112, 9716–9721. [Google Scholar] [CrossRef] [PubMed]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2017, 7, CD007807. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Capriglione, S.; Peterlunger, I.; La Rosa, V.L.; Vitagliano, A.; Noventa, M.; Valenti, G.; Sapia, F.; Angioli, R.; Lopez, S.; et al. The role of oxidative stress and membrane transport systems during endometriosis: A fresh look at a busy corner. Oxid. Med. Cell. Longev. 2018, 2018, 7924021. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Kolahdouz-Mohammadi, R. Curcumin and endometriosis: Review on potential roles and molecular mechanisms. Biomed. Pharmacother. 2018, 97, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G.; Schor, E.; Kopelman, A. Nutritional aspects related to endometriosis. Rev. Assoc. Med. Bras. 2015, 61, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, K.; Babu, K.N.; Singh, A.K.; Das, S.; Kumar, A.; Seal, S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomedicine 2013, 9, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; O, D.F.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, D.; Li, F.; Cosar, E.; Krikun, G.; Taylor, H.S. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin. Reprod. Med. 2015, 33, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.P.; Wang, K.H.; Chang, C.C.; Lee, J.N.; Long, C.Y.; Chen, H.S.; Tsai, C.F.; Hsieh, T.H.; Tsai, E.M. Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil. Steril. 2011, 95, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.W.; Ng, E.H.; Yeung, W.S. Identification of cells with colony-forming activity, self-renewal capacity, and multipotency in ovarian endometriosis. Am. J. Pathol. 2011, 178, 2832–2844. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, Y.; Zhu, H.; Jiang, Y.; Zhang, S. Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/beta-catenin pathway by paracrine production of TGF-beta1 and Wnt1. Hum. Reprod. 2016, 31, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Pittatore, G.; Moggio, A.; Benedetto, C.; Bussolati, B.; Revelli, A. Endometrial adult/progenitor stem cells: Pathogenetic theory and new antiangiogenic approach for endometriosis therapy. Reprod. Sci. 2014, 21, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Canosa, S.; Moggio, A.; Brossa, A.; Pittatore, G.; Marchino, G.L.; Leoncini, S.; Benedetto, C.; Revelli, A.; Bussolati, B. Angiogenic properties of endometrial mesenchymal stromal cells in endothelial co-culture: An in vitro model of endometriosis. Mol. Hum. Reprod. 2017, 23, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, G.; Balendran, S.; Pröstling, K.; Reischer, T.; Birner, P.; Wenzl, R.; Kuessel, L.; Streubel, B.; Husslein, H. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 204, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Moggio, A.; Pittatore, G.; Cassoni, P.; Marchino, G.L.; Revelli, A.; Bussolati, B. Sorafenib inhibits growth, migration, and angiogenic potential of ectopic endometrial mesenchymal stem cells derived from patients with endometriosis. Fertil. Steril. 2012, 98, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Abomaray, F.; Gidlof, S.; Gotherstrom, C. Mesenchymal stromal cells are more immunosuppressive in vitro if they are derived from endometriotic lesions than from eutopic endometrium. Stem Cells Int. 2017, 2017, 3215962. [Google Scholar] [CrossRef] [PubMed]
- Polymeri, A.; Giannobile, W.V.; Kaigler, D. Bone marrow stromal stem cells in tissue engineering and regenerative medicine. Horm. Metab. Res. 2016, 48, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sanchis, C.; Cervello, I.; Khurana, S.; Faus, A.; Verfaillie, C.; Simon, C. Contribution of different bone marrow-derived cell types in endometrial regeneration using an irradiated murine model. Fertil. Steril. 2015, 103, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, T.; Kyo, S.; Maida, Y.; Ozaki, S.; Takakura, M.; Nakao, S.; Inoue, M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am. J. Obstet. Gynecol. 2009, 201, 608. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.R.; Cousins, F.L.; Yang, X.; Mushafi, A.; Breault, D.T.; Gargett, C.E.; Deane, J.A. Bone marrow stem cells do not contribute to endometrial cell lineages in chimeric mouse models. Stem Cells 2018, 36, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mamillapalli, R.; Mutlu, L.; Du, H.; Taylor, H.S. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015, 15, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.; Naqvi, H.; Komm, B.; Taylor, H.S. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology 2014, 155, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, G.S.; Zolbin, M.M.; Cosar, E.; Mamillapalli, R.; Taylor, H.S. Medical therapies for endometriosis differentially inhibit stem cell recruitment. Reprod. Sci. 2017, 24, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Xia, W.; Huo, L.; Lim, S.O.; Wu, Y.; Hsu, J.L.; Chao, C.H.; Yamaguchi, H.; Yang, N.K.; Ding, Q.; et al. Epithelial–mesenchymal transition induced by TNF-alpha requires NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res. 2012, 72, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene 2015, 34, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Pappan, L.; Galliher-Beckley, A.; Shi, J. Il-1beta promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol. Cancer 2012, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Wu, Y.C.; Chi, C.C.; Su, W.C.; Chang, P.J.; Lee, K.F.; Tung, T.H.; Wang, J.; Liu, J.J.; Tung, S.Y.; et al. Activation of IL6/IGFIR confers poor prognosis of HBV-related hepatocellular carcinoma through induction of OCT4/NANOG expression. Clin. Cancer Res. 2015, 21, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S.; Chen, C.L.; Wu, Y.C.; Liu, J.J.; Kuo, Y.C.; Lee, K.F.; Lin, S.Y.; Lin, S.E.; Tung, S.Y.; Kuo, L.M.; et al. Inflammation promotes expression of stemness-related properties in HBV-related hepatocellular carcinoma. PLoS ONE 2016, 11, e0149897. [Google Scholar] [CrossRef] [PubMed]
- Lv, N.; Shan, Z.; Gao, Y.; Guan, H.; Fan, C.; Wang, H.; Teng, W. Twist1 regulates the epithelial–mesenchymal transition via the NF-kappaB pathway in papillary thyroid carcinoma. Endocrine 2016, 51, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-κB pathway and cancer stem cells. Cells 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Galoczova, M.; Coates, P.; Vojtesek, B. STAT3, stem cells, cancer stem cells and p63. Cell. Mol. Biol. Lett. 2018, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Sorjamaa, A.; Kangasniemi, M.; Sutinen, M.; Salo, T.; Liakka, A.; Lehenkari, P.; Tapanainen, J.S.; Vuolteenaho, O.; Chen, J.C.; et al. Niche matters: The comparison between bone marrow stem cells and endometrial stem cells and stromal fibroblasts reveal distinct migration and cytokine profiles in response to inflammatory stimulus. PLoS ONE 2017, 12, e0175986. [Google Scholar] [CrossRef] [PubMed]
- Pricola, K.L.; Kuhn, N.Z.; Haleem-Smith, H.; Song, Y.; Tuan, R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an erk1/2-dependent mechanism. J. Cell. Biochem. 2009, 108, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Fujisawa, T.; Ono, M.; Hara, E.S.; Pham, H.T.; Nakajima, R.; Sonoyama, W.; Kuboki, T. A short-term treatment with tumor necrosis factor-alpha enhances stem cell phenotype of human dental pulp cells. Stem Cell Res. Ther. 2014, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, N.; Zhou, J.; Tang, L.; Ding, B.; Duan, Y.; Jin, Y. Inflammatory environment induces gingival tissue-specific mesenchymal stem cells to differentiate towards a pro-fibrotic phenotype. Biol. Cell. 2013, 105, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Natali, L.; Nisi, M.; De Leo, M.; Daniele, S.; Costa, B.; Graziani, F.; Gabriele, M.; Braca, A.; Trincavelli, M.L.; et al. Negative effects of a high tumour necrosis factor-alpha concentration on human gingival mesenchymal stem cell trophism: The use of natural compounds as modulatory agents. Stem Cell Res. Ther. 2018, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Werkmeister, J.A.; Gargett, C.E. Inhibition of transforming growth factor-beta receptor signaling promotes culture expansion of undifferentiated human endometrial mesenchymal stem/stromal cells. Sci. Rep. 2015, 5, 15042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, W.; Li, N.; Liu, H.; He, H.; Du, Y.; Zhang, Z.; Liu, Y. Estrogen stabilizes hypoxia-inducible factor 1alpha through g protein-coupled estrogen receptor 1 in eutopic endometrium of endometriosis. Fertil. Steril. 2017, 107, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Xiong, W.; Zhang, L.; Liu, H.; Du, Y.; Li, N. Hypoxia-inducible factor 1alpha-induced epithelial–mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum. Reprod. 2016, 31, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, L.; Xiong, Y.; Liu, H.; Liu, Y. Hypoxia promotes invasion of endometrial stromal cells via hypoxia-inducible factor 1alpha upregulation-mediated beta-catenin activation in endometriosis. Reprod. Sci. 2016, 23, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Xiong, Y.; Li, N.; He, H.; Du, Y.; Liu, Y. Hypoxia-inducible factor-1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 2017, 153, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Seppanen, E.; Roy, E.; Ellis, R.; Bou-Gharios, G.; Fisk, N.M.; Khosrotehrani, K. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: Evidence from a murine fetal microchimerism model. PLoS ONE 2013, 8, e62662. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Leto Barone, A.A.; Khalifian, S.; Lee, W.P.; Brandacher, G. Immunomodulatory effects of adipose-derived stem cells: Fact or fiction? BioMed Res. Int. 2013, 2013, 383685. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian Kia, N.; Bahrami, A.R.; Ebrahimi, M.; Matin, M.M.; Neshati, Z.; Almohaddesin, M.R.; Aghdami, N.; Bidkhori, H.R. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J. Mol. Neurosci. 2011, 44, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.; Blanco, B.; Yanez, R.; Vazquez, M.; Herrero Sanchez, C.; Fernandez-Garcia, M.; Rodriguez Serrano, C.; Pescador, D.; Blanco, J.F.; Hernando-Rodriguez, M.; et al. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy 2016, 18, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Blanco, B.; Herrero-Sanchez, M.D.; Rodriguez-Serrano, C.; Garcia-Martinez, M.L.; Blanco, J.F.; Muntion, S.; Garcia-Arranz, M.; Sanchez-Guijo, F.; Del Canizo, C. Immunomodulatory effects of bone marrow versus adipose tissue-derived mesenchymal stromal cells on NK cells: Implications in the transplantation setting. Eur. J. Haematol. 2016, 97, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bochev, I.; Elmadjian, G.; Kyurkchiev, D.; Tzvetanov, L.; Altankova, I.; Tivchev, P.; Kyurkchiev, S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol. Int. 2008, 32, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Laranjeira, P.; Mendes, S.; Velada, I.; Leite, C.; Andrade, P.; Santos, F.; Henriques, A.; Graos, M.; Cardoso, C.M.; et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. Ther. 2013, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Castro-Manrreza, M.E.; Mayani, H.; Monroy-Garcia, A.; Flores-Figueroa, E.; Chavez-Rueda, K.; Legorreta-Haquet, V.; Santiago-Osorio, E.; Montesinos, J.J. Human mesenchymal stromal cells from adult and neonatal sources: A comparative in vitro analysis of their immunosuppressive properties against T cells. Stem Cells Dev. 2014, 23, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Yen, M.L.; Chen, Y.C.; Chien, C.C.; Huang, H.I.; Bai, C.H.; Yen, B.L. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells 2006, 24, 2466–2477. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Abomaray, F.M.; Alshabibi, M.A.; AlAskar, A.S.; Kalionis, B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 2017, 59, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chou, H.L.; Chang, Y.H.; Hung, W.T.; Liu, H.W.; Chu, T.Y. Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stroma-derived stem cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, Z.; Abedindo, A.; Omrani, M.D.; Ghaderian, S.M.H. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: A systematic review. Stem Cell Rev. 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Duan, H.; Xu, Q.; Tang, Y.-Q.; Li, J.-J.; Sun, F.-Q.; Wang, S. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 2017, 19, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Nagori, C.B.; Panchal, S.Y.; Patel, H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J. Hum. Reprod. Sci. 2011, 4, 43–48. [Google Scholar] [PubMed]
- Santamaria, X.; Cabanillas, S.; Cervelló, I.; Arbona, C.; Raga, F.; Ferro, J.; Palmero, J.; Remohí, J.; Pellicer, A.; Simón, C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: A pilot cohort study. Hum. Reprod. 2016, 31, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yang, T.; Li, J.; Yang, X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res. Ther. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Shalaby, S.M.; Abdelaziz, M.; Brakta, S.; Hill, W.D.; Ismail, N.; Al-Hendy, A. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod. Sci. 2018, 25, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, Q.; He, J.; She, H.; Zhang, Z.; Yin, C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res. Ther. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Taylor, H.S. Therapeutic strategies involving uterine stem cells in reproductive medicine. Curr. Opin. Obstet. Gynecol. 2018, 30, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.W.; Takashi, S.; Baik, G.H.; Shibata, W.; DiPrete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cheng, X.; Ding, Y.; Shi, J.; Jin, H.; Wang, H.; Wu, Y.; Ye, J.; Lu, Y.; Wang, T.C.; et al. Bone marrow-derived myofibroblasts promote colon tumorigenesis through the IL-6/JAK2/STAT3 pathway. Cancer Lett. 2014, 343, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Li, H.Y.; Zhao, X.P.; Jiao, J.Y.; Tang, D.X.; Yan, L.J.; Wan, Q.; Pan, C.B. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinoma cells. Cancer Sci. 2017, 108, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008, 68, 4331–4339. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Shu-Uin, G.; Kae-Siang, N.; Gauthaman, K.; Biswas, A.; Choolani, M.; Bongso, A.; Chui-Yee, F. Human umbilical cord Wharton’s jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Park, H.; Lim, E.H.; Lee, K.W. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int. J. Oncol. 2012, 40, 130–138. [Google Scholar] [PubMed]
- Chu, Y.; Wang, Y.; Peng, W.; Xu, L.; Liu, M.; Li, J.; Hu, X.; Li, Y.; Zuo, J.; Ye, Y. STAT3 activation by IL-6 from adipose-derived stem cells promotes endometrial carcinoma proliferation and metastasis. Biochem. Biophys. Res. Commun. 2018, 500, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, Y.; Yue, B.; Ma, X.; Zhang, G.; Xiang, H.; Liu, Y.; Wang, T.; Wu, X.; Chen, B. Adipose-derived mesenchymal stem cells promote osteosarcoma proliferation and metastasis by activating the STAT3 pathway. Oncotarget 2017, 8, 23803–23816. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: Implications for basic research and the clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tal, R.; Pluchino, N.; Mamillapalli, R.; Taylor, H.S. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J. Cell. Mol. Med. 2018, 22, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Tang, H.; Guo, Y.; Guo, J.; Huang, B.; Fang, F.; Cai, J.; Wang, Z. Adipose-derived mesenchymal stem cells promote cell proliferation and invasion of epithelial ovarian cancer. Exp. Cell Res. 2015, 337, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, K.; Yee, F.C.; Cheyyatraivendran, S.; Biswas, A.; Choolani, M.; Bongso, A. Human umbilical cord Wharton's jelly stem cell (hWJSC) extracts inhibit cancer cell growth in vitro. J. Cell. Biochem. 2012, 113, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Wang, Q.; Zhang, Q.; Sun, J.; He, B.; Xiang, C.; Liu, Z.; Lai, D. Human endometrial mesenchymal stem cells exhibit intrinsic anti-tumor properties on human epithelial ovarian cancer cells. Sci. Rep. 2016, 6, 37019. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.H.; Zhang, Y.; Solley, T.; Amaya-Manzanares, F.; Marini, F.; Andreeff, M.; Debeb, B.; Woodward, W.; Schmandt, R.; Broaddus, R.; et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin. Cancer Res. 2012, 18, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liang, T.; Wang, D.; Li, L.; Cheng, Y.; Guo, Q.; Zhang, G. IFNα-expressing amniotic fluid-derived mesenchymal stem cells migrate to and suppress HeLa cell-derived tumors in a mouse model. Stem Cells Int. 2018, 2018, 1241323. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.H.; Reagan, M.R.; Anderson, K.; Kaplan, D.L.; Rosenblatt, M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer Res. 2010, 70, 10044–10050. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Jiang, Q.; Deng, J.; Xu, F.; Chen, X.; Cheng, F.; Zhang, Y.; Yao, Y.; Xia, Z.; et al. Human adipose-derived mesenchymal stem cell-secreted CXCL1 and CXCL8 facilitate breast tumor growth by promoting angiogenesis. Stem Cells 2017, 35, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Chen, D.; Chen, F.; Chi, Y.; Han, Z.; Feng, X.; Li, X.; Han, Z. Human umbilical cord mesenchymal stem cells promote breast cancer metastasis by interleukin-8- and interleukin-6-dependent induction of CD44(+)/CD24(-) cells. Cell Transplant. 2015, 24, 2585–2599. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Wang, Y.; He, N.; Wang, D.; Zhao, Q.; Feng, G.; Su, W.; Xu, Y.; Han, Z.; Kong, D.; et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials 2014, 35, 5162–5170. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.H.; Yi, B.R.; Lim, S.Y.; Hwang, K.A.; Baek, Y.S.; Kang, K.S.; Choi, K.C. Human amniotic membrane-derived epithelial stem cells display anticancer activity in BALB/c female nude mice bearing disseminated breast cancer xenografts. Int. J. Oncol. 2012, 40, 2022–2028. [Google Scholar] [PubMed]
- Cheng, Y.; Li, L.; Wang, D.; Guo, Q.; He, Y.; Liang, T.; Sun, L.; Wang, X.; Cheng, Y.; Zhang, G. Characteristics of human endometrium-derived mesenchymal stem cells and their tropism to endometriosis. Stem Cells Int. 2017, 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Koippallil Gopalakrishnan, A.R.; Pandit, H.; Metkari, S.M.; Warty, N.; Madan, T. Adenoviral vector encoding soluble Flt-1 engineered human endometrial mesenchymal stem cells effectively regress endometriotic lesions in NOD/SCID mice. Gene Ther. 2016, 23, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Kondo, W.; Dal Lago, E.A.; Francisco, J.C.; Simeoni, R.B.; de Noronha, L.; Martins, A.P.; de Azevedo, M.L.; Ferreira, C.C.; Maestrelli, P.; Olandoski, M.; et al. Effect of the bone marrow derived-mononuclear stem cells transplantation in the growth, VEGF-R and TNF-alpha expression of endometrial implants in Wistar rats. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 158, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Abreu, J.P.; Rebelatto, C.L.K.; Savari, C.A.; Capriglione, L.G.A.; Miyague, L.; Noronha, L.; Amaral, V.F.D. The effect of mesenchymal stem cells on fertility in experimental retrocervical endometriosis. Rev. Bras. Ginecol. Obstet. 2017, 39, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Invitti, A.; Parreira, R.; Schor, E.; Silva, I.D.C.G.; Girão, M.J.B.C. Mesenchymal stem cells treatment improves the endometriosis proliferation in cell culture. FASEB J. 2013, 27, lb704. [Google Scholar]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Proposed Mechanism | Indication | Outcome | Comments |
|---|---|---|---|---|
| Anti-TNF-α Treatment | ||||
| Etanercept | Binds and inhibits TNF-α | Infertility | Significantly higher clinical pregnancy rate in patients receiving etanercept | Ref. [102] |
| Infliximab | Binds and inhibits TNF-α | Pain | No significant effect on pain or lesion size | Phase II trial Ref. [103] |
| Cytokine Treatment | ||||
| Recombinant interleukin-2 (rIL-2) | Enhances cytotoxic activity of macrophages and NK cells | Endometrioma postdrainage recurrence prevention | Significantly longer time to disease recurrence with favorable symptom improvement in rIL-2 and GnRH agonist combination group | Ref. [104] |
| Interferon-α-2b | Enhances cytotoxic activity of macrophages and NK cells | Postoperative recurrence prevention | No improvement in disease recurrence | Ref. [105] |
| Angiogenesis Inhibitor | ||||
| Simvastatin | Inhibits proliferation and angiogenesis | Postoperative pain | No significant difference from GnRH agonist group | Ref. [106] |
| Quinagolide | Dopamine receptor agonist for treatment of hyperprolactemia; also has VEGFR2 inhibition effect | Hyperprolactinemic patients with endometriosis | Decreased lesion size with downregulation of angiogenesis markers | Ref. [107] |
| Cabergoline | Dopamine receptor agonist for treatment of hyperprolactemia; also has VEGFR2 inhibition effect | Pain | N/A | Phase II trial; Recruiting; Clinical Trials.gov: NCT00115661 |
| Immunomodulatory and Anti-Inflammatory Agents | ||||
| Pentoxifylline | Nonselective phosphodiesterase inhibitor; reduces platelet aggregation through platelet phosphodiesterase inhibition; inhibits TNF-α and leukotriene synthesis | Infertility in mild/moderate endometriosis | Nonsignificant increase in cumulative probability of pregnancy in patients receiving pentoxifylline | Phase III trial; Clinical Trials.gov: NCT00632697; Ref. [108] |
| Infertility | No significant difference in pregnancy rate or disease recurrence | Ref. [109] | ||
| Postoperative pain | Improved pain score at 2–3 months after surgery | Ref. [110] | ||
| Pioglitazone | PPAR-γ agonist; inhibits inflammatory cytokine production and NFκB activity | Infertility | Nonsignificant increase in clinical pregnancy rate; significant increase in embryo implantation rate | Ref. [111] |
| Rosiglitazone | PPAR-γ agonist; inhibits inflammatory cytokine production and NFκB activity | Pain | Terminated/withdrawn due to adverse cardiovascular events | Clinical Trials.gov: NCT00115661/NCT00121953 |
| Metformin | Suppresses inflammatory response and aromatase activity; decreases local estrogen production | Pain and infertility | Improved pregnancy rate; improved symptom score | Ref. [112] |
| Resveratrol | Inhibits hypoxia-mediated ERK1/2, AKT, and MMP2/9 activity | Pain | Nonsignificant decrease in pain score and serum CA-125 level | Phase IV trial; Clinical Trials.gov: NCT02475564 |
| EGCg | Inhibits ROS-induced NFκB activation and MAPK, JNK, and p38 signaling; inhibits angiogenesis | Pain | N/A | Phase II trial; Recruiting; Clinical Trials.gov: NCT02832271 |
| Antioxidants | ||||
| Vitamins E and C | Antioxidative activity; decrease peritoneal inflammation | Pain | Improved pain score; decreased peritoneal RANTES, IL-6, and MCP-1 | Ref. [113] |
| Melatonin | Antioxidative activity | Pain | Improved pain score; decreased analgesic use; improved sleep quality | Phase II trial; Ref. [114] |
| Cancer type/MSC Source | Surface marker | Effect | Factors/Mechanisms | Ref. |
|---|---|---|---|---|
| Ovarian cancer | ||||
| Omental adipose tissue of healthy donor | CD73+ CD90+ CD105+ CD34− | Increased tumor growth and metastasis | ATMSC increased tumor cell secretion of MMP2 and MMP9 | [251] |
| Umbilical cord Wharton’s jelly of healthy donor | CD44+ CD90+ CD105+ CD34− HLA− | Decreased tumor growth | WJSC increased tumor cell apoptosis | [252] |
| Menstrual blood of healthy donor | CD73+ CD90+ CD34− | Decreased tumor growth and angiogenesis | emMSCs induced tumor cell cycle arrest, increased tumor cell apoptosis, decreased AKT phosphorylation, and promoted FoxO3a nuclear translocation of tumor cells | [253] |
| Endometrial Cancer | ||||
| Bone marrow of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ EpCAM− CD11b− CD34− CD45− | Increased tumor growth | BMMSC-secreted high level of VEGF, FGF, and SDF1-α; Tumor-secreted IL-8 and CXCL-1 attracted BMMSCs to the tumor site | [254] |
| Omental adipose tissue of healthy donor | CD73+ CD90+ CD105+ CD34− | Increased tumor growth and metastasis | ATMSC-secreted IL-6 activated STAT3 pathway in tumor cells | [247] |
| Omental adipose tissue of gynecologic cancer patients, subcutaneous adipose tissue of healthy donor | CD29+ CD44+ CD73+ CD90+ CD105+ EpCAM− CD11b− CD34− CD45− | Increased tumor growth (omental ATMSCs); No significant tumor promotion with subcutaneous ATMSCs | Omental ATMSCs secreted high level of VEGF, FGF, and SDF1-α; Tumor-secreted, IL-8 and CXCL-1 attracted ATMSCs to the tumor site | [254] |
| Cervical Cancer | ||||
| Amniotic fluid in second trimester of gestation | CD73+ CD90+ CD105+ CD14− CD34− CD45− HLA− | Increased tumor growth | Genetically modified IFN-α-expressing AFMSCs suppressed tumor growth | [255] |
| Breast Cancer | ||||
| Bone marrow of healthy donor | CD105+ CD31+ CD34− CD133− | Increased tumor growth and metastasis | Tumor increased BMMSC–secreted CCL-5 (RANTES) to increase cell motility, invasion, and metastasis | [256] |
| Bone marrow of healthy donor | Not mentioned | Varying effect in tumor growth and metastasis for different breast cancer cell lines | BMMSC-secreted IL-17 and tumor-expressed IL-17R may stimulate migration of metastatic cancer cells; Tumor-secreted TGF-β1 attracted BM-MSCs | [257] |
| Subcutaneous abdominal adipose tissue of healthy donor | CD29+ CD73+ CD90+ CD105+ CD166+ CD11b− CD31− CD34− CD45− HLA-DR− | Varying effect of tumor growth and angiogenesis for different breast cancer cell lines | ATMSC-secreted CXCL-1 and CXCL-8 promoted tumor angiogenesis | [258] |
| Umbilical cord of healthy donor | CD13+ CD29+ CD44+ CD73+ CD90+ CD105+ CD106+ CD166+ ABC+ HLA− CD14− CD31− CD34− CD38− CD45− HLA-DR− | Increased migration and metastasis (MCF-7) | UCMSC-secreted IL-6 and IL-8 promoted tumor cells to secret IL-6 and IL-8 to increase migration and mammosphere formation | [259] |
| Umbilical cord of healthy donor | CD29+ CD44+ CD54+ CD73+ CD90+ CD105+ CD11b− CD19− CD31− CD34− CD45− HLA-DR− | Decreased tumor growth and angiogenesis (MDA-MB-231) | Increased apoptosis | [260] |
| Umbilical cord Wharton’s jelly of healthy donor | CD44+ CD90+ CD105+ CD34− HLA− | Decreased tumor growth and migration | Increased apoptosis | [252] |
| Amniotic tissue of healthy donor | Not mentioned | Decreased tumor growth | AMESCs secreted TNF-α, TNF-β, TGF-β, IFN-γ, IL-2, IL-3, IL-4, M-CSF, and IL-8 | [261] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Chen, Y.-H.; Chang, H.-Y.; Au, H.-K.; Tzeng, C.-R.; Huang, Y.-H. Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. Int. J. Mol. Sci. 2018, 19, 2385. https://doi.org/10.3390/ijms19082385
Lin Y-H, Chen Y-H, Chang H-Y, Au H-K, Tzeng C-R, Huang Y-H. Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. International Journal of Molecular Sciences. 2018; 19(8):2385. https://doi.org/10.3390/ijms19082385
Chicago/Turabian StyleLin, Yi-Heng, Ya-Hsin Chen, Heng-Yu Chang, Heng-Kien Au, Chii-Ruey Tzeng, and Yen-Hua Huang. 2018. "Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies" International Journal of Molecular Sciences 19, no. 8: 2385. https://doi.org/10.3390/ijms19082385
APA StyleLin, Y.-H., Chen, Y.-H., Chang, H.-Y., Au, H.-K., Tzeng, C.-R., & Huang, Y.-H. (2018). Chronic Niche Inflammation in Endometriosis-Associated Infertility: Current Understanding and Future Therapeutic Strategies. International Journal of Molecular Sciences, 19(8), 2385. https://doi.org/10.3390/ijms19082385