Hydroxyl Radical-Suppressing Mechanism and Efficiency of Melanin-Mimetic Nanoparticles
Abstract
1. Introduction
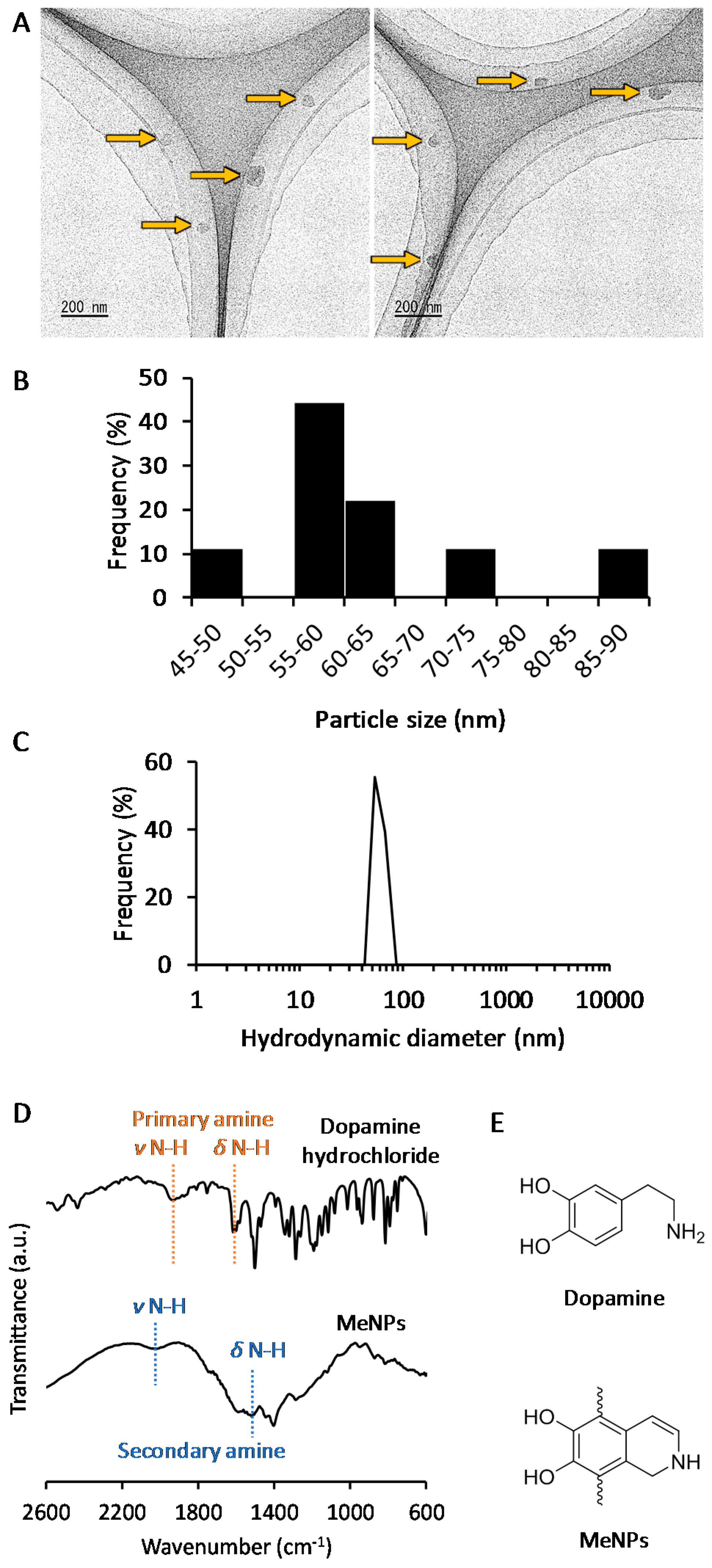
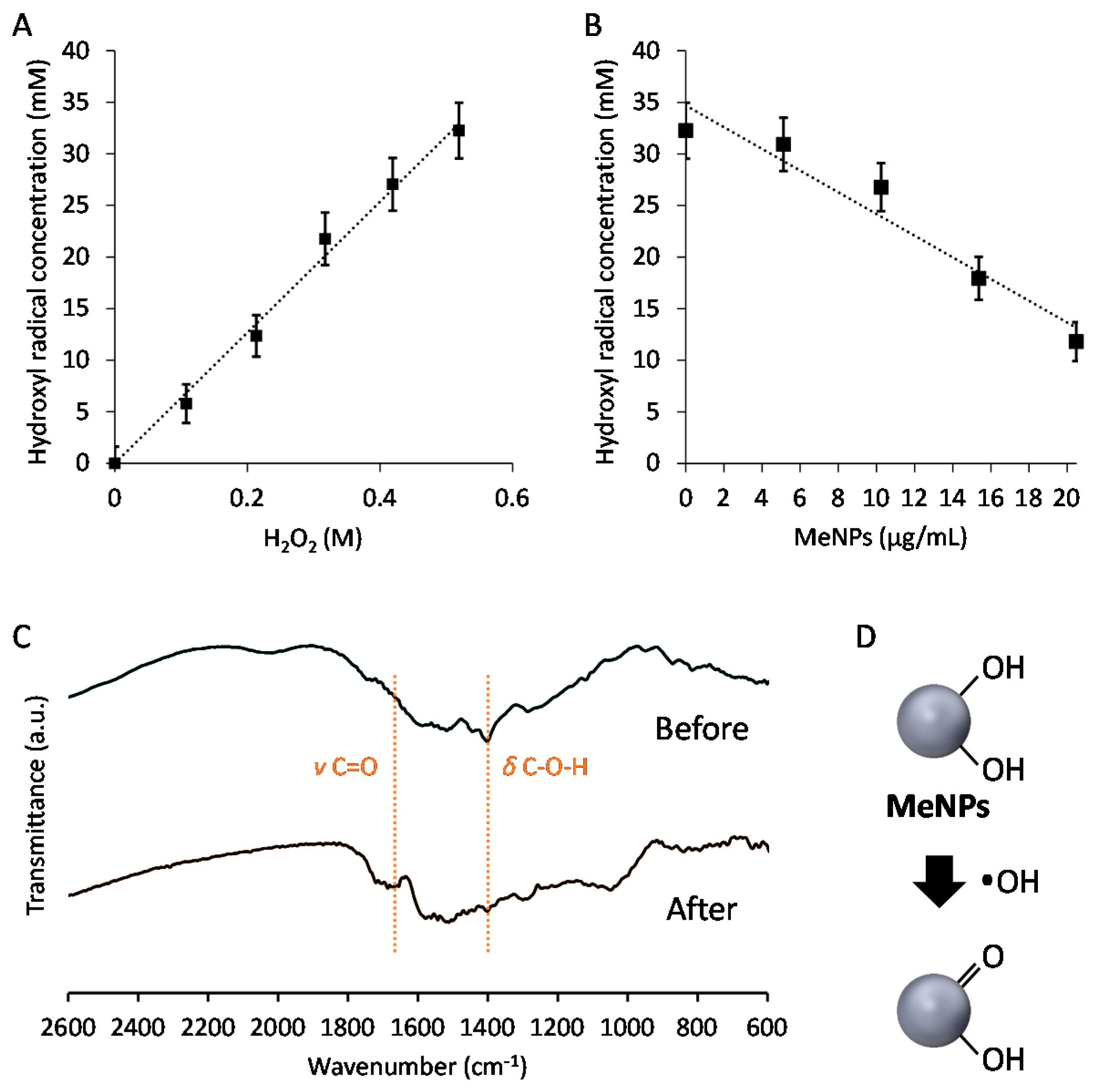
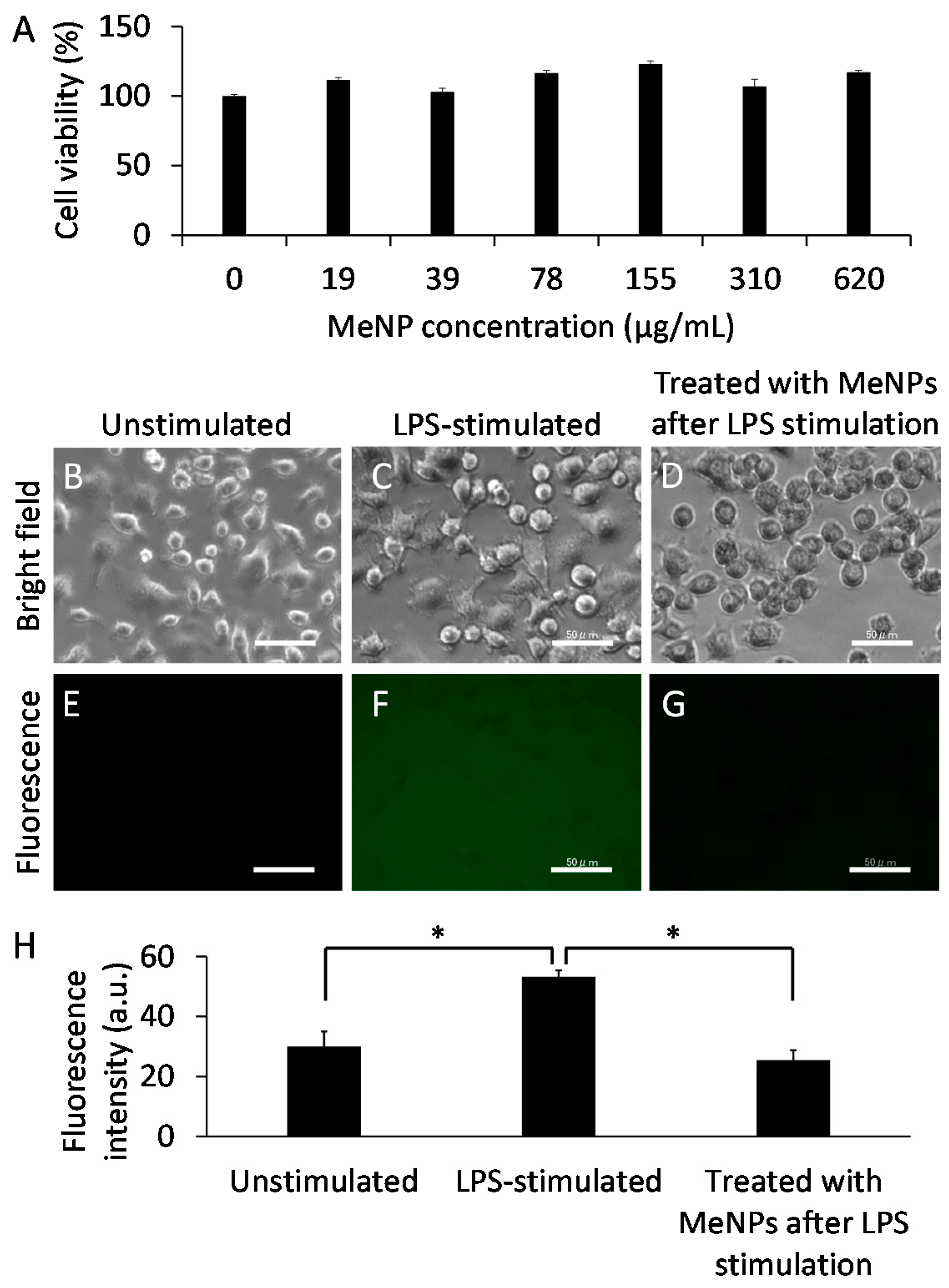
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of MeNPs
3.3. Characterization
3.4. Suppression of Hydroxyl Radicals Produced by the Fenton Reaction
3.5. Hydroxyl Radical Suppression Mechanism
3.6. Cytotoxicity
3.7. Suppression of Hydroxyl Radicals Produced by KCs
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Simon, J.D.; Peles, D.N. The red and the black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species and the central-nervous-system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Maruhashi, T.; Sakamoto, W.; Yogo, T. Organic–inorganic hybrid hollow nanoparticles suppress oxidative stress and repair damaged tissues for treatment of hepatic fibrosis. Adv. Funct. Mater. 2018, 28, 1706332. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamada, S.; Hayashi, H.; Sakamoto, W.; Yogo, T. Red blood cell-like particles with the ability to avoid lung and spleen accumulation for the treatment of liver fibrosis. Biomaterials 2018, 156, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-L.; Wang, P.-W.; Hung, C.-F.; Aljuffali, I.A.; Dai, Y.-S.; Fang, J.-Y. The impact of retinol loading and surface charge on the hepatic delivery of lipid nanoparticles. Colloids Surf. B 2016, 141, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, C.; Zha, Y.; Hu, W.; Gao, Z.; Zang, Y.; Chen, J.; Zhang, J.; Dong, L. Corona-directed nucleic acid delivery into hepatic stellate cells for liver fibrosis therapy. ACS Nano 2015, 9, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.H.; Park, K.; Lee, M.-Y.; Lee, H.; Sung, D.K.; Hahn, S.K. Cationic solid lipid nanoparticles derived from apolipoprotein-free LDLs for target specific systemic treatment of liver fibrosis. Biomaterials 2013, 34, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Q.; Su, H.; Chen, X.; Qin, X.-J.; Liu, J.-Y.; Zhu, Q.-G.; Hu, J.-H. Mannose 6-phosphate-modified bovine serum albumin nanoparticles for controlled and targeted delivery of sodium ferulate for treatment of hepatic fibrosis. J. Pharm. Pharmacol. 2009, 61, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Chen, S.-M.; Liu, J.-H.; Hsu, H.-W.; Lin, H.-Y.; Chen, S.-Y. Mechanical and photo-fragmentation processes for nanonization of melanin to improve its efficacy in protecting cells from reactive oxygen species stress. J. Appl. Phys. 2015, 117, 064701. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Ji, X.; Askhatova, D.; Du, R.; Lu, L.; Shi, J. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 2017, 139, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.R.; Sugnaux, C.; Lau, K.H.A.; Messersmith, P.B. Size Control and fluorescence labeling of polydopamine melanin-mimetic nanoparticles for intracellular imaging. Biomimetics 2017, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- European Chemicals Bureau. European Union Risk Assessment Report, Hydrogen Peroxide; 2nd Priority List; European Commission: Brussels, Belgium, 2003; Volume 38. [Google Scholar]
- Meng, F.; Lowell, C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases hck, fgr, and lyn. J. Exp. Med. 1997, 85, 1661–1670. [Google Scholar] [CrossRef]
- Soh, M.; Kang, D.-W.; Jeong, H.-G.; Kim, D.; Kim, D.Y.; Yang, W.; Song, C.; Baik, S.; Choi, I.-Y.; Ki, S.-K.; et al. Ceria–zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed. 2017, 56, 11399–11403. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Tokuda, A.; Sakamoto, W. Hydroxyl Radical-Suppressing Mechanism and Efficiency of Melanin-Mimetic Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2309. https://doi.org/10.3390/ijms19082309
Hayashi K, Tokuda A, Sakamoto W. Hydroxyl Radical-Suppressing Mechanism and Efficiency of Melanin-Mimetic Nanoparticles. International Journal of Molecular Sciences. 2018; 19(8):2309. https://doi.org/10.3390/ijms19082309
Chicago/Turabian StyleHayashi, Koichiro, Atsuto Tokuda, and Wataru Sakamoto. 2018. "Hydroxyl Radical-Suppressing Mechanism and Efficiency of Melanin-Mimetic Nanoparticles" International Journal of Molecular Sciences 19, no. 8: 2309. https://doi.org/10.3390/ijms19082309
APA StyleHayashi, K., Tokuda, A., & Sakamoto, W. (2018). Hydroxyl Radical-Suppressing Mechanism and Efficiency of Melanin-Mimetic Nanoparticles. International Journal of Molecular Sciences, 19(8), 2309. https://doi.org/10.3390/ijms19082309