Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins
Abstract
1. Introduction
2. Results
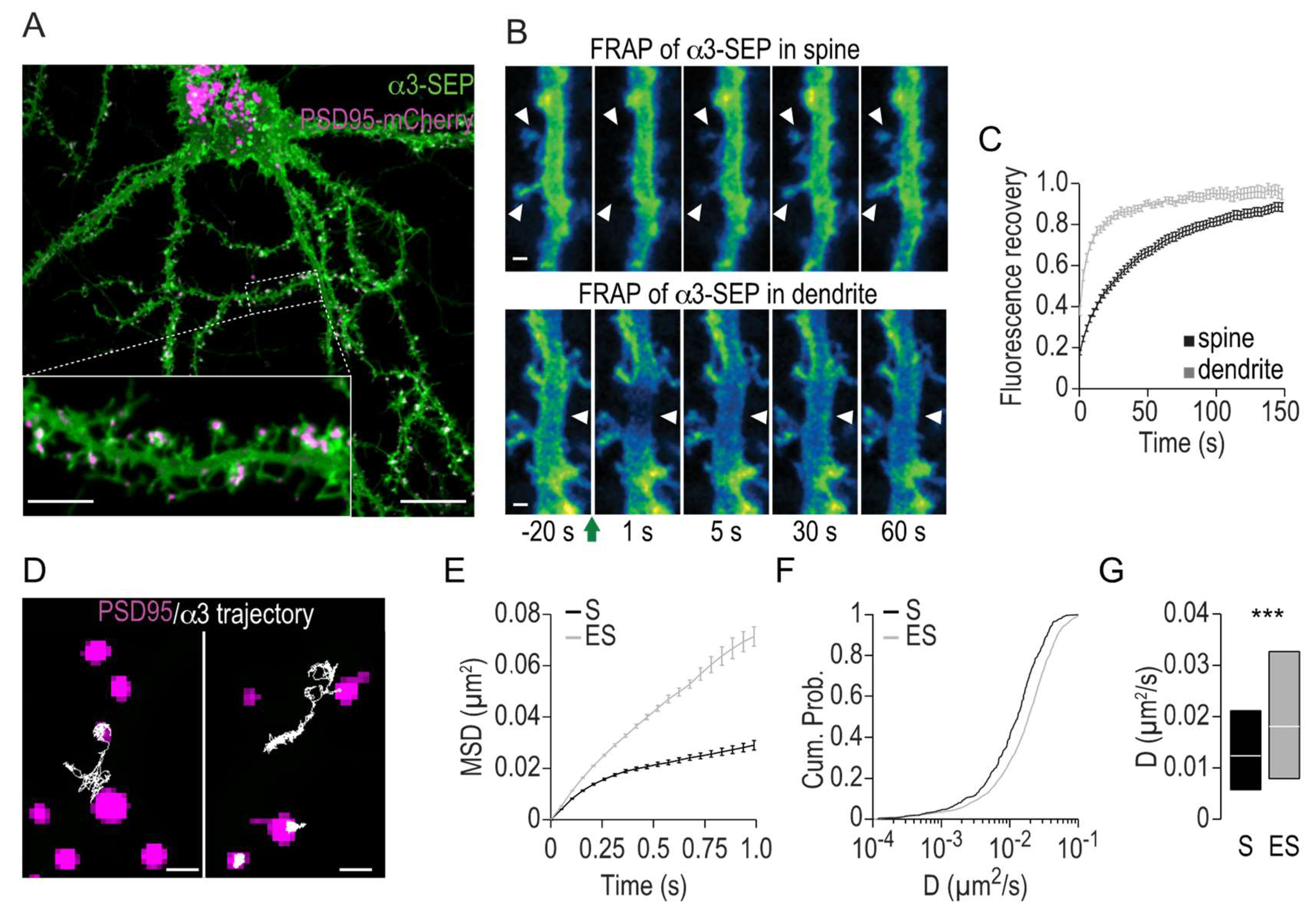
2.1. Lateral Mobility of Na,K-ATPase α3 in Hippocampal Neurons
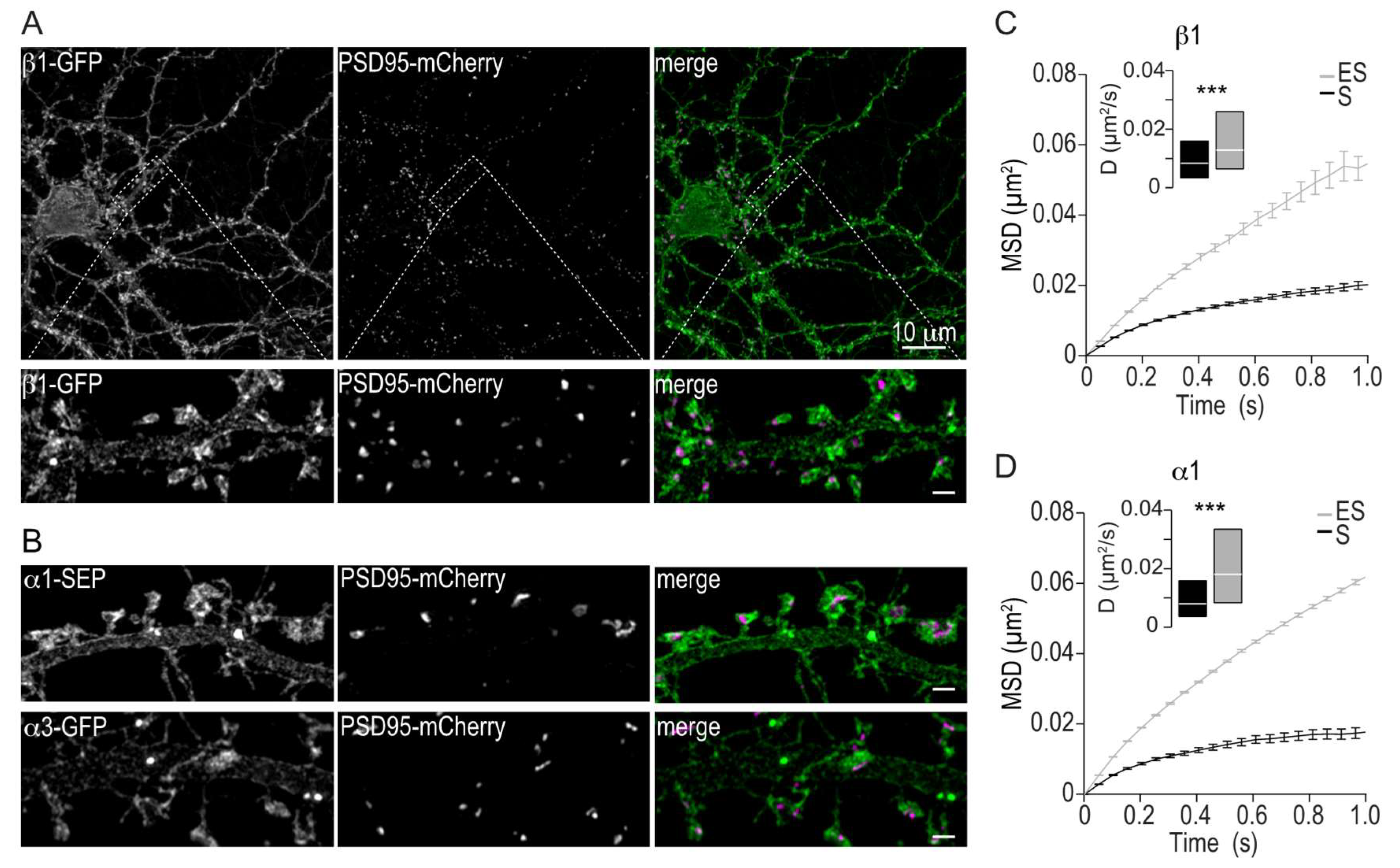
2.2. Membrane Distribution and Lateral Mobility of Na,K-ATPase α1 and β1
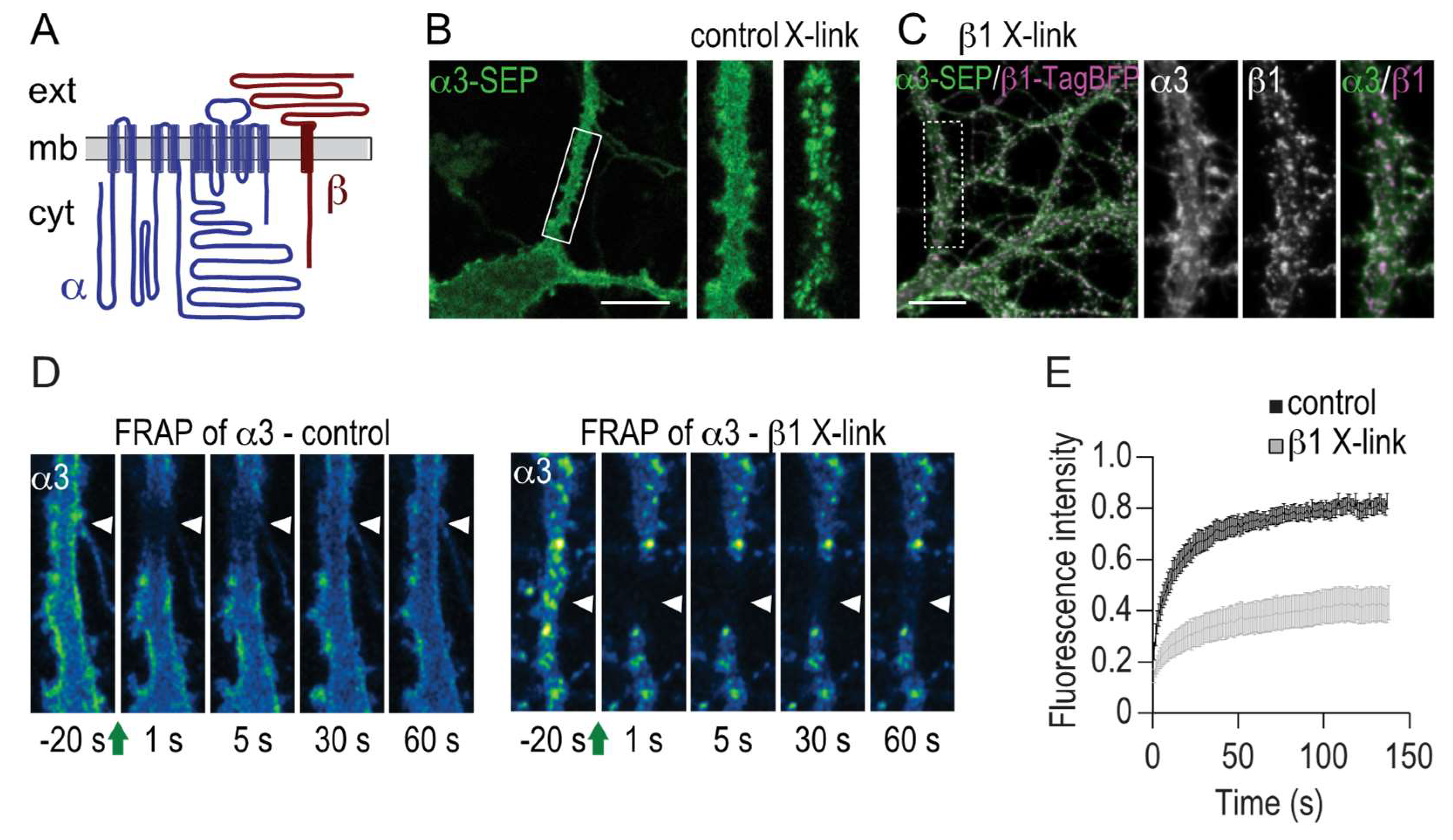
2.3. Immobilization of β1 Induces Clustering and Immobilization of α3
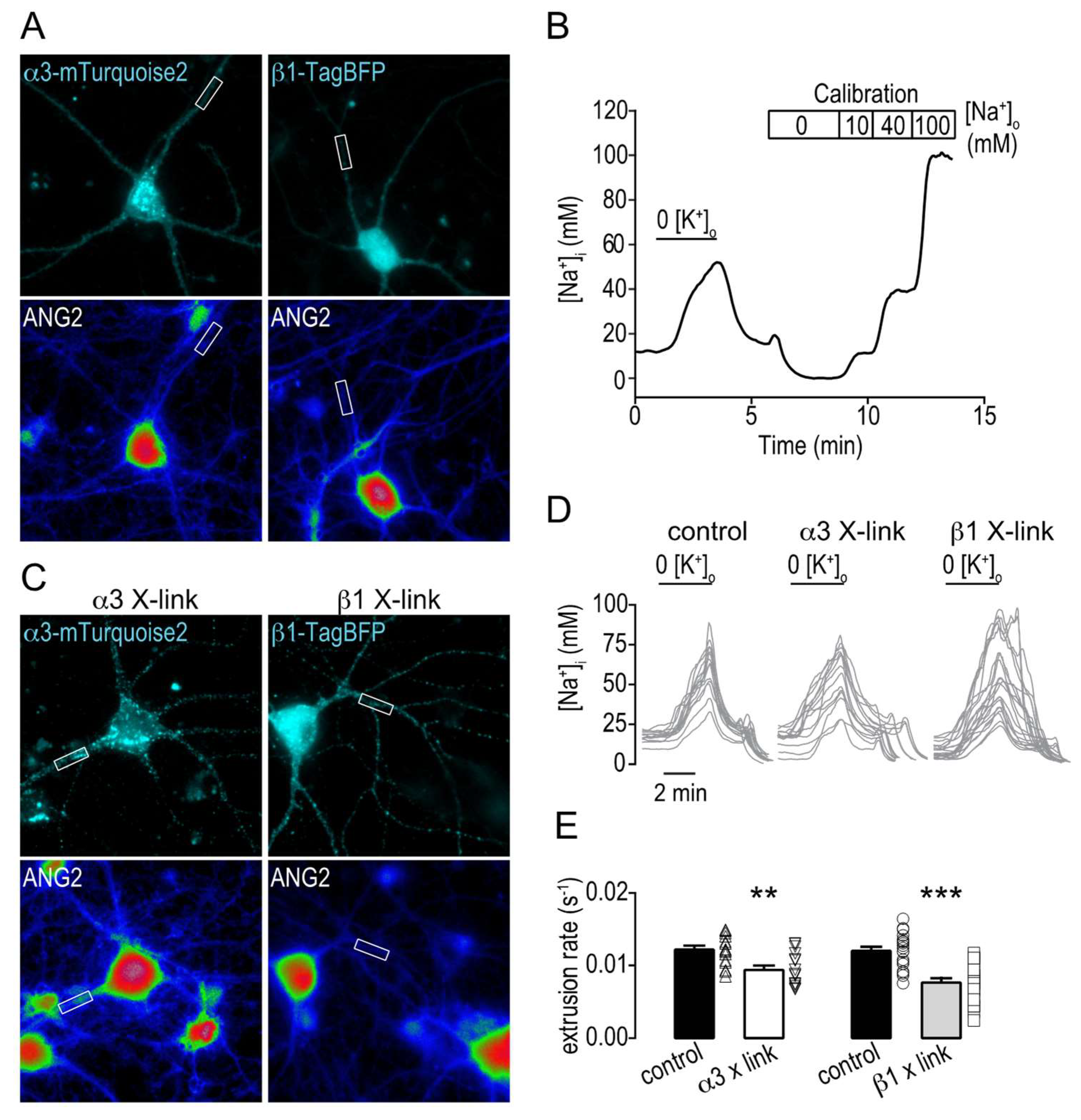
2.4. Clustering Na,K-ATPase Reduce the Na+ Extrusion Efficiency
2.5. Clustering of the Neuronal Na,K-ATPase Mediated by MONaKA Binding to the β1 Subunit
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Construct Design
4.3. Protein Purification
4.4. Microscopy
4.5. 3D Structured Illumination Microscopy (3D-SIM)
4.6. Immunocytochemistry and Live Cell Imaging
4.7. Fluorescence Recovery after Photobleaching (FRAP)
4.8. Single Particle Tracking
4.9. Na+ Extrusion Measurements
4.10. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Skou, J.C. Enzymatic basis for active transport of Na+ and K+ across cell membrane. Physiol. Rev. 1965, 45, 596–617. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C. Nobel Lecture. The identification of the sodium pump. Biosci. Rep. 1998, 18, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 2002, 71, 511–535. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Choquet, D.; Triller, A. The Dynamic Synapse. Neuron 2013, 80, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Subunit Assembly and Functional Maturation of Na,K-Atpase. J. Membr. Biol. 1990, 115, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.; Klein, L.; Shyjan, A.W.; Rakic, P.; Levenson, R. Neurons and astroglia express distinct subsets of Na,K-ATPase α and β subunits. Mol. Brain Res. 1994, 21, 333–343. [Google Scholar] [CrossRef]
- Zahler, R.; Zhang, Z.T.; Manor, M.; Boron, W.F. Sodium kinetics of Na,K-ATPase α isoforms in intact transfected HeLa cells. J. Gen. Physiol. 1997, 110, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R. Na+ signals at central synapses. Neuroscientist 2002, 8, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Azarias, G.; Kruusmagi, M.; Connor, S.; Akkuratov, E.E.; Liu, X.-L.; Lyons, D.; Brismar, H.; Broberger, C.; Aperia, A. A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3. J. Biol. Chem. 2013, 288, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Aguiar, P.P.; Sweadner, K.J.K.; Penniston, J.T.J.; Zaremba, J.J.; Liu, L.L.; Caton, M.M.; Linazasoro, G.; Borg, M.; Tijssen, M.A.; Bressman, S.B.; et al. Mutations in the Na+/K+-ATPase α3 Gene ATP1A3 Are Associated with Rapid-Onset Dystonia Parkinsonism. Neuron 2004, 43, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Heinzen, E.L.; Swoboda, K.J.; Hitomi, Y.; Gurrieri, F.; Nicole, S.; de Vries, B.; Tiziano, F.D.; Fontaine, B.; Walley, N.M.; Heavin, S.; et al. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat. Genet. 2012, 44, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Toustrup-Jensen, M.S.; Einholm, A.P.; Schack, V.R.; Nielsen, H.N.; Holm, R.; Sobrido, M.-J.; Andersen, J.P.; Clausen, T.; Vilsen, B. Relationship between Intracellular Na+ Concentration and Reduced Na+ Affinity in Na+,K+-ATPase Mutants Causing Neurological Disease. J. Biol. Chem. 2014, 289, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. The functional role of the β-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 1991, 285, 189–193. [Google Scholar] [CrossRef]
- Boldyrev, A.A. Na/K-ATPase as an Oligomeric Ensemble. Biochemistry 2001, 66, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Laughery, M.; Todd, M.; Kaplan, J.H. Oligomerization of the Na,K-ATPase in cell membranes. J. Biol. Chem. 2004, 279, 36339–36348. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Ferguson, T.S.; Cibulsky, S.M.; Holmqvist, M.; Ding, C.; Fei, H.; Levitan, I.B. MONaKA, a Novel Modulator of the Plasma Membrane Na,K-ATPase. J. Neurosci. 2005, 25, 7934–7943. [Google Scholar] [CrossRef] [PubMed]
- Bats, C.; Groc, L.; Choquet, D. The Interaction between Stargazin and PSD-95 Regulates AMPA Receptor Surface Trafficking. Neuron 2007, 53, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Gorokhova, S.S.; Bibert, S.S.; Geering, K.K.; Heintz, N.N. A novel family of transmembrane proteins interacting with beta subunits of the Na,K-ATPase. Hum. Mol. Genet. 2007, 16, 2394–2410. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Royal, C.; Dupuis, J.P.; Varela, J.A.; Panatier, A.; Pinson, B.; Baufreton, J.; Groc, L.; Oliet, S.H. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat. Neurosci. 2015, 18, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Ronnlund, D.; Scott, L.; Spicarova, Z.; Widengren, J.; Bondar, A.; Aperia, A.; Brismar, H. Spatial distribution of Na+-K+-ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy. BMC Neurosci. 2011, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, T.; Blom, H.; Aperia, A.; brismar, H. Nanoscale elucidation of Na,K-ATPase isoforms in dendritic spines. Opt. Nanoscopy 2013, 2, 6. [Google Scholar] [CrossRef]
- Tardin, C.; Cognet, L.; Bats, C.; Lounis, B.; Choquet, D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003, 22, 4656–4665. [Google Scholar] [CrossRef] [PubMed]
- Groc, L.; Heine, M.; Cognet, L.; Brickley, K.; Stephenson, F.A.; Lounis, B.; Choquet, D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 2004, 7, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious Effects of Amyloid beta Oligomers Acting as an Extracellular Scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.C.; Berg, D.K.; Gomez-Varela, D. Lateral Mobility of Nicotinic Acetylcholine Receptors on Neurons Is Determined by Receptor Composition, Local Domain, and Cell Type. J. Neurosci. 2010, 30, 8841–8851. [Google Scholar] [CrossRef] [PubMed]
- Chamma, I.; Heubl, M.; Chevy, Q.; Renner, M.; Moutkine, I.; Eugène, E.; Poncer, J.C.; Lévi, S. Activity-Dependent Regulation of the K/Cl Transporter KCC2 Membrane Diffusion, Clustering, and Function in Hippocampal Neurons. J. Neurosci. 2013, 33, 15488–15503. [Google Scholar] [CrossRef] [PubMed]
- Geering, K.; Jaunin, P.; Jaisser, F.; Merillat, A.M.; Horisberger, J.D.; Mathews, P.M.; Lemas, V.; Fambrough, D.M.; Rossier, B.C. Mutation of a Conserved Proline Residue in the Beta-Subunit Ectodomain Prevents Na+-K+-Atpase Oligomerization. Am. J. Physiol. 1993, 265, C1169–C1174. [Google Scholar] [CrossRef] [PubMed]
- Laughery, M.D.; Todd, M.L.; Kaplan, J.H. Mutational analysis of α-beta subunit interactions in the delivery of Na,K-ATPase heterodimers to the plasma membrane. J. Biol. Chem. 2003, 278, 34794–34803. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.J.; Kaplan, J.H. β-Subunit overexpression alters the stoicheometry of assembled Na-K-ATPase subunits in MDCK cells. Am. J. Physiol. Renal. 2008, 295, F1314–F1323. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.J.; Kaplan, J.H. Regulation of Na,K-ATPase subunit abundance by translational repression. J. Biol. Chem. 2009, 284, 22905–22915. [Google Scholar] [CrossRef] [PubMed]
- Larre, I.; Lazaro, A.; Contreras, R.G.; Balda, M.S.; Matter, K.; Flores-Maldonado, C.; Ponce, A.; Flores-Benitez, D.; Rincon-Heredia, R.; Padilla-Benavides, T.; et al. Ouabain modulates epithelial cell tight junction. Proc. Natl. Acad. Sci. USA 2010, 107, 11387–11392. [Google Scholar] [CrossRef] [PubMed]
- Tokhtaeva, E.; Sachs, G.; Souda, P.; Bassilian, S.; Whitelegge, J.P.; Shoshani, L.; Vagin, O. Epithelial Junctions Depend on Intercellular Trans-interactions between the Na,K-ATPase beta(1) Subunits. J. Biol. Chem. 2011, 286, 25801–25812. [Google Scholar] [CrossRef] [PubMed]
- Tokhtaeva, E.; Sachs, G.; Sun, H.; Dada, L.A.; Sznajder, J.I.; Vagin, O. Identification of the amino-acid region involved in the intercellular interaction between the Na,K-ATPase β1 subunits. J. Cell Sci. 2012, 125, 1065–1616. [Google Scholar] [CrossRef] [PubMed]
- Vagin, O.; Dada, L.A.; Tokhtaeva, E.; Sachs, G. The Na-K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am. J. Physiol. Cell 2012, 302, C1271–C1281. [Google Scholar] [CrossRef] [PubMed]
- Barwe, S.P.; Kim, S.; Rajasekaran, S.A.; Bowie, J.U.; Rajasekaran, A.K. Janus Model of The Na,K-ATPase β-Subunit Transmembrane Domain: Distinct Faces Mediate α/β Assembly and β-β Homo-oligomerization. J. Mol. Biol. 2007, 365, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Barwe, S.P.; Skay, A.; McSpadden, R.; Huynh, T.P.; Langhans, S.A.; Inge, L.J.; Rajasekaran, A.K. Na,K-ATPase β-subunit cis homo-oligomerization is necessary for epithelial lumen formation in mammalian cells. J. Cell Sci. 2012, 125, 5711–5720. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 1989, 1000, 439–446. [Google Scholar] [CrossRef]
- Skou, J.C. The identification of the sodium-pump as the membrane-bound Na+/K+-ATPase: A commentary on ‘The Influence of Some Cations on an Adenosine Triphosphatase from Peripheral Nerves’. Biochim. Biophys. Acta 1989, 1000, 435–438. [Google Scholar] [CrossRef]
- Pulver, S.R.; Griffith, L.C. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat. Neurosci. 2010, 13, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, A.T.; Dasari, S.; Onoue, K.; Stephens, E.K.; Hasse, J.M.; Avesar, D. A Sodium-Pump-Mediated Afterhyperpolarization in Pyramidal Neurons. J. Neurosci. 2013, 33, 13025–13041. [Google Scholar] [CrossRef] [PubMed]
- Clapcote, S.J.; Duffy, S.; Xie, G.; Kirshenbaum, G.; Bechard, A.R.; Schack, V.R.; Petersen, J.; Sinai, L.; Saab, B.J.; Lerch, J.P.; et al. Mutation I810N in the α 3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc. Natl. Acad. Sci. USA 2009, 106, 14085–14090. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Yanazawa, M.; Sasahara, T.; Kitamura, Y.; Hiroaki, H.; Fukazawa, Y.; Kii, I.; Nishiyama, T.; Kakita, A.; Takeda, H.; et al. Na, K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proc. Natl. Acad. Sci. USA 2015, 112, E4465–E4474. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.N.; Redeker, V.; Fritz, N.; Pieri, L.; Almeida, L.G.; Spolidoro, M.; Liebmann, T.; Bousset, L.; Renner, M.; Léna, C.; et al. α-synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO J. 2015, 34, 2408–2423. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Oxidative Stress and Energy Crises in Neuronal Dysfunction. Ann. N. Y. Acad. Sci. 2008, 1147, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Le Masson, G.; Przedborski, S.; Abbott, L.F. A computational model of motor neuron degeneration. Neuron 2014, 83, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, T.; Kruusmagi, M.; Sourial-Bassillious, N.; Bondar, A.; Svenningsson, P.; Flajolet, M.; Greengard, P.; Scott, L.; Brismar, H.; Aperia, A. A Noncanonical Postsynaptic Transport Route for a GPCR Belonging to the Serotonin Receptor Family. J. Neurosci. 2012, 32, 17998–18008. [Google Scholar] [CrossRef] [PubMed]
- Bannai, H.; Lévi, S.; Schweizer, C.; Dahan, M.; Triller, A. Imaging the lateral diffusion of membrane molecules with quantum dots. Nat. Protoc. 2006, 1, 2628–2634. [Google Scholar] [CrossRef] [PubMed]
- Saxton, M.J.; Jacobson, K. Single-particle tracking: Applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 373–399. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liebmann, T.; Fritz, N.; Kruusmägi, M.; Westin, L.; Bernhem, K.; Bondar, A.; Aperia, A.; Brismar, H. Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins. Int. J. Mol. Sci. 2018, 19, 2214. https://doi.org/10.3390/ijms19082214
Liebmann T, Fritz N, Kruusmägi M, Westin L, Bernhem K, Bondar A, Aperia A, Brismar H. Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins. International Journal of Molecular Sciences. 2018; 19(8):2214. https://doi.org/10.3390/ijms19082214
Chicago/Turabian StyleLiebmann, Thomas, Nicolas Fritz, Markus Kruusmägi, Linda Westin, Kristoffer Bernhem, Alexander Bondar, Anita Aperia, and Hjalmar Brismar. 2018. "Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins" International Journal of Molecular Sciences 19, no. 8: 2214. https://doi.org/10.3390/ijms19082214
APA StyleLiebmann, T., Fritz, N., Kruusmägi, M., Westin, L., Bernhem, K., Bondar, A., Aperia, A., & Brismar, H. (2018). Regulation of Neuronal Na,K-ATPase by Extracellular Scaffolding Proteins. International Journal of Molecular Sciences, 19(8), 2214. https://doi.org/10.3390/ijms19082214




