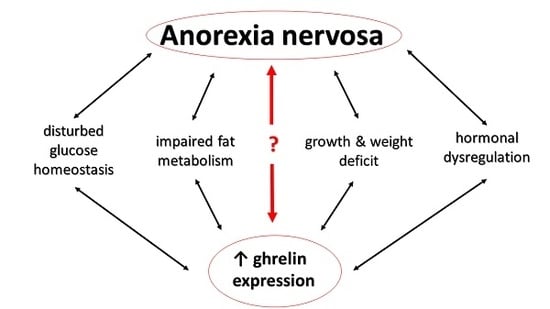
The Role of Ghrelin in Anorexia Nervosa
Abstract
1. Introduction
2. Alteration of Ghrelin in Anorexia Nervosa
2.1. Basal Circulating Total Ghrelin Levels
2.2. Expression of Ghrelin
2.3. Acyl and Desacyl Ghrelin
2.4. Nutrient-Related Alterations of Ghrelin
2.5. Treatment-Related Alterations of Ghrelin
3. Genetics Contributing to Altered Ghrelin Signaling in Anorexia Nervosa
4. Effects of Exogenous Ghrelin in Anorexia Nervosa
4.1. Effects of Ghrelin Administration
4.2. Effects of Ghrelin-Related Products and Ghrelin Receptor Agonists
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GHSR | growth hormone secretagogue receptor |
| AgRP | agouti-related peptide |
| NPY | neuropeptide Y |
| GOAT | ghrelin-O-acyl transferase |
| GIP | gastric inhibitory polypeptide/glucose-dependent insulinotropic peptide |
| mRNA | messenger ribonucleic acid |
| mTORC1/S6K1 | mechanistic target of rapamycin complex 1/p70 ribosomal protein kinase 1 |
| AN | anorexia nervosa |
| BMI | body mass index |
| ABA | activity-based anorexia |
| AN-BP | anorexia nervosa of bingeing/purging subtype |
| AN-R | anorexia nervosa of restrictive subtype |
| BULIT-R | bulimia test—DSM-III-R |
| GH | growth hormone |
| IGF | insulin-like growth factor |
| HOMA-IR | homeostatic model assessment—insulin resistance |
| T3 | triiodothyronine |
| ELISA | enzyme-linked immunosorbent assay |
| Ig | immune globulin |
| IGF-1 | insulin-like growth factor 1 |
| oGTT | oral glucose tolerance test |
| SNP | single nucleotide polymorphism |
| ACTH | adrenocorticotropic hormone |
| POMC | pro-opiomelanocortin |
References
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Konturek, S.J.; Polus, A.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; et al. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J. Physiol. Pharmacol. 2006, 57, 425–437. [Google Scholar] [PubMed]
- Willesen, M.G.; Kristensen, P.; Rømer, J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 1999, 70, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Goebel-Stengel, M.; Stengel, A.; Taché, Y. Continued controversy on obestatin as a gut hormone influencing food intake and gastrointestinal motility. Obes. Metab. 2008, 4, 143–148. [Google Scholar]
- Druce, M.R.; Wren, A.M.; Park, A.J.; Milton, J.E.; Patterson, M.; Frost, G.; Ghatei, M.A.; Small, C.; Bloom, S.R. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 2005, 29, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Arvat, E.; Benso, A.; Gottero, C.; Muccioli, G.; Papotti, M.; van der Lely, A.J.; Deghenghi, R.; Ghigo, E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5083–5086. [Google Scholar] [CrossRef] [PubMed]
- Tack, J.; Depoortere, I.; Bisschops, R.; Delporte, C.; Coulie, B.; Meulemans, A.; Janssens, J.; Peeters, T. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut 2006, 55, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Inhoff, T.; Mönnikes, H.; Noetzel, S.; Stengel, A.; Goebel, M.; Dinh, Q.T.; Riedl, A.; Bannert, N.; Wisser, A.S.; Wiedenmann, B.; et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides 2008, 29, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, G.; Cabral, A.; Cornejo, M.P.; De Francesco, P.N.; Garcia-Romero, G.; Reynaldo, M.; Perello, M. Des-acyl ghrelin directly targets the arcuate nucleus in a ghrelin-receptor independent manner and impairs the orexigenic effect of ghrelin. J. Neuroendocrinol. 2016, 28, 12349. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Piechowski, C.L.; Müller, A.; Ottaway, N.; Sisley, S.; Smiley, D.L.; Habegger, K.M.; Pfluger, P.T.; Dimarchi, R.; Biebermann, H.; et al. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes 2014, 63, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Gauna, C.; van de Zande, B.; van Kerkwijk, A.; Themmen, A.P.; van der Lely, A.J.; Delhanty, P.J. Unacylated ghrelin is not a functional antagonist but a full agonist of the type 1a growth hormone secretagogue receptor (GHS-R). Mol. Cell. Endocrinol. 2007, 274, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Fujimiya, M.; Sakamaki, R.; Shinfuku, N.; Ueta, Y.; Meguid, M.M.; Kasuga, M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005, 54, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S.; et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Nakazato, M.; Hashiguchi, S.; Dezaki, K.; Mondal, M.S.; Hosoda, H.; Kojima, M.; Kangawa, K.; Arima, T.; Matsuo, H.; et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 2002, 51, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Kola, B.; Grossman, A.; Korbonits, M. The expression of ghrelin o-acyltransferase (GOAT) in human tissues. Endocr. J. 2011, 58, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Goebel, M.; Wang, L.; Taché, Y.; Sachs, G.; Lambrecht, N.W. Differential distribution of ghrelin-o-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem. Biophys. Res. Commun. 2010, 392, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Goebel-Stengel, M.; Hofmann, T.; Elbelt, U.; Teuffel, P.; Ahnis, A.; Kobelt, P.; Lambrecht, N.W.; Klapp, B.F.; Stengel, A. The ghrelin activating enzyme ghrelin-o-acyltransferase (GOAT) is present in human plasma and expressed dependent on body mass index. Peptides 2013, 43, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Solenberg, P.J.; Perkins, D.R.; Willency, J.A.; Knierman, M.D.; Jin, Z.; Witcher, D.R.; Luo, S.; Onyia, J.E.; Hale, J.E. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 2008, 105, 6320–6325. [Google Scholar] [CrossRef] [PubMed]
- González, C.R.; Vázquez, M.J.; López, M.; Diéguez, C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J. Mol. Endocrinol. 2008, 41, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Sakata, I.; Nakamura, K.; Yamazaki, M.; Matsubara, M.; Hayashi, Y.; Kangawa, K.; Sakai, T. Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 2002, 23, 531–536. [Google Scholar] [CrossRef]
- Iwakura, H.; Ariyasu, H.; Hosoda, H.; Yamada, G.; Hosoda, K.; Nakao, K.; Kangawa, K.; Akamizu, T. Oxytocin and dopamine stimulate ghrelin secretion by the ghrelin-producing cell line, MGN3-1 in vitro. Endocrinology 2011, 152, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Engelstoft, M.S.; Park, W.M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nøhr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.; Laermans, J.; Verhulst, P.J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and alpha gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA. 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Date, Y.; Mondal, M.S.; Toshinai, K.; Shimbara, T.; Fukunaga, K.; Murakami, N.; Miyazato, M.; Kangawa, K.; Yoshimatsu, H.; et al. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem. Biophys. Res. Commun. 2003, 302, 520–525. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel-Stengel, M.; Wang, L.; Shaikh, A.; Lambrecht, N.W.; Rivier, J.; Taché, Y. Abdominal surgery inhibits circulating acyl ghrelin and ghrelin-O-acyltransferase levels in rats: Role of the somatostatin receptor subtype 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G239–G248. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembiński, A. Peptidyl hormones of endocrine cells origin in the gut—Their discovery and physiological relevance. J. Physiol. Pharmacol. 2015, 66, 11–27. [Google Scholar] [PubMed]
- Weibert, E.; Stengel, A. The X/A-like cell revisited—Spotlight on the peripheral effects of NUCB2/nesfatin-1 and ghrelin. J. Physiol. Pharmacol. 2017, 68, 497–520. [Google Scholar] [PubMed]
- Zhang, Q.; Huang, C.; Meng, B.; Tang, T.; Shi, Q.; Yang, H. Acute effect of ghrelin on ischemia/reperfusion injury in the rat spinal cord. Int. J. Mol. Sci. 2012, 13, 9864–9876. [Google Scholar] [CrossRef] [PubMed]
- Frascarelli, S.; Ghelardoni, S.; Ronca-Testoni, S.; Zucchi, R. Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res. Cardiol. 2003, 98, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Nishimatsu, H.; Suzuki, E.; Satonaka, H.; Nagata, D.; Oba, S.; Sata, M.; Takahashi, M.; Yamamoto, Y.; Terauchi, Y.; et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Ceranowicz, P.; Dembiński, A.; Cieszkowski, J.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2010, 61, 419–427. [Google Scholar] [PubMed]
- Ceranowicz, D.; Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Role of hormonal axis, growth hormone—IGF-1, in the therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis. J. Physiol. Pharmacol. 2010, 61, 599–606. [Google Scholar] [PubMed]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Dembiński, A. Therapeutic effect of ghrelin in the course of ischemia/reperfusion-induced acute pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Cieszkowski, J.; Pawlik, W.W.; Tomaszewska, R.; Kuśnierz-Cabala, B.; Naskalski, J.W.; Kuwahara, A.; Kato, I. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm. IGF Res. 2006, 16, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Ceranowicz, D.; Kusnierz-Cabala, B.; Pedziwiatr, M.; Dembiński, M.; Ambrozy, T.; Kaczmarzyk, T.; Pihut, M.; et al. Therapeutic effect of exogenous ghrelin in the healing of gingival ulcers is mediated by the release of endogenous growth hormone and insulin-like growth factor-1. J. Physiol. Pharmacol. 2017, 68, 609–617. [Google Scholar] [PubMed]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Dembiński, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. 2013, 64, 657–668. [Google Scholar] [PubMed]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Dembinski, M.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med. Sci. Monit. 2012, 18, Br181–Br187. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef] [PubMed]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Dembiński, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar] [PubMed]
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The influence of ghrelin on the development of dextran sodium sulfate-induced colitis in rats. BioMed. Res. Int. 2015, 718314. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Wawarta, R.; Riepl, R.L.; Friedrich, S.; Bidlingmaier, M.; Landgraf, R.; Folwaczny, C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Investig. 2001, 24, Rc19–Rc21. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Bencivenga, R.; Longobardi, N.; Serritella, C.; Maj, M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Atsuchi, K.; Asakawa, A.; Matsuda, N.; Fujimura, M.; Inui, A.; Kato, I.; Fujimiya, M. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G974–G980. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Garcia-Touza, M.; Hijazi, R.A.; Taffet, G.; Epner, D.; Mann, D.; Smith, R.G.; Cunningham, G.R.; Marcelli, M. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J. Clin. Endocrinol. Metab. 2005, 90, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Cuntz, U.; Fruehauf, E.; Wawarta, R.; Folwaczny, C.; Riepl, R.L.; Heiman, M.L.; Lehnert, P.; Fichter, M.; Tschöp, M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur. J. Endocrinol. 2001, 145, 669–673. [Google Scholar] [PubMed]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, D.; Trajkovic, V.; Müller-Lühlhoff, S.; Brandt, E.; Abplanalp, W.; Bumke-Vogt, C.; Liehl, B.; Wiedmer, P.; Janjetovic, K.; Starcevic, V.; et al. Ghrelin-induced food intake and adiposity depend on central mTORC1/S6K1 signaling. Mol. Cell. Endocrinol. 2013, 381, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Sim, L.A.; McAlpine, D.E.; Grothe, K.B.; Himes, S.M.; Cockerill, R.G.; Clark, M.M. Identification and treatment of eating disorders in the primary care setting. Mayo Clin. Proc. 2010, 85, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Lindvall Dahlgren, C.; Wisting, L.; Rø, Ø. Feeding and eating disorders in the DSM-5 era: A systematic review of prevalence rates in non-clinical male and female samples. J. Eat. Disord. 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.E.; Pincelli, A.I.; Corra, B.; Viarengo, R.; Bonomo, S.M.; Galimberti, D.; Scacchi, M.; Scarpini, E.; Cavagnini, F.; Müller, E.E. Plasma ghrelin concentrations in elderly subjects: Comparison with anorexic and obese patients. J. Endocrinol. 2002, 175, R1–R5. [Google Scholar] [CrossRef] [PubMed]
- Méquinion, M.; Caron, E.; Zgheib, S.; Stievenard, A.; Zizzari, P.; Tolle, V.; Cortet, B.; Lucas, S.; Prévot, V.; Chauveau, C.; et al. Physical activity: Benefit or weakness in metabolic adaptations in a mouse model of chronic food restriction? Am. J. Physiol. Endocrinol. Metab. 2015, 308, E241–E255. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, L.A.; Egecioglu, E.; Luijendijk, M.C.; Hillebrand, J.J.; Adan, R.A.; Dickson, S.L. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur. Neuropsychopharmacol. 2011, 21, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Elbelt, U.; Haas, V.; Ahnis, A.; Klapp, B.F.; Rose, M.; Stengel, A. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite 2017, 108, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.; Leichner, P.; Wong, A.C.; Ghatei, M.A.; Kieffer, T.J.; Bloom, S.R.; Chanoine, J.P. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J. Clin. Endocrinol. Metab. 2005, 90, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Naruo, T.; Yasuhara, D.; Tatebe, Y.; Nagai, N.; Shiiya, T.; Nakazato, M.; Matsukura, S.; Nozoe, S. Fasting plasma ghrelin levels in subtypes of anorexia nervosa. Psychoneuroendocrinology 2003, 28, 829–835. [Google Scholar] [CrossRef]
- Tanaka, M.; Naruo, T.; Nagai, N.; Kuroki, N.; Shiiya, T.; Nakazato, M.; Matsukura, S.; Nozoe, S. Habitual binge/purge behavior influences circulating ghrelin levels in eating disorders. J. Psychiatr. Res. 2003, 37, 17–22. [Google Scholar] [CrossRef]
- Troisi, A.; Di Lorenzo, G.; Lega, I.; Tesauro, M.; Bertoli, A.; Leo, R.; Iantorno, M.; Pecchioli, C.; Rizza, S.; Turriziani, M.; et al. Plasma ghrelin in anorexia, bulimia, and binge-eating disorder: Relations with eating patterns and circulating concentrations of cortisol and thyroid hormones. Neuroendocrinology 2005, 81, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakahara, T.; Kojima, S.; Nakano, T.; Muranaga, T.; Nagai, N.; Ueno, H.; Nakazato, M.; Nozoe, S.; Naruo, T. Effect of nutritional rehabilitation on circulating ghrelin and growth hormone levels in patients with anorexia nervosa. Regul. Pept. 2004, 122, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Serritella, C.; Martiadis, V.; Maj, M. Deranged secretion of ghrelin and obestatin in the cephalic phase of vagal stimulation in women with anorexia nervosa. Biol. Psychiatry 2008, 64, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Tolle, V.; Kadem, M.; Bluet-Pajot, M.T.; Frere, D.; Foulon, C.; Bossu, C.; Dardennes, R.; Mounier, C.; Zizzari, P.; Lang, F.; et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 2003, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Germain, N.; Galusca, B.; Le Roux, C.W.; Bossu, C.; Ghatei, M.A.; Lang, F.; Bloom, S.R.; Estour, B. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. Am. J. Clin. Nutr. 2007, 85, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Langius, J.A.; Heijboer, A.C.; Becker, A.; de van der Schueren, M.A.; Verheul, H.M. Plasma ghrelin levels are associated with anorexia but not cachexia in patients with NSCLC. Front. Physiol. 2017, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Miller, K.K.; Kuo, K.; Griffin, K.; Stewart, V.; Hunter, E.; Herzog, D.B.; Klibanski, A. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E347–E356. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J.; Hattori, Y.; Ishizaki, Y.; Kaneko, K. Surged leptin/ghrelin secretion associated with anorexia nervosa. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Grimberg, A. Insulin-like growth factor-I is a marker for the nutritional state. Pediatr. Endocrinol. Rev. 2015, 13, 499–511. [Google Scholar] [PubMed]
- Baumgard, L.H.; Hausman, G.J.; Sanz Fernandez, M.V. Insulin: Pancreatic secretion and adipocyte regulation. Domest. Anim. Endocrinol. 2016, 54, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, F.; Scheithauer, B.W.; Syro, L.V.; Rotondo, A.; Kovacs, K. Pituitary immunoexpression of ghrelin in anorexia nervosa. Pituitary 2012, 15, 533–538. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Barde, S.; Achamrah, N.; Breton, J.; do Rego, J.C.; Coëffier, M.; Hökfelt, T.; Déchelotte, P.; Fetissov, S.O. The number of preproghrelin mRNA expressing cells is increased in mice with activity-based anorexia. Neuropeptides 2015, 51, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Roca-Rivada, A.; Al-Massadi, O.; Seoane, L.M.; Camiña, J.P.; Casanueva, F.F. Peripheral leptin and ghrelin receptors are regulated in a tissue-specific manner in activity-based anorexia. Peptides 2010, 31, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Dieguez, C.; Scanlon, M.F. Effects of hypothyroidism, tri-iodothyronine and glucocorticoids on growth hormone responses to growth hormone-releasing hormone and His-D-Trp-Ala-Trp-D-Phe-Lys-NH2. J. Endocrinol. 1989, 121, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Hosoda, H.; Nin, K.; Ooya, C.; Hayashi, H.; Akamizu, T.; Kangawa, K. Plasma levels of active form of ghrelin during oral glucose tolerance test in patients with anorexia nervosa. Eur. J. Endocrinol. 2003, 149, R1–R3. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Hosoda, H.; Nin, K.; Ooya, C.; Hayashi, H.; Akamizu, T.; Kangawa, K. Short-term secretory regulation of the active form of ghrelin and total ghrelin during an oral glucose tolerance test in patients with anorexia nervosa. Eur. J. Endocrinol. 2004, 150, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Ohwada, R.; Katakami, H.; Shibasaki, T.; Hizuka, N.; Takano, K. Plasma levels of intact and degraded ghrelin and their responses to glucose infusion in anorexia nervosa. J. Clin. Endocrinol. Metab. 2004, 89, 5707–5712. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Yamanaka, T.; Yamashita, S.; Gondo, M.; Morita, C.; Arimura, C.; Nozaki, T.; Takii, M.; Kubo, C. Somatic and psychological factors related to the body mass index of patients with anorexia nervosa. Eat. Weight Disord. 2008, 13, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Nakahara, T.; Yasuhara, D.; Kojima, S.; Sagiyama, K.; Amitani, H.; Laviano, A.; Naruo, T.; Inui, A. Obestatin, acyl ghrelin, and des-acyl ghrelin responses to an oral glucose tolerance test in the restricting type of anorexia nervosa. Biol. Psychiatry 2008, 63, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Germain, N.; Galusca, B.; Grouselle, D.; Frere, D.; Tolle, V.; Zizzari, P.; Lang, F.; Epelbaum, J.; Estour, B. Ghrelin/obestatin ratio in two populations with low body weight: Constitutional thinness and anorexia nervosa. Psychoneuroendocrinology 2009, 34, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Germain, N.; Galusca, B.; Grouselle, D.; Frere, D.; Billard, S.; Epelbaum, J.; Estour, B. Ghrelin and obestatin circadian levels differentiate bingeing-purging from restrictive anorexia nervosa. J. Clin. Endocrinol. Metab. 2010, 95, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.I.; Yasuhara, D.; Nakahara, T.; Harada, T.; Uehara, M.; Ushikai, M.; Asakawa, A.; Inui, A. Changes in acyl ghrelin, des-acyl ghrelin, and ratio of acyl ghrelin to total ghrelin with short-term refeeding in female inpatients with restricting-type anorexia nervosa. Horm. Metab. Res. 2010, 42, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Terashi, M.; Asakawa, A.; Harada, T.; Ushikai, M.; Coquerel, Q.; Sinno, M.H.; Déchelotte, P.; Inui, A.; Fetissov, S.O. Ghrelin reactive autoantibodies in restrictive anorexia nervosa. Nutrition 2011, 27, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Beauloye, V.; Diene, G.; Kuppens, R.; Zech, F.; Winandy, C.; Molinas, C.; Faye, S.; Kieffer, I.; Beckers, D.; Nergardh, R.; et al. High unacylated ghrelin levels support the concept of anorexia in infants with prader-willi syndrome. Orphanet J. Rare Dis. 2016, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Nedvídková, J.; Krykorková, I.; Barták, V.; Papezová, H.; Gold, P.W.; Alesci, S.; Pacak, K. Loss of meal-induced decrease in plasma ghrelin levels in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 2003, 88, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Sedláčkova, D.; Kopečková, J.; Papežová, H.; Vybíral, S.; Kvasnčcková, H.; Hill, M.; Nedvídková, J. Changes of plasma obestatin, ghrelin and NPY in anorexia and bulimia nervosa patients before and after a high-carbohydrate breakfast. Physiol. Res. 2011, 60, 165–173. [Google Scholar] [PubMed]
- Misra, M.; Tsai, P.; Anderson, E.J.; Hubbard, J.L.; Gallagher, K.; Soyka, L.A.; Miller, K.K.; Herzog, D.B.; Klibanski, A. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am. J. Clin. Nutr. 2006, 84, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nakashima, M.; Kojima, M.; Yamashita, S.; Takakura, S.; Shimizu, M.; Kubo, C.; Sudo, N. Ghrelin activation and neuropeptide Y elevation in response to medium chain triglyceride administration in anorexia nervosa patients. Clin. Nutr. ESPEN 2017, 17, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kollai, M.; Bonyhay, I.; Jokkel, G.; Szonyi, L. Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur. Heart J. 1994, 15, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Maria Monteleone, A.; Monteleone, P.; Dalle Grave, R.; Nigro, M.; El Ghoch, M.; Calugi, S.; Cimino, M.; Maj, M. Ghrelin response to hedonic eating in underweight and short-term weight restored patients with anorexia nervosa. Psychiatry Res. 2016, 235, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Holsen, L.M.; Lawson, E.A.; Christensen, K.; Klibanski, A.; Goldstein, J.M. Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa. Psychiatry Res. 2014, 223, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Paslakis, G.; Westphal, S.; Hamann, B.; Gilles, M.; Lederbogen, F.; Deuschle, M. Unstimulated and glucose-stimulated ghrelin in depressed patients and controls. J. Psychopharmacol. 2014, 28, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tatebe, Y.; Nakahara, T.; Yasuhara, D.; Sagiyama, K.; Muranaga, T.; Ueno, H.; Nakazato, M.; Nozoe, S.; Naruo, T. Eating pattern and the effect of oral glucose on ghrelin and insulin secretion in patients with anorexia nervosa. Clin. Endocrinol. (Oxf.) 2003, 59, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Ohwada, R.; Akamizu, T.; Shibasaki, T.; Kangawa, K. Therapeutic potential of ghrelin in restricting-type anorexia nervosa. Methods Enzymol. 2012, 514, 381–398. [Google Scholar] [PubMed]
- Misra, M.; Miller, K.K.; Herzog, D.B.; Ramaswamy, K.; Aggarwal, A.; Almazan, C.; Neubauer, G.; Breu, J.; Klibanski, A. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J. Clin. Endocrinol. Metab. 2004, 89, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Barrios, V.; Campos-Barros, A.; Argente, J. Ghrelin levels in obesity and anorexia nervosa: Effect of weight reduction or recuperation. J. Pediatr. 2004, 144, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Janas-Kozik, M.; Krupka-Matuszczyk, I.; Malinowska-Kolodziej, I.; Lewin-Kowalik, J. Total ghrelin plasma level in patients with the restrictive type of anorexia nervosa. Regul. Pept. 2007, 140, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Tschöp, M.; Frühauf, E.; Heldwein, W.; Fichter, M.; Otto, C.; Cuntz, U. Postprandial ghrelin release in anorectic patients before and after weight gain. Psychoneuroendocrinology 2005, 30, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Janas-Kozik, M.; Krupka-Matuszczyk, I.; Tomasik-Krótki, J. Total plasma ghrelin level in anorexia nervosa female. Wiad. Lek. 2006, 59, 311–316. [Google Scholar] [PubMed]
- Beranová, L.; Sedlácková, D.; Kopecková, J.; Hainer, V.; Papezová, H.; Kvasnicková, H.; Nedvídková, J. Neuropeptide Y, ghrelin and leptin plasma levels in anorexia nervosa patients and their changes during six-week refeeding. Vnitr. Lek. 2009, 55, 925–928. [Google Scholar] [PubMed]
- Uehara, M.; Yasuhara, D.; Nakahara, T.; Harada, T.; Koyama, K.I.; Ushikai, M.; Asakawa, A.; Inui, A. Increase in energy intake leads to a decrease in obestatin in restricting-type of anorexia nervosa. Exp. Clin. Endocrinol. Diabetes 2011, 119, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kojima, S.; Tanaka, M.; Yasuhara, D.; Harada, T.; Sagiyama, K.; Muranaga, T.; Nagai, N.; Nakazato, M.; Nozoe, S.; et al. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J. Psychiatr. Res. 2007, 41, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, F.; Monteleone, P.; Maj, M. Olanzapine-induced weight gain in anorexia nervosa: Iof leptin and ghrelin secretion? Psychoneuroendocrinology 2007, 32, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, O.; Ravussin, E.; Jacobson, P.; Snyder, E.E.; Chagnon, M.; Sjöström, L.; Bouchard, C. Mutations in the preproghrelin/ghrelin gene associated with obesity in humans. J. Clin. Endocrinol. Metab. 2001, 86, 3996–3999. [Google Scholar] [CrossRef] [PubMed]
- Hinney, A.; Hoch, A.; Geller, F.; Schäfer, H.; Siegfried, W.; Goldschmidt, H.; Remschmidt, H.; Hebebrand, J. Ghrelin gene: Identification of missense variants and a frameshift mutation in extremely obese children and adolescents and healthy normal weight students. J. Clin. Endocrinol. Metab. 2002, 87, 2716. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Tortorella, A.; Castaldo, E.; Di Filippo, C.; Maj, M. No association of the Arg51Gln and Leu72Met polymorphisms of the ghrelin gene with anorexia nervosa or bulimia nervosa. Neurosci. Lett. 2006, 398, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Cellini, E.; Nacmias, B.; Brecelj-Anderluh, M.; Badía-Casanovas, A.; Bellodi, L.; Boni, C.; Di Bella, D.; Estivill, X.; Fernandez-Aranda, F.; Foulon, C.; et al. Case-control and combined family trios analysis of three polymorphisms in the ghrelin gene in european patients with anorexia and bulimia nervosa. Psychiatr. Genet. 2006, 16, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Dardennes, R.M.; Zizzari, P.; Tolle, V.; Foulon, C.; Kipman, A.; Romo, L.; Iancu-Gontard, D.; Boni, C.; Sinet, P.M.; Thérèse Bluet, M.; et al. Family trios analysis of common polymorphisms in the obestatin/ghrelin, BDNF and AGRP genes in patients with anorexia nervosa: Association with subtype, body-mass index, severity and age of onset. Psychoneuroendocrinology 2007, 32, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Komaki, G.; Nishimura, H.; Naruo, T.; Okabe, K.; Kawai, K.; Takii, M.; Oka, T.; Kodama, N.; Nakamoto, C.; et al. A ghrelin gene variant may predict crossover rate from restricting-type anorexia nervosa to other phenotypes of eating disorders: A retrospective survival analysis. Psychiatr. Genet. 2010, 20, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Tschöp, M.H.; Jarick, I.; Ehrlich, S.; Scherag, S.; Herpertz-Dahlmann, B.; Zipfel, S.; Herzog, W.; de Zwaan, M.; Burghardt, R.; et al. Genetic variation of the ghrelin activator gene ghrelin o-acyltransferase (GOAT) is associated with anorexia nervosa. J. Psychiatr. Res. 2011, 45, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Legrand, R.; Lucas, N.; Breton, J.; Azhar, S.; do Rego, J.C.; Déchelotte, P.; Coëffier, M.; Fetissov, S.O. Ghrelin treatment prevents development of activity based anorexia in mice. Eur. Neuropsychopharmacol. 2016, 26, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Legrand, R.; Asakawa, A.; Amitani, H.; François, M.; Tennoune, N.; Coëffier, M.; Claeyssens, S.; do Rego, J.C.; Déchelotte, P.; et al. Anti-ghrelin immunoglobulins modulate ghrelin stability and its orexigenic effect in obese mice and humans. Nat. Commun. 2013, 4, 2685. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Gianotti, L.; Destefanis, S.; Fassino, S.; Abbate Daga, G.; Mondelli, V.; Lanfranco, F.; Gottero, C.; Gauna, C.; Hofland, L.; et al. The endocrine response to acute ghrelin administration is blunted in patients with anorexia nervosa, a ghrelin hypersecretory state. Clin. Endocrinol. 2004, 60, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Miljic, D.; Pekic, S.; Djurovic, M.; Doknic, M.; Milic, N.; Casanueva, F.F.; Ghatei, M.; Popovic, V. Ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 2006, 91, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Weikel, J.C.; Wichniak, A.; Ising, M.; Brunner, H.; Friess, E.; Held, K.; Mathias, S.; Schmid, D.A.; Uhr, M.; Steiger, A. Ghrelin promotes slow-wave sleep in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E407–E415. [Google Scholar] [CrossRef] [PubMed]
- Miljic, D.; Djurovic, M.; Pekic, S.; Doknic, M.; Stojanovic, M.; Milic, N.; Casanueva, F.F.; Ghatei, M.; Popovic, V. Glucose metabolism during ghrelin infusion in patients with anorexia nervosa. J. Endocrinol. Investig. 2007, 30, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Ohwada, R.; Akamizu, T.; Shibasaki, T.; Takano, K.; Kangawa, K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: A pilot study. Endocr. J. 2009, 56, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Maejima, Y.; Muroya, S.; Yada, T. Rikkunshito and isoliquiritigenin counteract 5-HT-induced 2C receptor-mediated activation of pro-opiomelanocortin neurons in the hypothalamic arcuate nucleus. Neuropeptides 2013, 47, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, C.; Muto, S.; Nakagawa, K.; Ohnishi, S.; Saegusa, Y.; Nahata, M.; Hattori, T.; Asaka, M.; Takeda, H. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem. Biophys. Res. Commun. 2011, 412, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Watari, H.; Kanno, M.; Ohba, Y.; Takeuchi, S.; Miyaji, T.; Oyamada, S.; Nomura, E.; Kato, H.; Sugiyama, T.; et al. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting, and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase II study (JORTC KMP-02). J. Gynecol. Oncol. 2017, 28, e44. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Lawson, E.A.; Faje, A.T.; Eddy, K.T.; Lee, H.; Fiedorek, F.T.; Breggia, A.; Gaal, I.M.; DeSanti, R.; Klibanski, A. Treatment with a ghrelin agonist in outpatient women with anorexia nervosa: A randomized clinical trial. J. Clin. Psychiatry 2018, 79, 1. [Google Scholar] [CrossRef] [PubMed]
 growth hormone secretagogue receptor 1a;
growth hormone secretagogue receptor 1a;  mechanisms contributing to insufficient stimulation of food intake irrespective of high ghrelin levels; = no alteration; ↑ increase/stimulation; ↓ decrease/inhibition, - - > indirect effect; ACTH, adrenocorticotropic hormone; BBB, brain-blood barrier; BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor-1; T3, triiodothyronine.
mechanisms contributing to insufficient stimulation of food intake irrespective of high ghrelin levels; = no alteration; ↑ increase/stimulation; ↓ decrease/inhibition, - - > indirect effect; ACTH, adrenocorticotropic hormone; BBB, brain-blood barrier; BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor-1; T3, triiodothyronine.
 growth hormone secretagogue receptor 1a;
growth hormone secretagogue receptor 1a;  mechanisms contributing to insufficient stimulation of food intake irrespective of high ghrelin levels; = no alteration; ↑ increase/stimulation; ↓ decrease/inhibition, - - > indirect effect; ACTH, adrenocorticotropic hormone; BBB, brain-blood barrier; BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor-1; T3, triiodothyronine.
mechanisms contributing to insufficient stimulation of food intake irrespective of high ghrelin levels; = no alteration; ↑ increase/stimulation; ↓ decrease/inhibition, - - > indirect effect; ACTH, adrenocorticotropic hormone; BBB, brain-blood barrier; BMI, body mass index; GH, growth hormone; IGF-1, insulin-like growth factor-1; T3, triiodothyronine.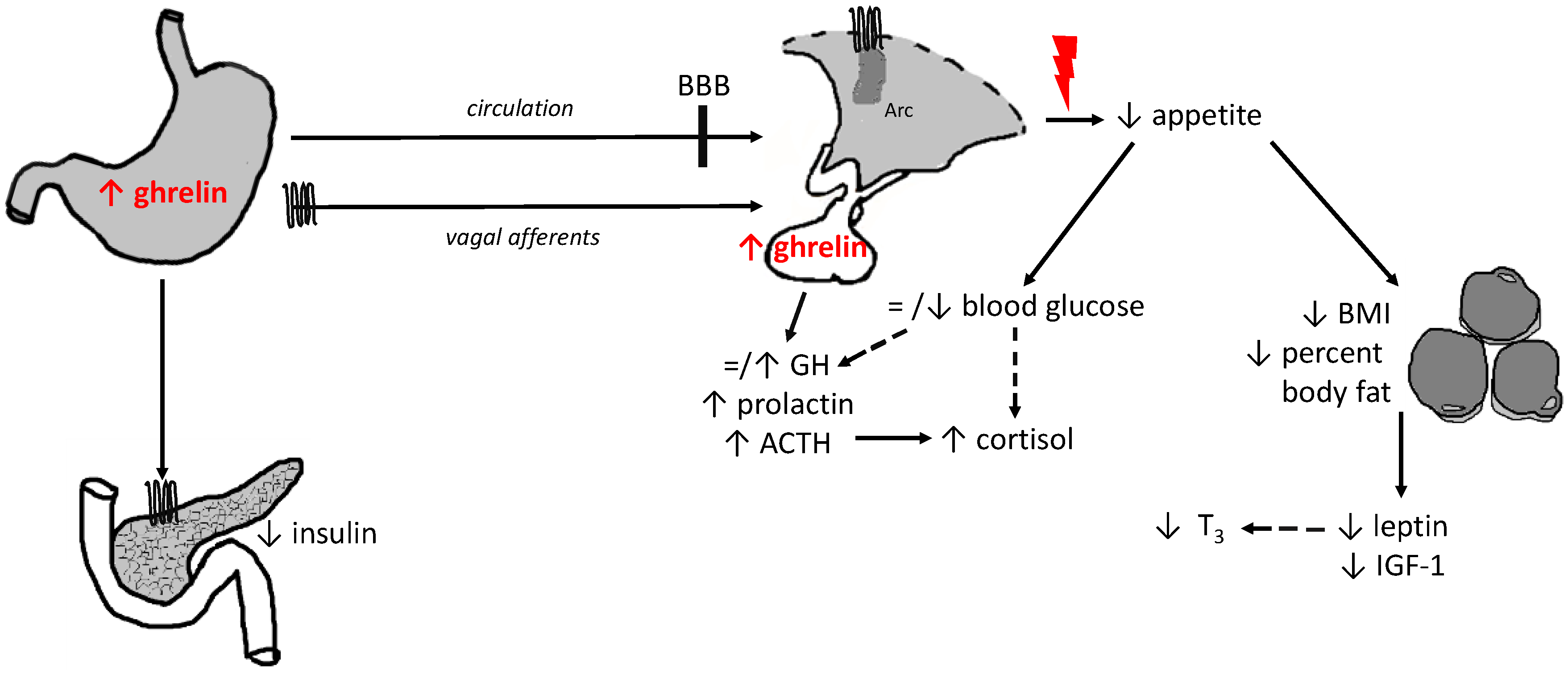
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schalla, M.A.; Stengel, A. The Role of Ghrelin in Anorexia Nervosa. Int. J. Mol. Sci. 2018, 19, 2117. https://doi.org/10.3390/ijms19072117
Schalla MA, Stengel A. The Role of Ghrelin in Anorexia Nervosa. International Journal of Molecular Sciences. 2018; 19(7):2117. https://doi.org/10.3390/ijms19072117
Chicago/Turabian StyleSchalla, Martha A., and Andreas Stengel. 2018. "The Role of Ghrelin in Anorexia Nervosa" International Journal of Molecular Sciences 19, no. 7: 2117. https://doi.org/10.3390/ijms19072117
APA StyleSchalla, M. A., & Stengel, A. (2018). The Role of Ghrelin in Anorexia Nervosa. International Journal of Molecular Sciences, 19(7), 2117. https://doi.org/10.3390/ijms19072117