Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine
Abstract
1. Introduction
2. Result and Discussion
2.1. Effects on Growth Performance and Nutrient Metabolism
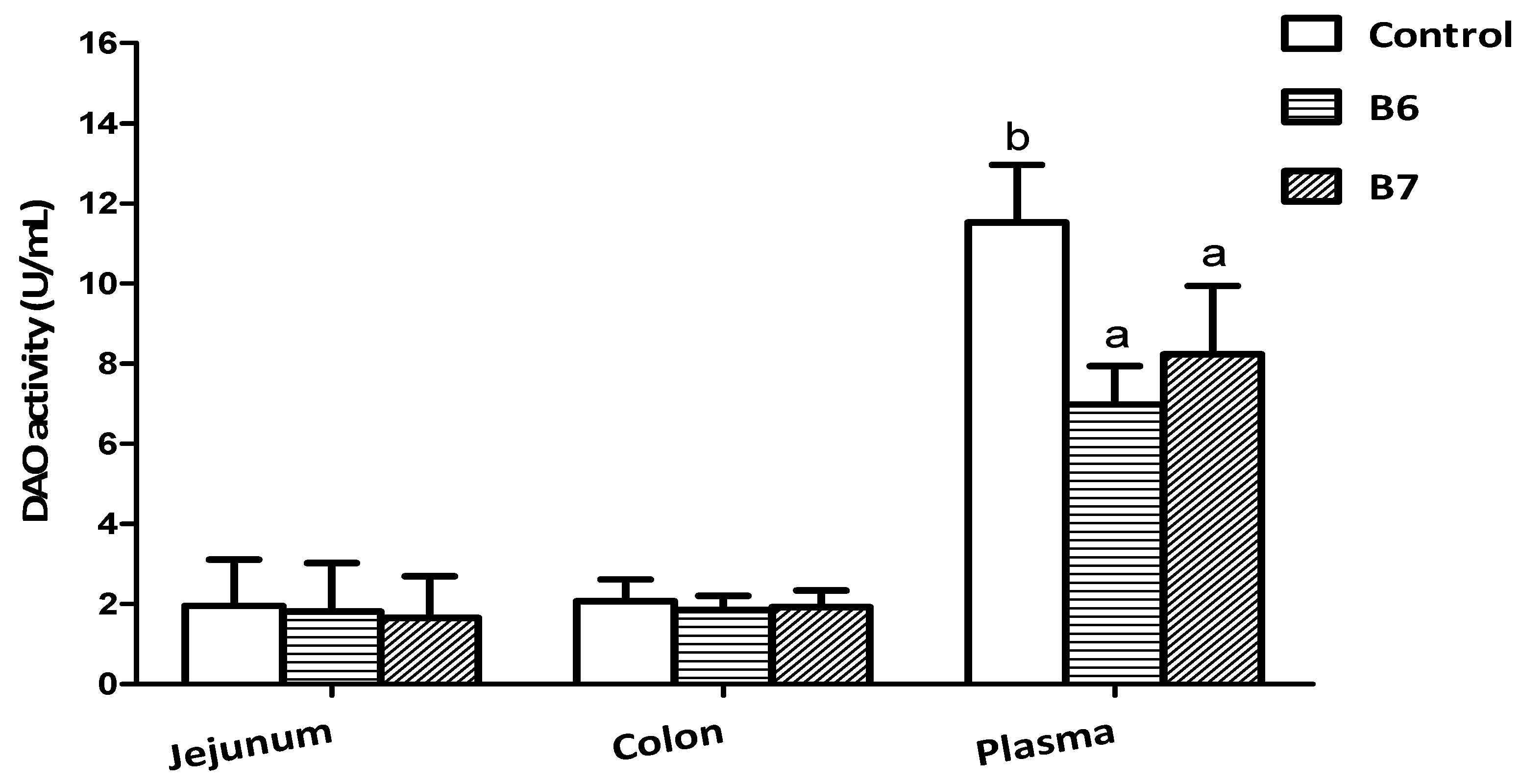
2.2. Effects on Intestinal Integrity and Redox Status
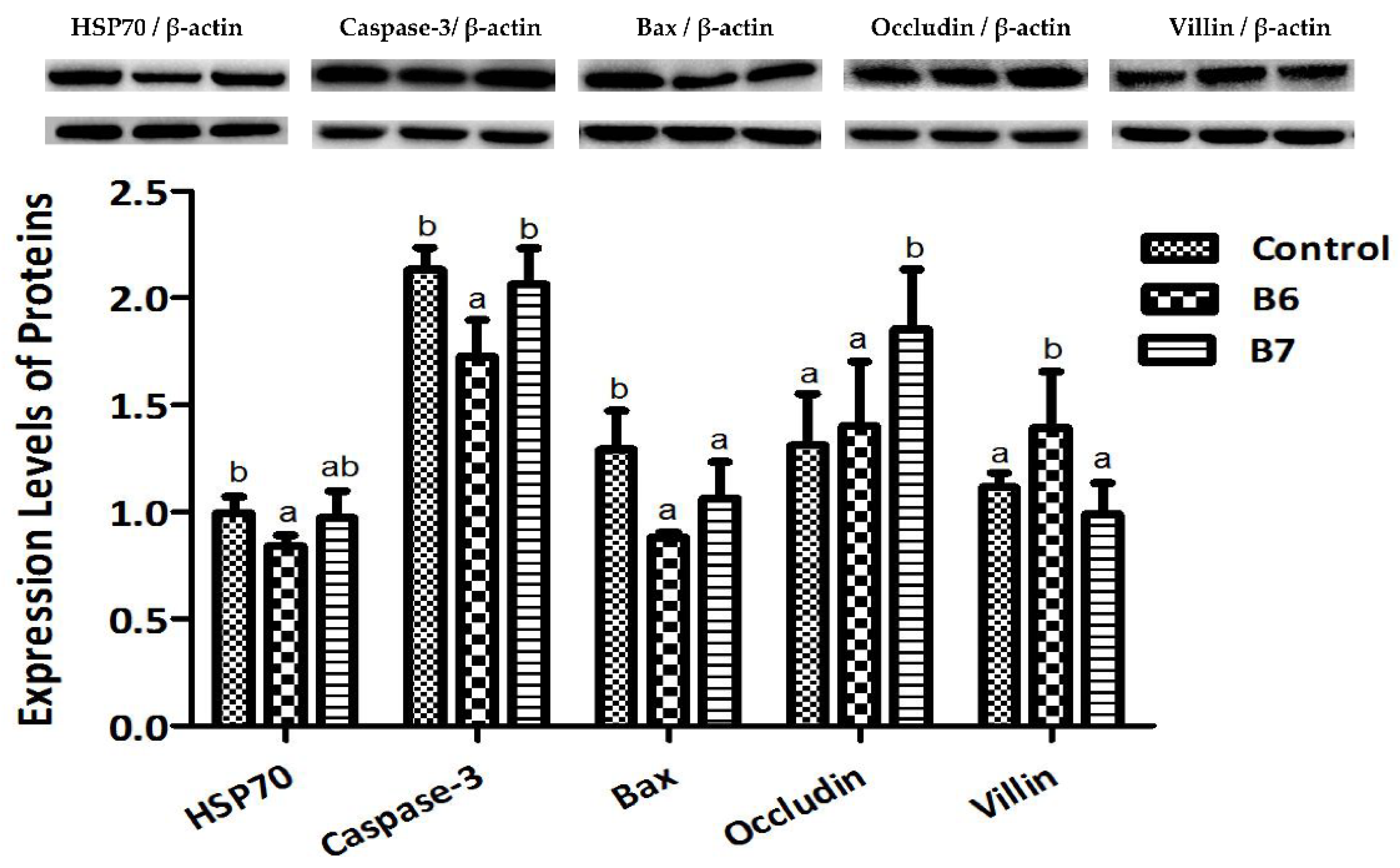
2.3. Regulation of Protein Expression
2.4. Regulation of Gene Expression
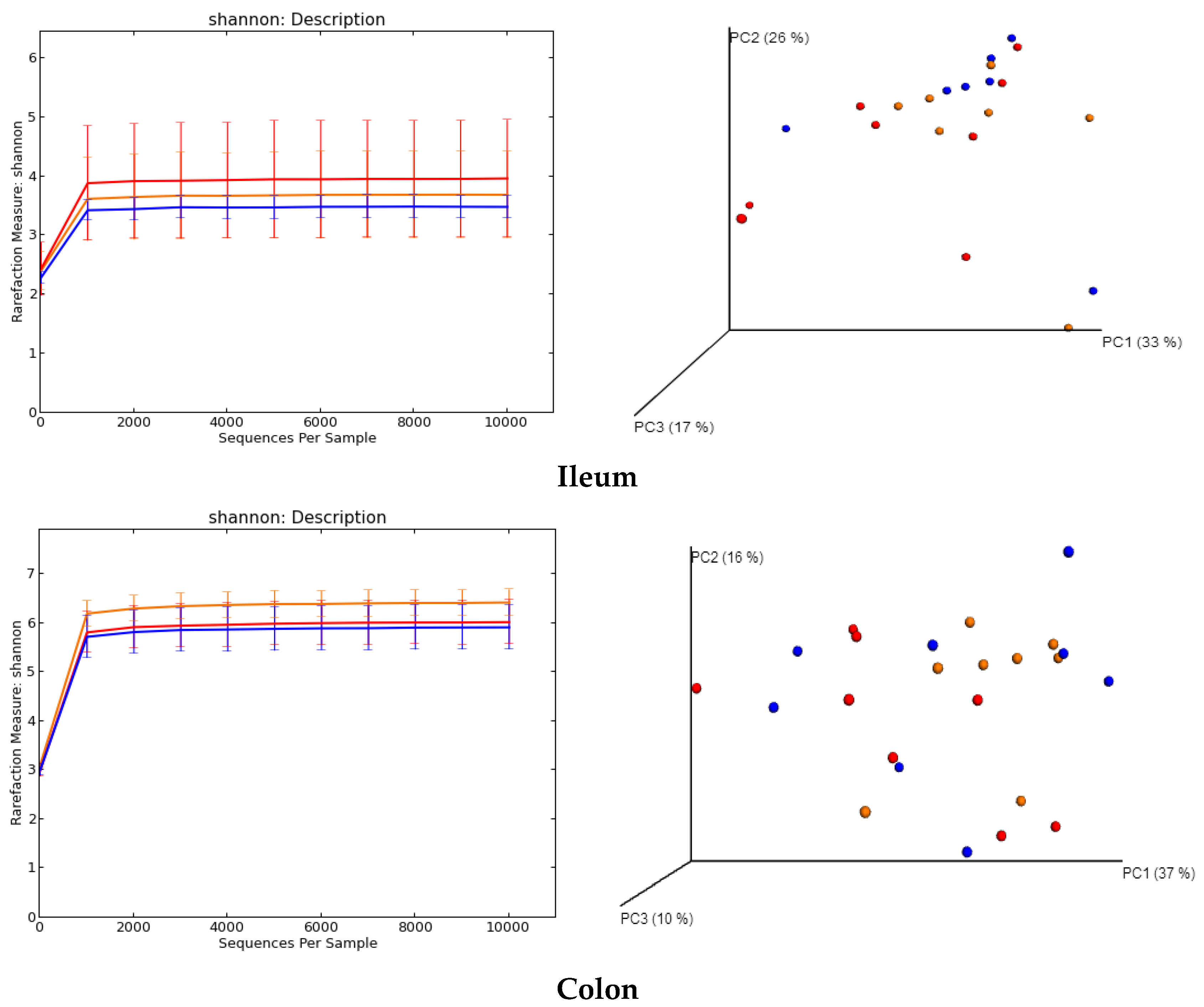
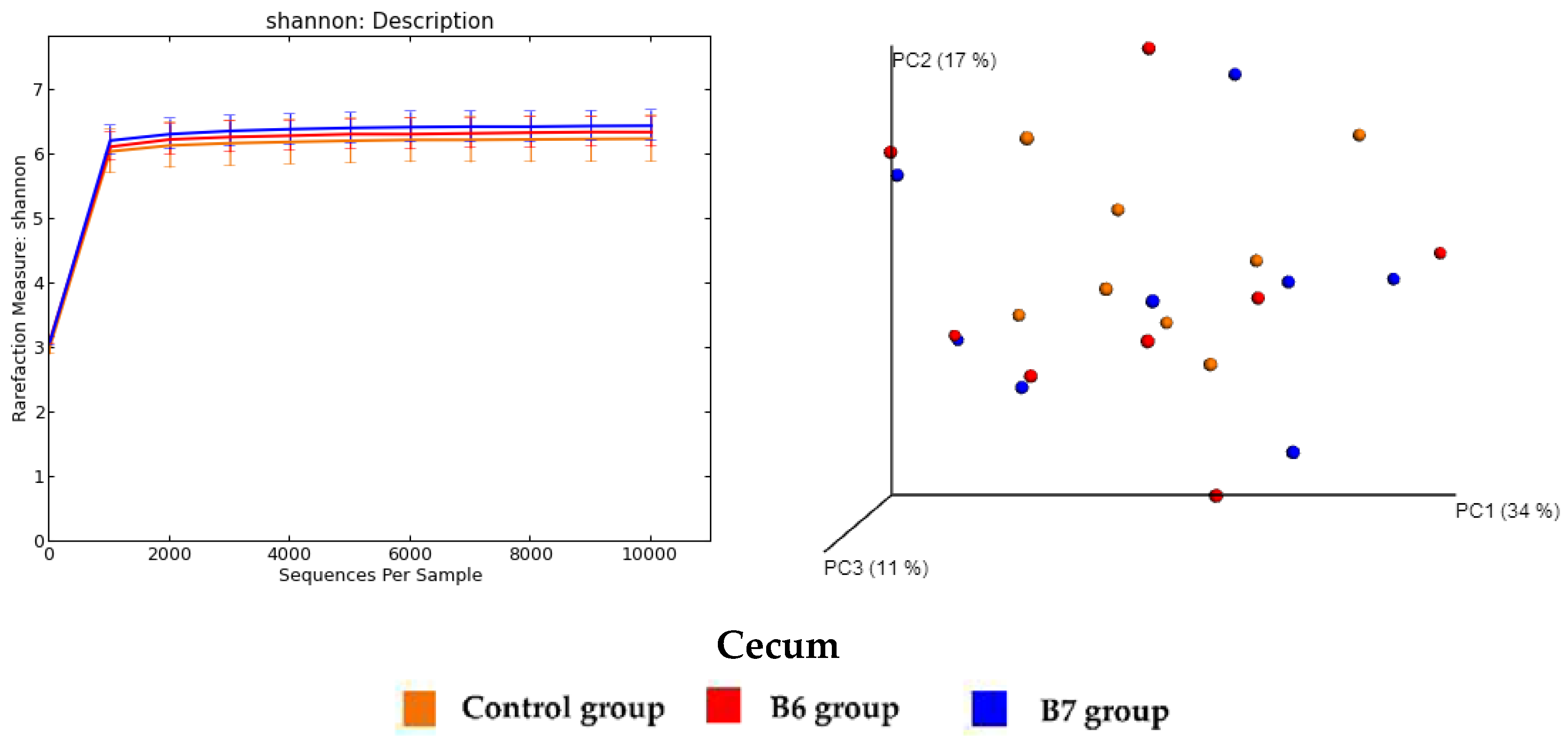
2.5. Regulation of Gut Microbiota
3. Material and Methods
3.1. Experimental Design and Sample Collection
3.2. Plasma Biochemical Indicators and Intestinal Redox Status
3.3. Intestinal Morphology
3.4. Expression Levels of Proteins
3.5. Expression Levels of Genes
3.6. Analysis of Gut Microbiota
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pluske, J.R.; Hampson, D.J.; Williams, I.H. Factors influencing the structure and function of the small intestine in the weaned pig: A review. Livest. Prod. Sci. 1997, 51, 215–236. [Google Scholar] [CrossRef]
- De, G.A.; Resink, J.W.; VanHees, H.M.; Ruuls, L.; Klaassen, G.J.; Rouwers, S.M.; Stockhofe-Zurwieden, N. Supplementation of piglets with nutrient-dense complex milk replacer improves intestinal development and microbial fermentation. J. Anim. Sci. 2016, 94, 1012–1019. [Google Scholar]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pig. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Boudry, G.; Favier, C.; Le Huërou-Luron, I.; Lallès, J.P.; Sève, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Moeser, A.J.; Ryan, K.A.; Nighot, P.K.; Blikslager, A.T. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G413–G421. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, R.D.; Seerley, R.W.; Wyatt, R.D. The effect of diet on performance, digestibility, blood composition and intestinal microflora of weaned pigs. J. Anim. Sci. 1984, 58, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Hamard, A.; Sève, B.; Le, F.N. Intestinal development and growth performance of early-weaned piglets fed a low-threonine diet. Animal 2007, 1, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G.; Wu, G. Intestinal nitrogen recycling and utilization in health and disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.L.; Li, X.L.; Xi, P.B.; Zhang, J.; Wu, G.; Zhu, W.Y. L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013, 45, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A truly functional amino acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Huang, J.J.; Hou, Y.Q.; Zhu, H.L.; Zhao, S.J.; Ding, B.Y.; Yin, Y.L.; Yi, G.F.; Shi, J.Y.; Fan, W. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 2008, 100, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino acid metabolism in intestinal bacteria: Links between gut ecology and host health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Xin, W.; Zheng, R.; Kong, X.F.; Tang, Z.R.; Dong, X.H. Functions of microbiota in monogastric gastrointestinal tract and new practices in animal production. J. Food Agric. Environ. 2010, 8, 695–702. [Google Scholar]
- Arunachalam, K.D. Role of Bifidobacteria in nutrition, medicine and technology. Nutr. Res. 1999, 19, 1559–1597. [Google Scholar] [CrossRef]
- Bon, M.L.; Davies, H.E.; Glynn, C.; Thompson, C.; Madden, M.; Wiseman, J.; Dodd, C.; Hurdidge, L.; Payne, G.; Treut, Y.L.; et al. Influence of probiotics on gut health in the weaned pig. Livest. Sci. 2010, 133, 179–181. [Google Scholar] [CrossRef]
- Xuan, Z.N.; Kim, J.D.; Heo, K.N.; Jung, H.J.; Lee, J.H.; Han, Y.K.; Kim, Y.Y.; Han, I.K. Study on the development of a probiotics complex for weaned pigs. Asian Aust. J. Anim. Sci. 2001, 14, 1425–1428. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yin, F.G.; Zhu, C.; Yu, H.; Niven, S.J.; DeLange, C.F.M.; Gong, J. Evaluation of probiotic bacteria for their effects on the growth performance and intestinal microbiota of newly-weaned pigs fed fermented high-moisture maize. Livest. Sci. 2012, 145, 79–86. [Google Scholar] [CrossRef]
- Payot, T.; Chemaly, Z.; Fick, M. Lactic acid production by Bacillus coagulans—Kinetic studies and optimization of culture medium for batch and continuous fermentations. Enzyme Microb. Technol. 1999, 24, 191–199. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Gu, Q.; Li, W. Effect of dietary probiotic, Bacillus coagulans, on growth performance, chemical composition, and meat quality of Guangxi Yellow chicken. Poul. Sci. 2010, 89, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.T.; Lin, S.Y.; Yang, T.Y.; Chou, C.K.; Liu, H.C.; Lu, J.J.; Wang, B.; Chen, S.Y.; Lien, T.F. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Anim. Prod. Sci. 2012, 52, 874–879. [Google Scholar] [CrossRef]
- Ripamonti, B.; Agazzi, A.; Baldi, A.; Balzaretti, C.; Bersani, C.; Pirani, S.; Rebucci, R.; Savoini, G.; Stella, S.; Stenico, A.; et al. Administration of Bacillus coagulans, in calves: Recovery from faecal samples and evaluation of functional aspects of spores. Vet. Res. Commun. 2009, 33, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Hyronimus, B.; Marrec, C.L.; Urdaci, M.C. Coagulin, a bacteriocin-like inhibitory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 1998, 85, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Baron, M. A patented strain of Bacillus coagulans increased immune response to viral challenge. Postgrad. Med. 2009, 121, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Kristal-Boneh, E.; Harari, G.; Green, M.S. Circannual Variations in Blood Cholesterol Levels. Chronobiol. Int. 1993, 10, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Choi, M.K.; Kim, M.H. Manganese supplementation reduces the blood cholesterol levels in Ca-deficient ovariectomized rats. Biol. Trace Elem. Res. 2011, 141, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Stole, E.; Smith, T.K.; Manning, J.M.; Meister, A. Interaction of gamma-glutamyl transpeptidase with acivicin. J. Biol. Chem. 1994, 269, 21435–21439. [Google Scholar] [PubMed]
- Braun, J.P.; Benard, P.; Burgat, V.; Rico, A.G. Gamma Glutamyl Transferase in domestic animals. Vet. Res. Commun. 1983, 6, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Agúndez, J.A.; Ayuso, P.; Cornejo-García, J.A.; Blanca, M.; Torres, M.J.; Doña, I.; Salas, M.; Blanca-López, N.; Canto, G.; Rondon, C.; et al. The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLoS ONE 2012, 7, e47571. [Google Scholar] [CrossRef] [PubMed]
- Kitanaka, J.; Kitanaka, N.; Tsujimura, T.; Terada, N.; Takemura, M. Expression of diamine oxidase (histaminase) in guinea-pig tissues. Eur. J. Pharmacol. 2002, 437, 179–185. [Google Scholar] [CrossRef]
- Pluske, J.R.; Williams, I.H.; Aherne, F.X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996, 62, 131–144. [Google Scholar] [CrossRef]
- Cera, K.R.; Mahan, D.C.; Cross, R.F.; Reinhart, G.A.; Whitmoyer, R.E. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J. Anim. Sci. 1988, 66, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Verrugio, C.; Ruiz-Ortega, M.; Mosqueira, M.; Simon, F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Regulation of antioxidant enzymes. J. Nutr. 1992, 122, 625–626. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Buchmeier, N.A.; Heffron, F. Induction of Salmonella stress proteins upon infection of macrophages. Science 1990, 248, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, S.; Smith, E.D.; Prieto, G.A.; Cotman, C.W. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci. Bull. 2012, 28, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, R.A.; Saavedra, G.M.; Cummings, B.S.; Franklin, J.L. Bax regulates production of superoxide in both apoptotic and nonapoptotic neurons: Role of caspases. J. Neurosci. 2010, 30, 16114–16127. [Google Scholar] [CrossRef] [PubMed]
- Friederich, E.; Pringault, E.; Arpin, M.; Louvard, D. From the structure to the function of villin, an actin-binding protein of the brush border. Bioessays 1990, 12, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 2005, 23, 307–336. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Yanai, H. Interferon signalling network in innate defence. Cell. Microbiol. 2006, 8, 907–922. [Google Scholar] [CrossRef] [PubMed]
- García-Sastre, A.; Biron, C.A. Type 1 interferons and the virus-host relationship: A lesson in Détente. Science 2006, 312, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, T.; Hayakari, R.; Narita, N.; Ito, R.; Kon, T.; Kubota, K.; Sakaki, H.; Yoshida, H.; Imaizumi, T.; Kobayashi, W.; et al. Role of type I- and type II-interferon in expression of melanoma differentiation-associated gene-5 in HSC-3 oral squamous carcinoma cells. Biomed. Res. 2014, 35, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Lisboa, P.C.; Lima, N.S.; Amaral, T.A.; Peixoto-Silva, N.; Resende, A.C.; Oliveira, E.; Passos, M.C.; Moura, E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013, 24, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Villehuchet, A.M.; Brack, M.; Dreyfus, G.; Oussar, Y.; Bonnefont-Rousselot, D.; Chapman, M.J.; Kontush, A. A machine-learning approach to the prediction of oxidative stress in chronic inflammatory disease. Redox. Rep. 2009, 14, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Lu, C.Y.; Wei, C.Y.; Ma, Y.S.; Lee, H.C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin. J. Physiol. 2001, 44, 1–12. [Google Scholar] [PubMed]
- Jiang, X.C.; Moulin, P.; Quinet, E.; Goldberg, I.J.; Yacoub, L.K.; Agellon, L.B.; Compton, D.; Schnitzer-Polokoff, R.; Tall, A.R. Mammalian adipose tissue and muscle are major sources of lipid transfer protein mRNA. J. Biol. Chem. 1991, 266, 4631–4639. [Google Scholar] [PubMed]
- Sarfstein, R.; Werner, H. Insulin receptor (INSR) and insulin-like growth factor-I receptor (IGF-GR) translocate to nucleus and regulate IGF-GR gene expression in breast cancer cells. Growth Horm. IGF Res. 2012, 22, S4. [Google Scholar] [CrossRef]
- Arnspang, E.C.; Sundbye, S.; Nelson, W.J.; Nejsum, L.N. Aquaporin-3 and aquaporin-4 are sorted differently and separately in the trans-Golgi network. PLoS ONE 2013, 8, e73977. [Google Scholar] [CrossRef] [PubMed]
- Tavakkolizadeh, A.; Berger, U.V.; Shen, K.R.; Levitsky, L.L.; Zinner, M.J.; Hediger, M.A.; Ashley, S.W.; Whang, E.E.; Rhoads, D.B. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G209–G215. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Dave, M.H.; Bacic, D.; Ristic, Z.; Camargo, S.M.; Loffing, J.; Wagner, C.A.; Verrey, F. Luminal kidney and intestine SLC6 amino acid transporters of B0AT-cluster and their tissue distribution in Mus musculus. Am. J. Physiol. Ren. Physiol. 2006, 290, F376–F383. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.; Andersen, A.D.; Molbak, L.; Stagsted, J.; Boye, M. Changes in the gut microbiota of cloned and non-cloned control pigs during development of obesity: Gut microbiota during development of obesity in cloned pigs. BMC Microbiol. 2013, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Z.; Xu, H.; Laflamme, D.; Czarnecki-Maulden, G.; Li, Q.J.; Labuda, J.; Bourqui, B. Fecal Microbiota of Cats with Naturally Occurring Chronic Diarrhea Assessed Using 16S rRNAGene 454-Pyrosequencing before and after Dietary Treatment. J. Vet. Int. Med. 2014, 28, 59–65. [Google Scholar] [CrossRef]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking longterm dietary patterns with gut microbial enterotypes. Science 2011, 333, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Sabine, G.; Sabine, W.; Alla, L.; Goegelel, H.; Sifri, C.D.; Martin, K.; Hinder, R.; Nguyen, J.H. Complete genome sequence of Veillonella parvula type strain (Te3). Stand. Genom. Sci. 2010, 2, 57–65. [Google Scholar]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Skerman, V.B.D.; McGowan, V.; Sneath, P.H.A. Approved lists of bacterial names. Int. J. Syst. Bacteriol. 1980, 30, 225–420. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Wang, L.; Ding, B.Y. Dietary α-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharidechallenged piglets. Amino Acids 2010, 39, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Dawson, H.D.; Wasmuth, E.V.; Roneker, C.A.; Chen, C.; Urban, J.F.; Welch, R.M.; Miller, D.D.; Lei, X.G. Supplemental dietary inulin influences expression of iron and inflammation related genes in young pigs. J. Nutr. 2009, 139, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yao, K.; Wang, L.; Ding, B.; Fu, D.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Kang, P.; et al. Effects of α-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide. Br. J. Nutr. 2011, 106, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Hou, Y.; Wang, L.; Zhao, D.; Ding, B.; Wu, T.; Chen, H.; Liu, Y.; Kang, P.; Wu, G. Gene expression profiles in the intestine of lipopolysaccharide-challenged piglets. Front. Biosci. 2016, 21, 487–501. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
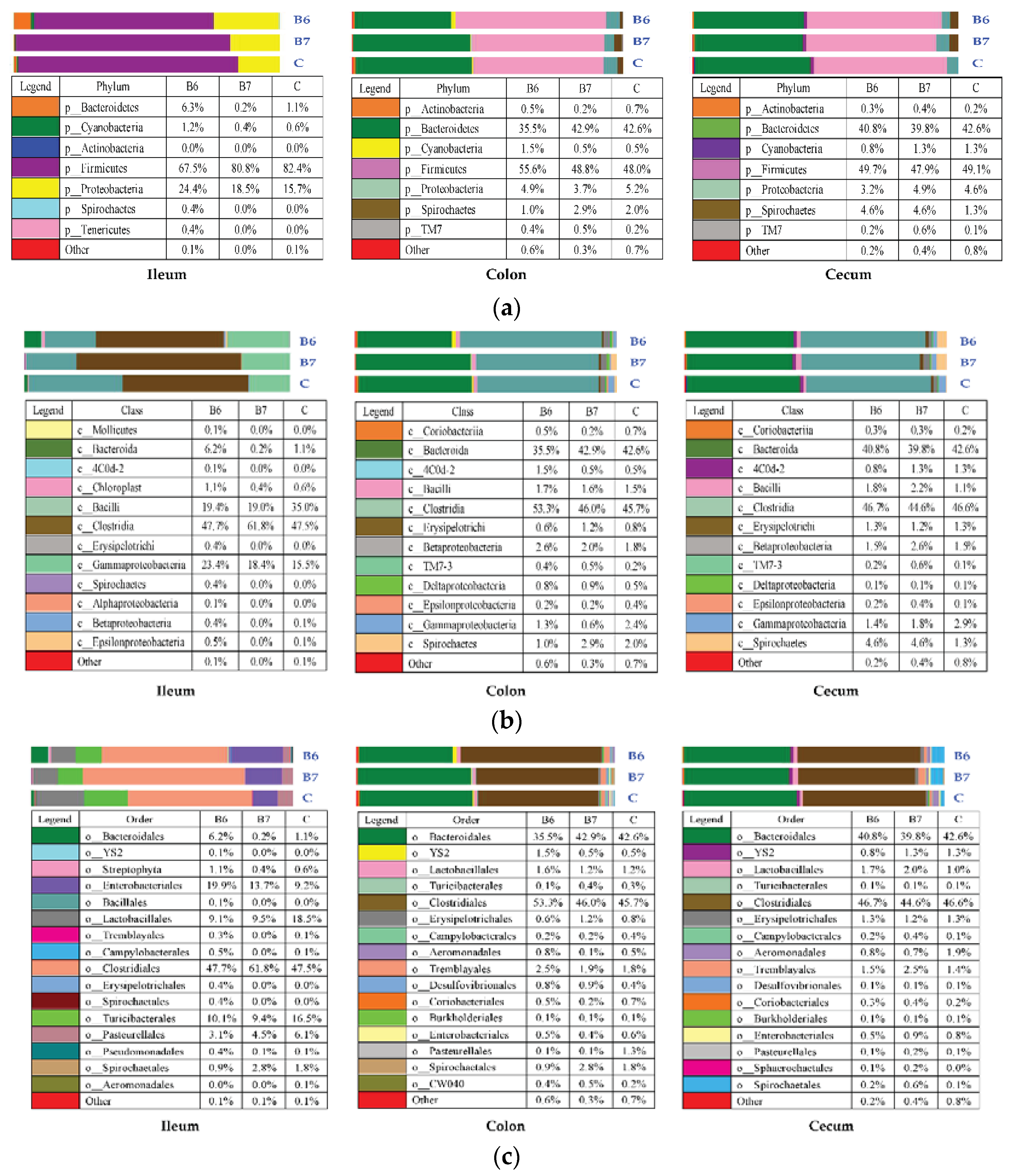
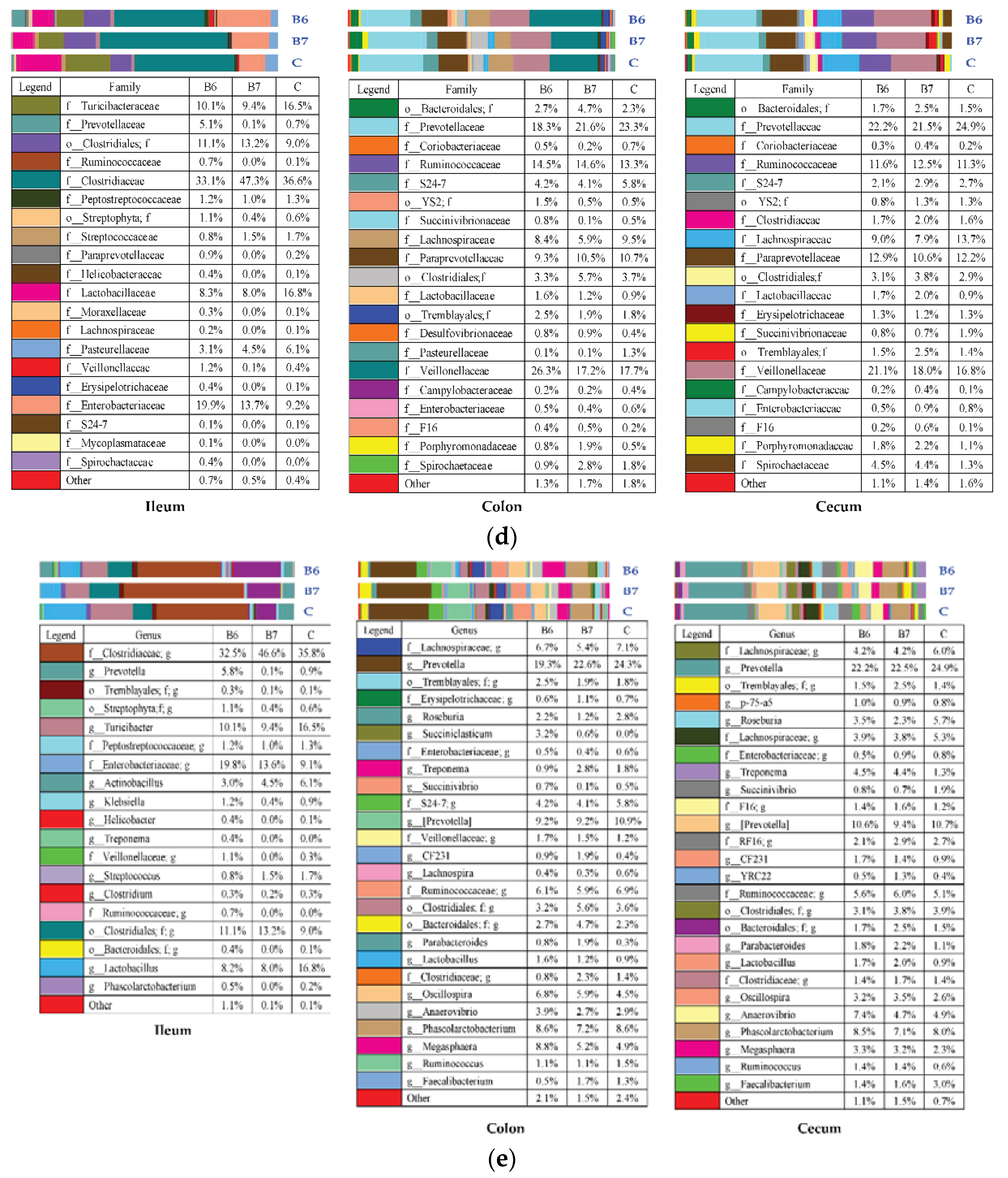
| Item | Control | B6 | B7 |
|---|---|---|---|
| Day 0–10 | |||
| ADG/g | 295.35 ± 70.08 | 334.07 ± 51.97 | 344.68 ± 65.62 |
| ADFI/g | 239.40 ± 84.79 | 263.20 ± 62.74 | 267.60 ± 77.96 |
| F/G | 1.28 ± 0.21 | 1.28 ± 0.12 | 1.29 ± 0.04 |
| Diarrhea rate/% | 27.0 b | 9.0 a | 5.0 a |
| Day 10–21 | |||
| ADG/g | 592.02 ± 122.77 | 642.62 ± 99.67 | 635.90 ± 96.64 |
| ADFI/g | 403.27 ± 119.01 | 432.27 ± 119.55 | 409.82 ± 166.78 |
| F/G | 1.48 ± 0.06 | 1.53 ± 0.22 | 1.56 ± 0.11 |
| Diarrhea rate/% | 11.8 b | 0.9 a | 5.5 b |
| Day 0–21 | |||
| ADG/g | 450.72 ± 96.11 | 497.22 ± 77.65 | 495.68 ± 72.39 |
| ADFI/g | 325.24 ± 97.15 | 342.10 ± 81.62 | 354.95 ± 81.05 |
| F/G | 1.40 ± 0.10 | 1.46 ± 0.06 | 1.41 ± 0.09 |
| Diarrhea rate/% | 19.5 b | 5.2 a | 5.7 a |
| Item | Control | B6 | B7 |
|---|---|---|---|
| TP (g/L) | 49.68 ± 2.70 | 48.86 ± 4.17 | 48.16 ± 3.31 |
| CHOL (mmol/L) | 1.77 ± 0.29 b | 1.71 ± 0.19 ab | 1.51 ± 0.23 a |
| TG (mmol/L) | 0.37 ± 0.02 a | 0.41 ± 0.09 ab | 0.46 ± 0.08 b |
| GLU (mmol/L) | 5.54 ± 0.84 | 5.48 ± 0.16 | 5.51 ± 0.85 |
| GGT (mmol/L) | 36.23 ± 8.94 b | 32.48 ± 4.08 ab | 28.67 ± 5.25 a |
| Item | Jejunum | Ileum | ||||
|---|---|---|---|---|---|---|
| Control | B6 | B7 | Control | B6 | B7 | |
| villus height (μm) | 318.7 ± 35.0 | 350.1 ± 41.4 | 336.6 ± 41.2 | 242.1 ± 22.8 a | 273.3 ± 19.8 b | 285.2 ± 30.7 b |
| crypt depth (μm) | 213.6 ± 18.2 b | 168.7 ± 15.8 a | 175.4 ± 20.2 a | 161.0 ± 11.1 | 168.0 ± 13.3 | 173.2 ± 19.5 |
| villus height/crypt depth | 1.49 ± 0.11 a | 2.08 ± 0.17c | 1.92 ± 0.15 b | 1.51 ± 0.09 a | 1.63 ± 0.12 b | 1.65 ± 0.08 b |
| villous surface area (cm2) | 29677 ± 3031 | 31738 ± 3633 | 29,540 ± 4078 | 27,520 ± 932 | 28,396 ± 3715 | 28,502 ± 2870 |
| Item | Control | B6 | B7 | Control | B6 | B7 |
|---|---|---|---|---|---|---|
| Duodenum | Ileum | |||||
| SOD (U/mg) | 42.25 ± 3.76 a | 54.89 ± 4.19 b | 45.48 ± 3.30 a | 83.04 ± 5.79 | 78.95 ± 3.00 | 79.68 ± 4.52 |
| CAT (U/mg) | 17.92 ± 4.41 | 16.61 ± 2.73 | 15.59 ± 3.64 | 9.54 ± 3.08 | 7.93 ± 1.72 | 7.92 ± 1.40 |
| MDA (nmol/mg) | 4.52 ± 0.77 | 5.82 ± 1.77 | 5.69 ± 0.93 | 6.00 ± 2.40 b | 3.52 ± 1.11 a | 4.21 ± 1.40 ab |
| H2O2 (nmol/mg) | 5.06 ± 1.10 ab | 4.12 ± 1.01a | 5.35 ± 1.21 b | 8.34 ± 1.80 | 7.77 ± 3.20 | 7.49 ± 1.82 |
| Jejunum | Colon | |||||
| SOD (U/mg) | 81.55 ± 10.51 a | 90.53 ± 5.43 b | 94.21 ± 6.36 b | 100.54 ± 25.07 | 95.62 ± 4.92 | 92.56 ± 12.95 |
| CAT (U/mg) | 7.64 ± 1.23 a | 7.55 ± 1.11 a | 11.00 ± 2.48 b | 6.56 ± 1.15 a | 10.58 ± 2.40 b | 11.79 ± 2.89 b |
| MDA (nmol/mg) | 11.71 ± 4.75 | 9.61 ± 4.82 | 13.39 ± 3.96 | 3.66 ± 1.74 b | 1.64 ± 0.45 a | 2.30 ± 0.69 a |
| H2O2 (nmol/mg) | 30.75 ± 10.31 b | 19.40 ± 5.24 a | 23.08 ± 5.72 ab | 25.25 ± 6.43 b | 19.61 ± 5.00 a | 16.80 ± 3.04 a |
| Item | Ileum | Colon | ||||
|---|---|---|---|---|---|---|
| Control | B6 | B7 | Control | B6 | B7 | |
| IFN-α | 1.000 ± 0.156 a | 1.393 ± 0.211 b | 2.124 ± 0.383 c | 1.000 ± 0.218 | 1.041 ± 0.220 | 1.016 ± 0.323 |
| IFN-β | 1.000 ± 0.239 a | 1.247 ± 0.219 ab | 1.337 ± 0.313 b | 1.000 ± 0.141 a | 2.436 ± 0.483 b | 0.905 ± 0.205 a |
| IFN-γ | 1.000 ± 0.167 a | 1.276 ± 0.202 b | 1.191 ± 0.302 ab | 1.000 ± 0.223 b | 0.825 ± 0.159 a | 0.682 ± 0.078 a |
| MX1 | 1.000 ± 0.135 | 1.000 ± 0.230 | 1.196 ± 0.271 | 1.000 ± 0.172 a | 0.937 ± 0.159 a | 1.194 ± 0.188 b |
| MX2 | 1.000 ± 0.144 a | 2.015 ± 0.264 b | 2.649 ± 0.482 c | 1.000 ± 0.239 | 0.806 ± 0.203 | 0.976 ± 0.236 |
| OAS1 | 1.000 ± 0.148 a | 1.437 ± 0.313 b | 1.467 ± 0.354 b | 1.000 ± 0.130 | 1.088 ± 0.212 | 1.001 ± 0.263 |
| IL-1β | 1.000 ± 0.214 | 1.022 ± 0.125 | 0.846 ± 0.185 | 1.000 ± 0.257 b | 0.984 ± 0.113 b | 0.708 ± 0.130 a |
| IL-4 | 1.000 ± 0.265 a | 1.540 ± 0.300 b | 1.291 ± 0.285 ab | 1.000 ± 0.168 b | 0.759 ± 0.166 a | 0.870 ± 0.229 ab |
| CXCL-9 | 1.000 ± 0.253 b | 0.868 ± 0.119 ab | 0.787 ± 0.158 a | 1.000 ± 0.204 b | 1.102 ± 0.269 b | 0.729 ± 0.186 a |
| CCL-2 | 1.000 ± 0.205 a | 1.360 ± 0.325 b | 1.143 ± 0.275 ab | 1.000 ± 0.250 b | 0.646 ± 0.096 a | 0.862 ± 0.168 b |
| AQP3 | 1.000 ± 0.217 a | 2.643 ± 0.708 b | 2.382 ± 0.602 b | 1.000 ± 0.233 a | 0.923 ± 0.230 a | 1.287 ± 0.265 b |
| SGLT-1 | 1.000 ± 0.232 ab | 0.843 ± 0.132 a | 1.199 ± 0.268 b | 1.000 ± 0.203 a | 1.340 ± 0.273 b | 1.358 ± 0.223 b |
| LPL | 1.000 ± 0.203 a | 1.307 ± 0.276 b | 1.156 ± 0.216 ab | 1.000 ± 0.195 a | 0.857 ± 0.127 a | 1.250 ± 0.333 b |
| INSR | 1.000 ± 0.244 | 1.104 ± 0.275 | 1.037 ± 0.206 | 1.000 ± 0.203 a | 1.016 ± 0.186 a | 1.390 ± 0.311 b |
| b0,+AT | 1.000 ± 0.257 | 1.017 ± 0.219 | 1.228 ± 0.211 | 1.000 ± 0.215 a | 1.678 ± 0.390 b | 1.521 ± 0.370 b |
| OTU | p Value | FDR p Value | Relative Abundance | Taxonomy | ||
|---|---|---|---|---|---|---|
| C | B6 | B7 | ||||
| Ileum | ||||||
| 4406684 | 0.003 | 0.845 | 6.000 | 5.125 | 0.143 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| 299382 | 0.019 | 0.877 | 0.143 | 46.875 | 0.000 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| OTU8146 | 0.020 | 0.877 | 0.714 | 0.000 | 1.857 | o_Clostridiales; f_Clostridiaceae; g_ |
| 289257 | 0.038 | 0.877 | 2.857 | 13.875 | 1.571 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella; |
| Colon | ||||||
| 355450 | 0.012 | 0.943 | 22.250 | 14.000 | 2.000 | o_Clostridiales; f_Ruminococcaceae; g_ |
| OTU128 | 0.013 | 0.943 | 0.000 | 48.500 | 4.250 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| 613933 | 0.015 | 0.943 | 3.500 | 7.375 | 1.375 | o_Clostridiales; f_; g_ |
| 187350 | 0.017 | 0.943 | 2.375 | 0.125 | 3.125 | o_Clostridiales; f_; g_ |
| 325236 | 0.021 | 0.943 | 0.375 | 2.625 | 5.875 | o_Clostridiales; f_; g_ |
| 300859 | 0.022 | 0.943 | 61.125 | 31.625 | 30.875 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| 36705 | 0.025 | 0.943 | 2.625 | 1.625 | 1.250 | o_Clostridiales; f_Clostridiaceae; g_Clostridium |
| 4392188 | 0.026 | 0.943 | 7.375 | 1.375 | 1.875 | o_Clostridiales; f_Lachnospiraceae; g_ |
| OTU16964 | 0.031 | 0.943 | 0.500 | 2.875 | 0.000 | o_Clostridiales; f_Veillonellaceae; g_Acidaminococcus |
| 301253 | 0.032 | 0.943 | 2.250 | 1.250 | 0.000 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| 174019 | 0.033 | 0.943 | 1.125 | 10.625 | 1.250 | o_Clostridiales; f_Lachnospiraceae; g_Coprococcus |
| 4481427 | 0.034 | 0.943 | 7.625 | 0.125 | 0.000 | o_Clostridiales; f_Lachnospiraceae; g_Roseburia |
| 151623 | 0.038 | 0.943 | 6.250 | 42.250 | 12.875 | o_Clostridiales; f_Veillonellaceae; g_Megasphaera |
| 4416951 | 0.038 | 0.943 | 0.375 | 9.500 | 4.500 | o_Clostridiales; f_Ruminococcaceae; g_Ruminococcus |
| 4295618 | 0.041 | 0.943 | 7.750 | 3.000 | 0.750 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| 295835 | 0.047 | 0.943 | 7.625 | 19.500 | 0.125 | o_Clostridiales; f_; g_ |
| OTU1089 | 0.049 | 0.943 | 0.250 | 1.375 | 3.500 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| Cecum | ||||||
| OTU100 | 0.012 | 0.999 | 0.500 | 10.625 | 8.250 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| OTU102 | 0.013 | 0.999 | 0.250 | 14.625 | 9.875 | o_Bacteroidales; f_S24-7; g_ |
| 174153 | 0.016 | 0.999 | 0.375 | 3.750 | 4.000 | o_Clostridiales; f_Lachnospiraceae; g_ |
| 196800 | 0.019 | 0.999 | 0.250 | 6.625 | 5.250 | o_Bacteroidales; f_Prevotellaceae; g_Prevotellastercorea |
| 3275562 | 0.020 | 0.999 | 79.625 | 24.000 | 25.375 | o_Clostridiales; f_Lachnospiraceae; g_ |
| 190320 | 0.028 | 0.999 | 71.500 | 19.500 | 22.250 | o_Clostridiales; f_Lachnospiraceae; g_Roseburiafaecis |
| 4301511 | 0.034 | 0.999 | 3.625 | 0.125 | 0.000 | o_Clostridiales; f_; g_ |
| 310301 | 0.037 | 0.999 | 0.000 | 8.625 | 15.000 | o_Clostridiales; f_Ruminococcaceae; g_Faecalibacteriumprausnitzii |
| 183030 | 0.038 | 0.999 | 6.500 | 0.000 | 0.000 | o_Clostridiales; f_; g_ |
| 4416951 | 0.038 | 0.999 | 0.000 | 3.375 | 3.750 | o_Clostridiales; f_Ruminococcaceae; g_Ruminococcus |
| 4326866 | 0.039 | 0.999 | 0.750 | 4.250 | 3.000 | o_Clostridiales; f_Ruminococcaceae; g_ |
| OTU34 | 0.039 | 0.999 | 0.000 | 50.750 | 38.500 | o_Bacteroidales; f_Prevotellaceae; g_Prevotella |
| 4392188 | 0.046 | 0.999 | 17.000 | 3.500 | 2.750 | o_Clostridiales; f_Lachnospiraceae; g_ |
| 1110312 | 0.047 | 0.999 | 11.250 | 2.250 | 3.125 | o_Clostridiales; f_; g_ |
| 22697 | 0.049 | 0.999 | 0.500 | 82.125 | 82.625 | o_Clostridiales; f_Veillonellaceae; g_Succiniclasticum |
| Gene | Forward | Reverse |
|---|---|---|
| RPL4 | GAGAAACCGTCGCCGAAT | GCCCACCAGGAGCAAGTT |
| IFN-α | ACTCCATCCTGGCTGTGAGGAAAT | ATCTCATGACTTCTGCCCTGACGA |
| IFN-β | ATGTCAGAAGCTCCTGGGACAGTT | AGGTCATCCATCTGCCCATCAAGT |
| IFN-γ | TCTGGGAAACTGAATGACTTCG | GACTTCTCTTCCGCTTTCTTAGGTT |
| MX1 | AGTGCGGCTGTTTACCAAG | TTCACAAACCCTGGCAACTC |
| MX2 | CGCATTCTTTCACTCGCATC | CCTCAACCCACCAACTCACA |
| OAS1 | TGGTGGTGGAGACACACACA | CCAACCAGAGACCCATCCA |
| IL-1β | CAACGTGCAGTCTATGGAGT | GAGGTGCTGATGTACCAGTTG |
| IL-4 | AGGAGCCACACGTGCTTGA | TTGCCAAGCTGTTGAGATTCC |
| CXCL-9 | CTTGCTTTTGGGTATCATCTTCCT | TCATCCTTTGGCTGGTGTTG |
| CCL-2 | CATAAGCCACCTGGACAAGAAAA | GGGTATTTAGGGCAAGTTAGAAGGA |
| AQP3 | AAGCTGTCCCAAGTAAAGCACAA | GCCCTACTTCCTGTTTCACCAC |
| SGLT-1 | CCCAAATCAGAGCATTCCATTCA | AAGTATGGTGTGGTGGCCGGTT |
| LPL | AGCCTGAGTTGGACCCATGT | CTCTGTTTTCCCTTCCTCTCTCC |
| INSR | GGGGCTAAAGAGGAACTATGAGG | AGAGGAAAGCGAAGACAGGAAA |
| b0,+AT | CGAGTACCCGTACCTGATGGA | TGCGTAGAAGGGCGAAGAA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Zhang, Y.; Lv, Y.; Li, P.; Yi, D.; Wang, L.; Zhao, D.; Chen, H.; Gong, J.; Hou, Y. Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. Int. J. Mol. Sci. 2018, 19, 2084. https://doi.org/10.3390/ijms19072084
Wu T, Zhang Y, Lv Y, Li P, Yi D, Wang L, Zhao D, Chen H, Gong J, Hou Y. Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. International Journal of Molecular Sciences. 2018; 19(7):2084. https://doi.org/10.3390/ijms19072084
Chicago/Turabian StyleWu, Tao, Yue Zhang, Yang Lv, Peng Li, Dan Yi, Lei Wang, Di Zhao, Hongbo Chen, Joshua Gong, and Yongqing Hou. 2018. "Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine" International Journal of Molecular Sciences 19, no. 7: 2084. https://doi.org/10.3390/ijms19072084
APA StyleWu, T., Zhang, Y., Lv, Y., Li, P., Yi, D., Wang, L., Zhao, D., Chen, H., Gong, J., & Hou, Y. (2018). Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. International Journal of Molecular Sciences, 19(7), 2084. https://doi.org/10.3390/ijms19072084






