Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis
Abstract
:1. Introduction
2. Results
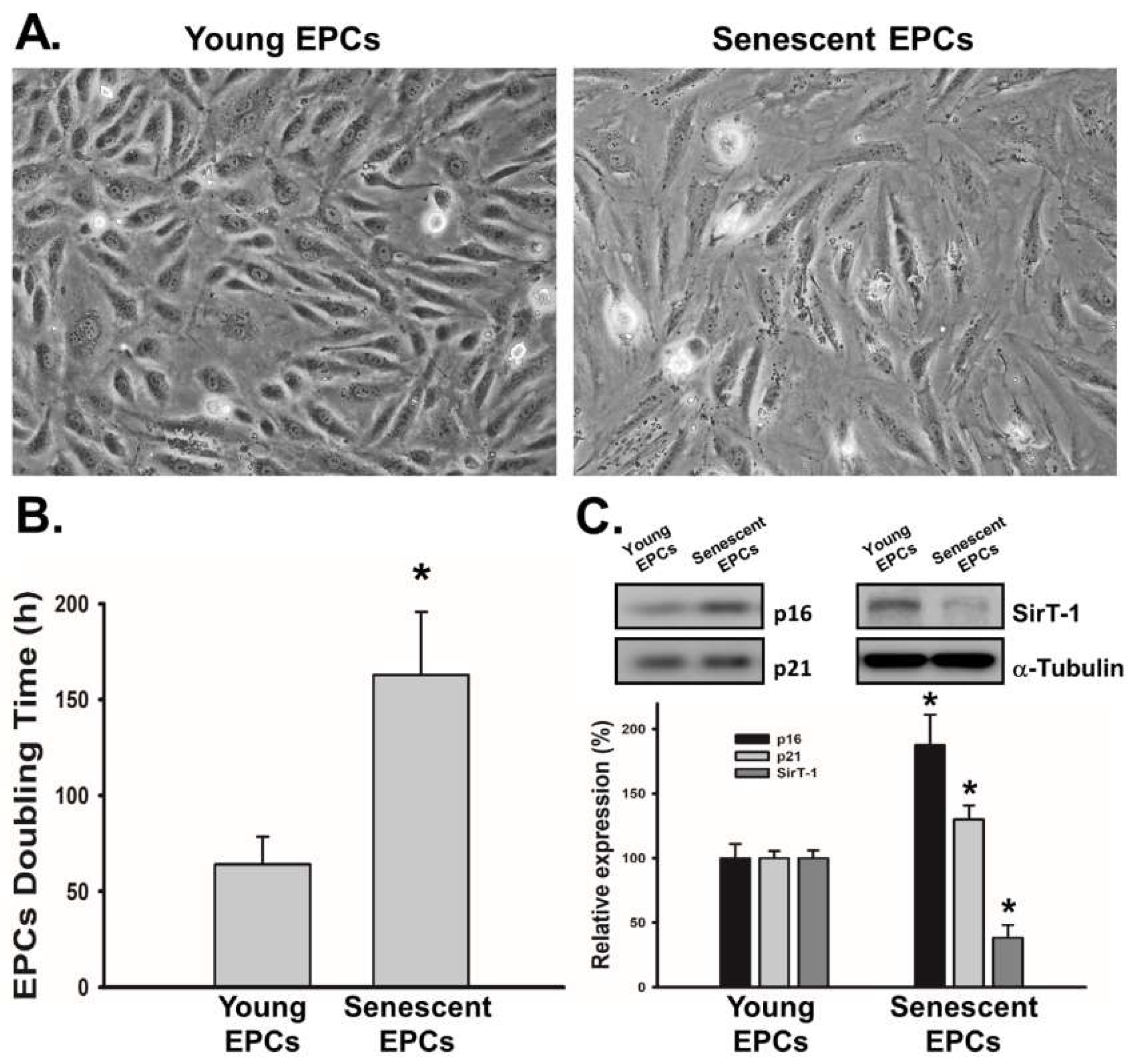
2.1. EPCs with Serial Passaging Express Characters of Senescence
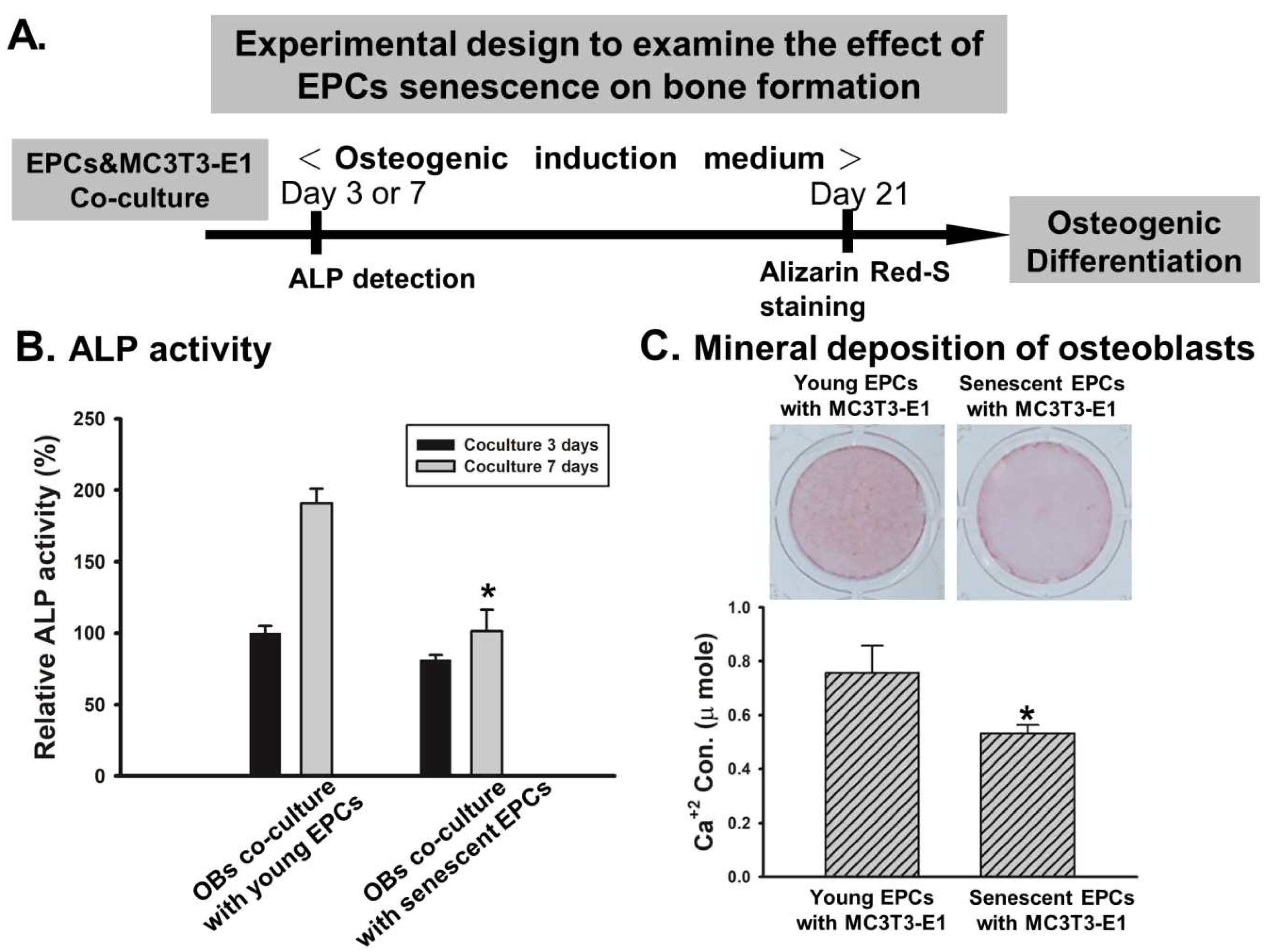
2.2. EPCs’ Senescence Represses Bone Formation of Osteoblasts
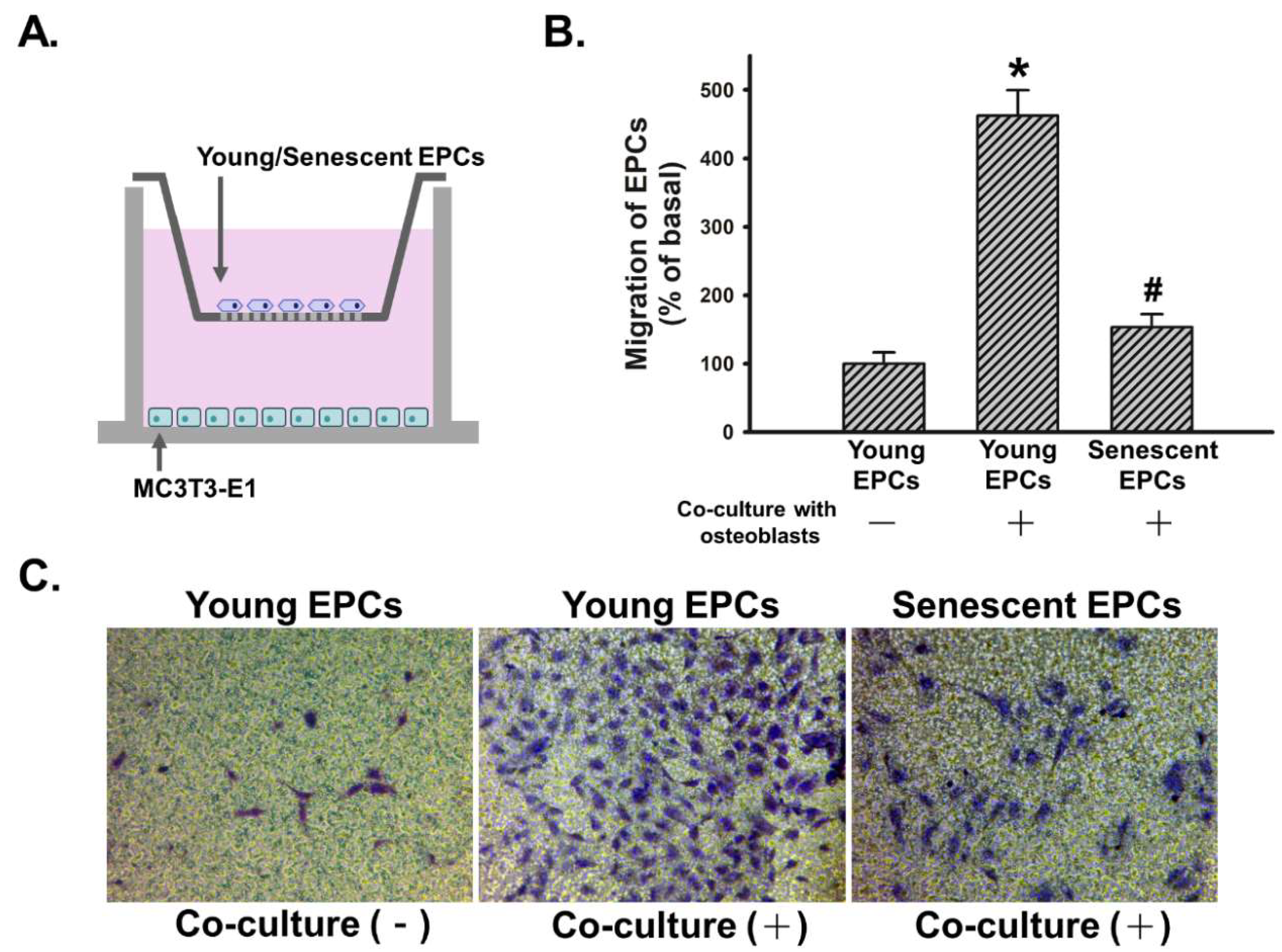
2.3. Senescence Impairs Osteoblast-Attracted EPCs Migration
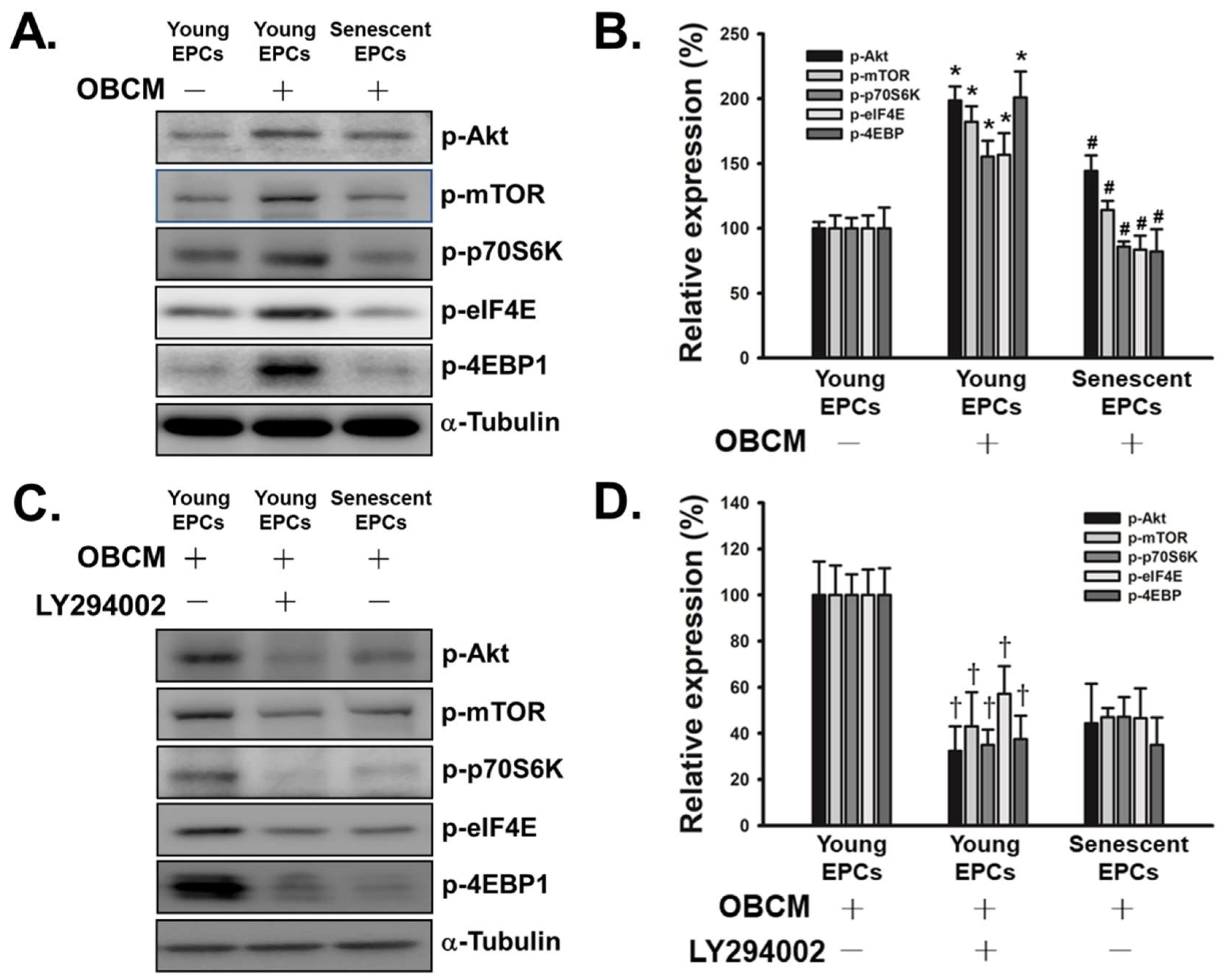
2.4. Senescence Inhibits OBCM-Induced Akt/mTOR Translational Pathway in EPCs
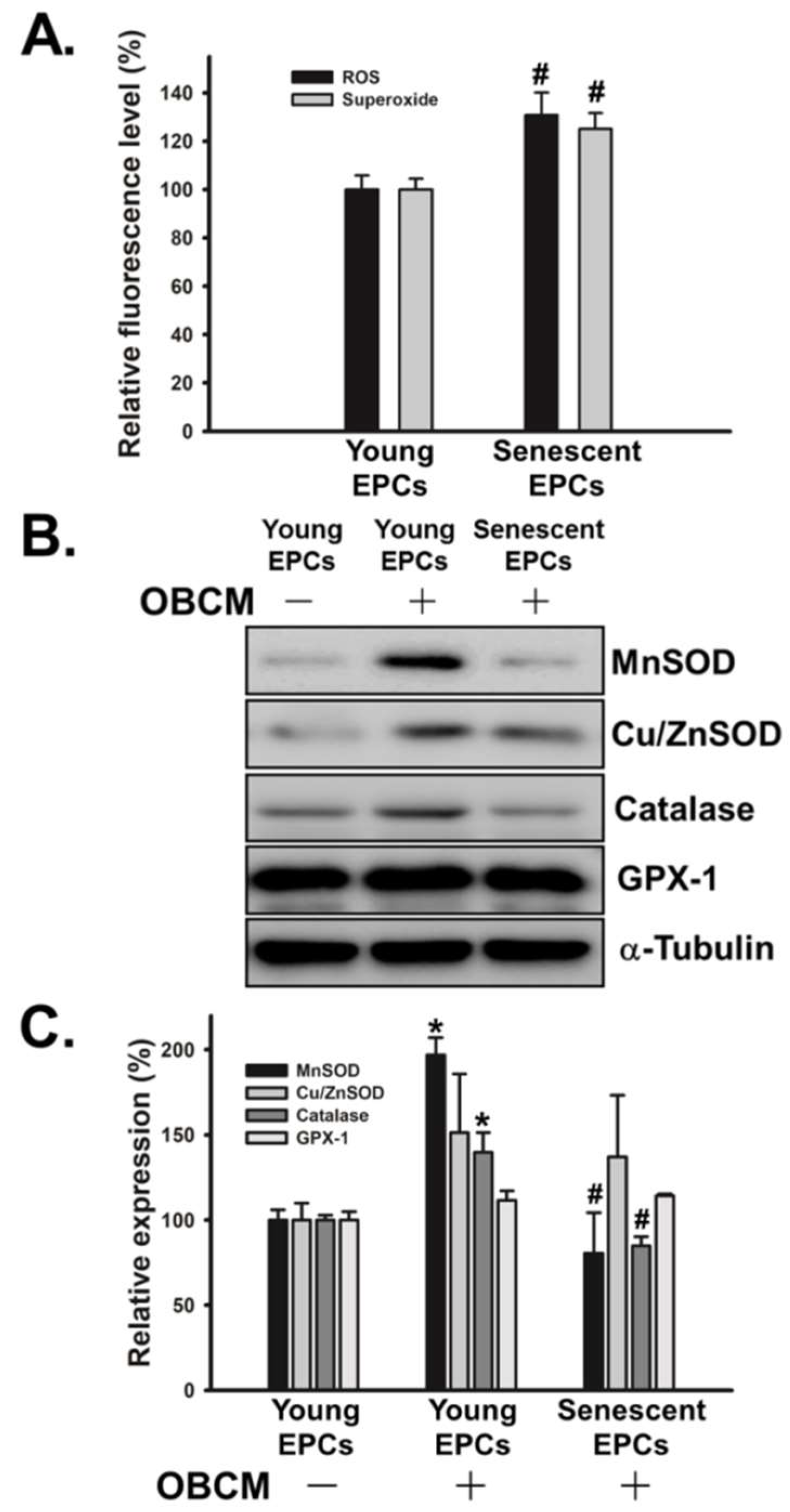
2.5. Senescence Alters OBCM-Mediated Reduction-Oxidation Balance in EPCs
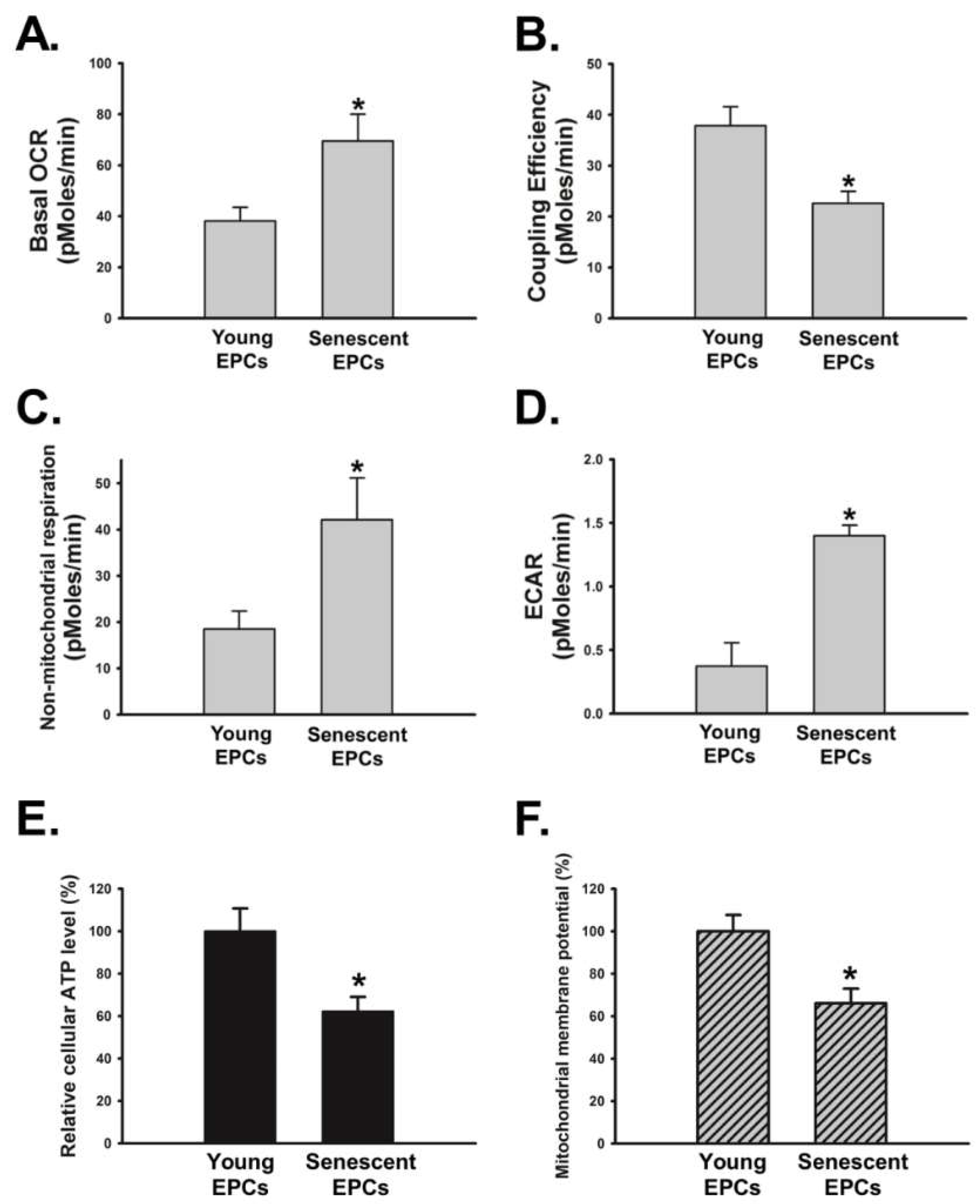
2.6. Senescence Disrupts OBCM-Regulated Bioenergetic Homeostasis in EPCs
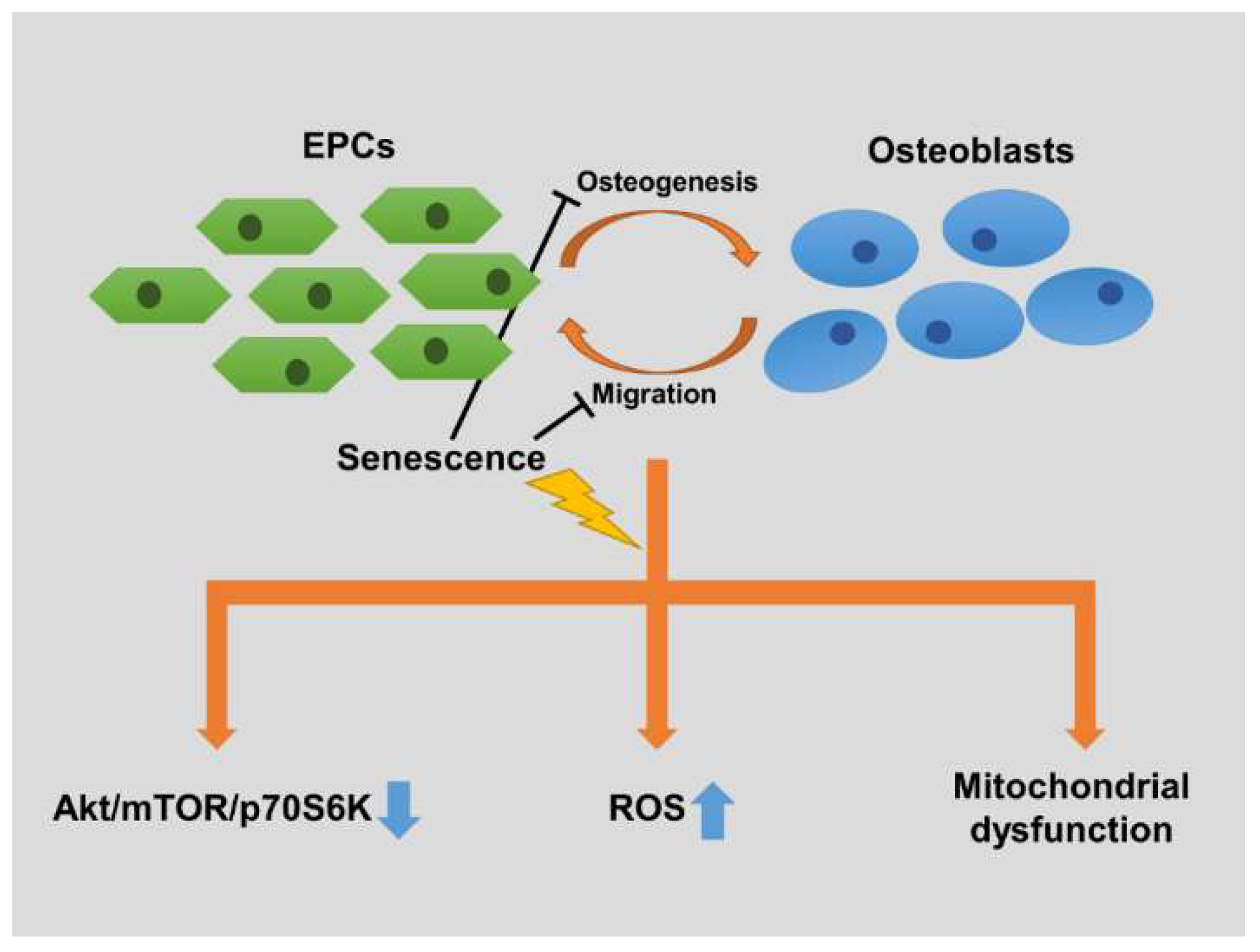
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Obtainment of Senescent EPCs
4.3. Co-Culture Model
4.4. .Measurement of ALP Activity
4.5. Mineral Deposition Analysis
4.6. EPCs Migration Assay
4.7. Preparation of Osteoblast Conditioned Medium (OBCM)
4.8. Determination of Reactive Oxygen Species (ROS) and Superoxide Production
4.9. Western Blot Analysis
4.10. Bioenergetic Function Analysis
4.11. Measurement of Intracellular ATP Content
4.12. Mitochondrial Membrane Potential (MMP) Analysis
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Milovanovic, P.; Rakocevic, Z.; Djonic, D.; Zivkovic, V.; Hahn, M.; Nikolic, S.; Amling, M.; Busse, B.; Djuric, M. Nano-structural, compositional and micro-architectural signs of cortical bone fragility at the superolateral femoral neck in elderly hip fracture patients vs. healthy aged controls. Exp. Gerontol. 2014, 55, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Wronski, T.J.; Iwaniec, U.; Phleger, L.; Kurimoto, P.; Boudignon, B.; Halloran, B.P. Aging Increases Stromal/Osteoblastic Cell-Induced Osteoclastogenesis and Alters the Osteoclast Precursor Pool in the Mouse. J. Bone Miner. Res. 2005, 20, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, G. Bone and fat connection in aging bone. Curr. Opin. Rheumatol. 2008, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Kenan, S.; Gold, A.; Salai, M.; Steinberg, E.; Ankory, R.; Chechik, O. Long-Term Outcomes Following Reduction and Fixation of Displaced Subcapital Hip Fractures in the Young Elderly. Isr. Med. Assoc. J. IMAJ 2015, 17, 341–345. [Google Scholar] [PubMed]
- Mills, L.A.; Simpson, A.H.R. The relative incidence of fracture non-union in the Scottish population (5.17 million): A 5-year epidemiological study. BMJ Open 2013, 3, e002276. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Xia, W.B.; He, S.L.; Xu, L.; Liu, A.M.; Jiang, Y.; Li, M.; Wang, O.; Xing, X.P.; Sun, Y.; Cummings, S.R. Rapidly increasing rates of hip fracture in Beijing, China. J. Bone Miner. Res. 2012, 27, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Simon, U.; Liedert, A.; Claes, L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos. Int. 2005, 16, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Grellier, M.; Bordenave, L.; Amedee, J. Cell-to-cell communication between osteogenic and endothelial lineages: Implications for tissue engineering. Trends Biotechnol. 2009, 27, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Keramaris, N.; Calori, G.; Nikolaou, V.; Schemitsch, E.; Giannoudis, P. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Saran, U.; Piperni, S.G.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.D. Endothelial progenitor cells—An evolving story. Microvasc. Res. 2010, 79, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.J.; Ciarrocchi, A.; Mellick, A.S.; Jaggi, J.S.; Bambino, K.; Gupta, S.; Heikamp, E.; McDevitt, M.R.; Scheinberg, D.A.; Benezra, R. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007, 21, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Ii, M.; Matsumoto, T.; Kuroda, R.; Kuroda, T.; Kwon, S.M.; Kawamoto, A.; Akimaru, H.; Mifune, Y.; Shoji, T. SDF-1/CXCR4 Axis in Tie2-Lineage Cells Including Endothelial Progenitor Cells Contributes to Bone Fracture Healing. J. Bone Miner. Res. 2015, 30, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wang, Y.; Yang, Z.; Tu, C.; Xu, M.; Wang, J. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J. Hum. Hypertens. 2006, 20, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.Y.; Cho, T.-J.; Kim, J.A.; Lee, H.R.; Yoo, W.J.; Chung, C.Y.; Choi, I.H. Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis. Bone 2008, 42, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Sun, X.L.; Wan, C.Y.; Ma, J.X.; Tian, P. Significance of circulating endothelial progenitor cells in patients with fracture healing process. J. Orthop. Res. 2012, 30, 1860–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Kuroda, R.; Mifune, Y.; Kawamoto, A.; Shoji, T.; Miwa, M.; Asahara, T.; Kurosaka, M. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone 2008, 43, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Atesok, K.; Li, R.; Stewart, D.J.; Schemitsch, E.H. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J. Orthop. Res. 2010, 28, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, D.; Hadjiargyrou, M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone 2004, 34, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Tombran-Tink, J.; Barnstable, C. Osteoblasts and osteoclasts express PEDF, VEGF-A isoforms, and VEGF receptors: Possible mediators of angiogenesis and matrix remodeling in the bone. Biochem. Biophys. Res. Commun. 2004, 316, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, M.; Spreafico, A.; Bardelli, M.; Rocchigiani, M.; Salameh, A.; Nucciotti, S.; Capperucci, C.; Frediani, B.; Oliviero, S. Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J. Biol. Chem. 2006, 281, 17961–17967. [Google Scholar] [CrossRef] [PubMed]
- Melero-Martin, J.M.; Khan, Z.A.; Picard, A.; Wu, X.; Paruchuri, S.; Bischoff, J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 2007, 109, 4761–4768. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Keymel, S.; Niesler, U.; Ziemann, J.; Kelm, M.; Kalka, C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 2005, 45, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Hoeber, S.; Froese, S.; Klink, I.; Stichtenoth, D.O.; Galuppo, P.; Jakob, M.; Tsikas, D.; Anker, S.D.; Poole-Wilson, P.A. Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1. Circ. Res. 2007, 100, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, M.; Masuda, H.; Kwon, S.; Shirakura, K.; Shizuno, T.; Ito, R.; Kobori, M.; Asahara, T. Lesion-targeted thrombopoietin potentiates vasculogenesis by enhancing motility and enlivenment of transplanted endothelial progenitor cells via activation of Akt/mTOR/p70S6kinase signaling pathway. J. Mol. Cell. Cardiol. 2008, 45, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.F.; Simoncini, S.; Ligi, I.; Chateau, A.-L.; Bachelier, R.; Robert, S.; Morere, J.; Fernandez, S.; Guillet, B.; Marcelli, M. Accelerated senescence of cord blood endothelial progenitor cells in premature neonates is driven by SIRT1 decreased expression. Blood 2014, 123, 2116–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschalaki, K.E.; Starke, R.D.; Hu, Y.; Mercado, N.; Margariti, A.; Gorgoulis, V.G.; Randi, A.M.; Barnes, P.J. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells 2013, 31, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Fu, G.; Dai, T.; Huang, H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1α/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2007, 50, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Punn, A.; Chen, J.; Randeva, H.S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008, 78, 356–365. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Boveris, A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007, 292, C670–C686. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.-F.; Rabinovitch, P.S.; Ungvari, Z. Mitochondria and cardiovascular aging. Circ. Res. 2012, 110, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assmus, B.; Urbich, C.; Aicher, A.; Hofmann, W.K.; Haendeler, J.; Rössig, L.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ. Res. 2003, 92, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Lähteenvuo, J.; Rosenzweig, A. Effects of aging on angiogenesis. Circ. Res. 2012, 110, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; di Fagagna, F.D.A. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. School 2010, 77, 4–12. [Google Scholar] [CrossRef]
- Joensuu, K.; Uusitalo, L.; Alm, J.; Aro, H.; Hentunen, T.; Heino, T. Enhanced osteoblastic differentiation and bone formation in co-culture of human bone marrow mesenchymal stromal cells and peripheral blood mononuclear cells with exogenous VEGF. Orthop. Traumatol. Surg. Res. 2015, 101, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zampetaki, A.; Kirton, J.P.; Xu, Q. Vascular repair by endothelial progenitor cells. Cardiovasc. Res. 2008, 78, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Wang, J.; Schneider, A.; Sun, Y.-X.; Koh-Paige, A.; Osman, N.; McCauley, L.; Taichman, R. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 2006, 38, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Miriuka, S.; Rao, V.; Peterson, M.; Tumiati, L.; Delgado, D.; Mohan, R.; Ramzy, D.; Stewart, D.; Ross, H.; Waddell, T. mTOR inhibition induces endothelial progenitor cell death. Am. J. Transplant. 2006, 6, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, H.; Guo, M.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species in vascular formation and development. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Labinskyy, N.; Gupte, S.; Chander, P.N.; Edwards, J.G.; Csiszar, A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2121–H2128. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Jimenez, R.; Pinto, J.T.; Ballabh, P.; Losonczy, G.; Pearson, K.J.; de Cabo, R.; Ungvari, Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech. Ageing Dev. 2009, 130, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieke, S.M.; Phillips, D.; McCoy, J.P.; Aponte, A.M.; Shen, R.-F.; Balaban, R.S.; Finkel, T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 2006, 281, 27643–27652. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Schreiber, S.L. Direct control of mitochondrial function by mTOR. Proc. Natl. Acad. Sci. USA 2009, 106, 22229–22232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-C.; Lee, Y.-C.; Yu, G.; Cheng, C.-J.; Zhou, X.; Chu, K.; Murshed, M.; Le, N.-T.; Baseler, L.; Abe, J.-I. Endothelial-to-osteoblast conversion generates osteoblastic metastasis of prostate cancer. Dev. Cell 2017, 41, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Kalluri, R. Endothelial–Mesenchymal Transition and Its Contribution to the Emergence of Stem Cell Phenotype; Seminars in Cancer Biology; Elsevier: New York, NY, USA, 2012; pp. 379–384. [Google Scholar]
- Lee, D.H.; Park, B.J.; Lee, M.-S.; Lee, J.W.; Kim, J.K.; Yang, H.-C.; Park, J.-C. Chemotactic migration of human mesenchymal stem cells and MC3T3-E1 osteoblast-like cells induced by COS-7 cell line expressing rhBMP-7. Tissue Eng. 2006, 12, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.C.; Hamdy, R.C.; Chua, B.H. Mechanism of transforming growth factor-β1-induced expression of vascular endothelial growth factor in murine osteoblastic MC3T3-E1 cells. BBA Mol. Cell Res. 2000, 1497, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-H.; Chang, C.-H.; Chen, S.-S.; Wang, H.-H.; Yen, J.-Y.; Hsiao, C.-J.; Wu, N.-L.; Chen, Y.-L.; Huang, T.-F.; Wang, P.-C. Butein inhibits angiogenesis of human endothelial progenitor cells via the translation dependent signaling pathway. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chung, C.-H.; Lu, Y.-C.; Wu, M.-H.; Chou, P.-H.; Yen, J.-Y.; Lai, Y.-W.; Wang, G.-S.; Liu, S.-C.; Cheng, J.-K. BMP-2 induces angiogenesis by provoking integrin α6 expression in human endothelial progenitor cells. Biochem. Pharmacol. 2018, 150, 256–266. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.-S.; Shen, Y.-S.; Chou, W.-Y.; Tang, C.-H.; Yeh, H.-I.; Wang, L.-Y.; Yen, J.-Y.; Huang, T.-Y.; Liu, S.-C.; Yang, C.-Y.; et al. Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis. Int. J. Mol. Sci. 2018, 19, 1997. https://doi.org/10.3390/ijms19071997
Wang G-S, Shen Y-S, Chou W-Y, Tang C-H, Yeh H-I, Wang L-Y, Yen J-Y, Huang T-Y, Liu S-C, Yang C-Y, et al. Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis. International Journal of Molecular Sciences. 2018; 19(7):1997. https://doi.org/10.3390/ijms19071997
Chicago/Turabian StyleWang, Guo-Shou, Yung-Shuen Shen, Wen-Yi Chou, Chih-Hsin Tang, Hung-I Yeh, Li-Yu Wang, Juei-Yu Yen, Te-Yang Huang, Shih-Chia Liu, Chen-Yu Yang, and et al. 2018. "Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis" International Journal of Molecular Sciences 19, no. 7: 1997. https://doi.org/10.3390/ijms19071997
APA StyleWang, G.-S., Shen, Y.-S., Chou, W.-Y., Tang, C.-H., Yeh, H.-I., Wang, L.-Y., Yen, J.-Y., Huang, T.-Y., Liu, S.-C., Yang, C.-Y., Lin, T.-Y., Chen, C., & Wang, S.-W. (2018). Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis. International Journal of Molecular Sciences, 19(7), 1997. https://doi.org/10.3390/ijms19071997




