The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins
Abstract
1. Introduction
2. Results
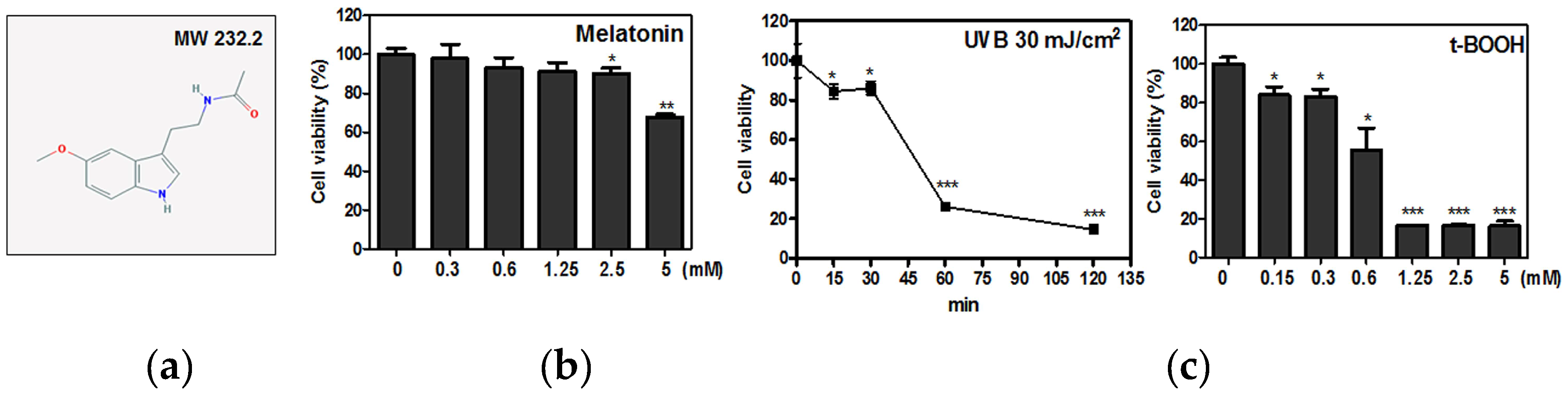
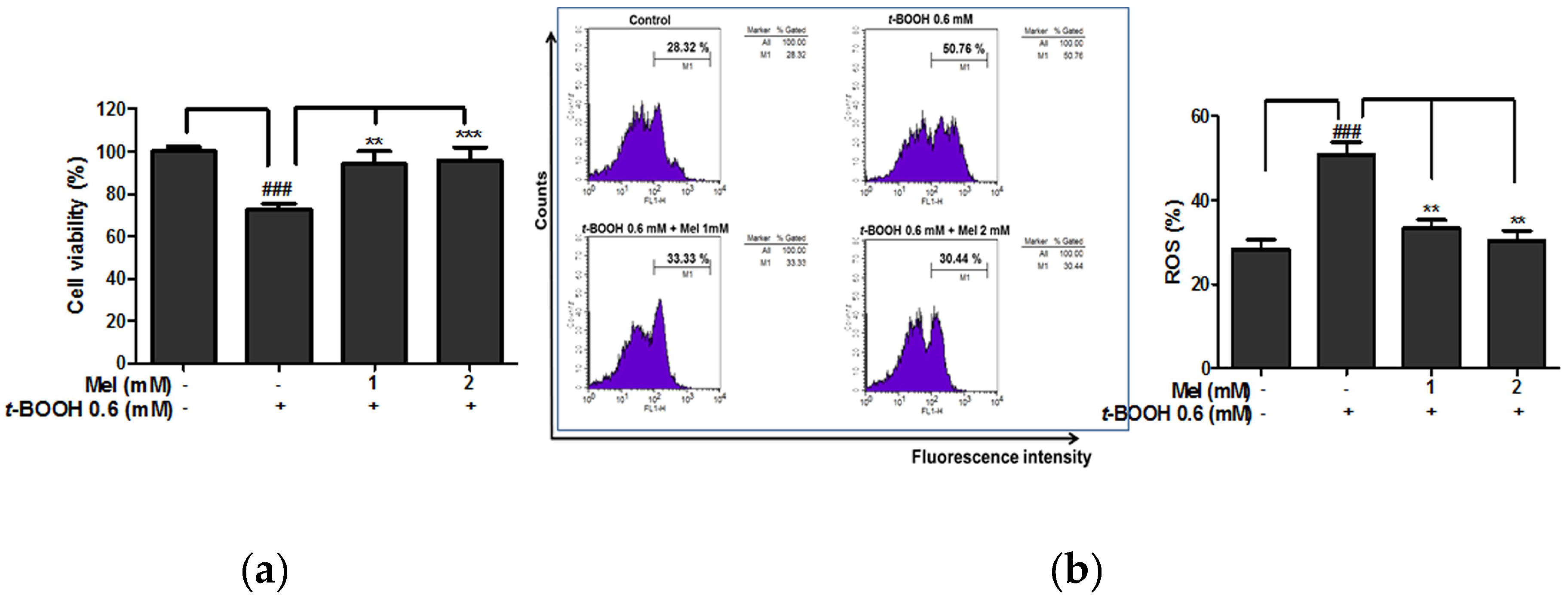
2.1. Melatonin Protected Against t-BOOH Induced ROS Production and Cytotoxicity in HaCaT Keratinocytes
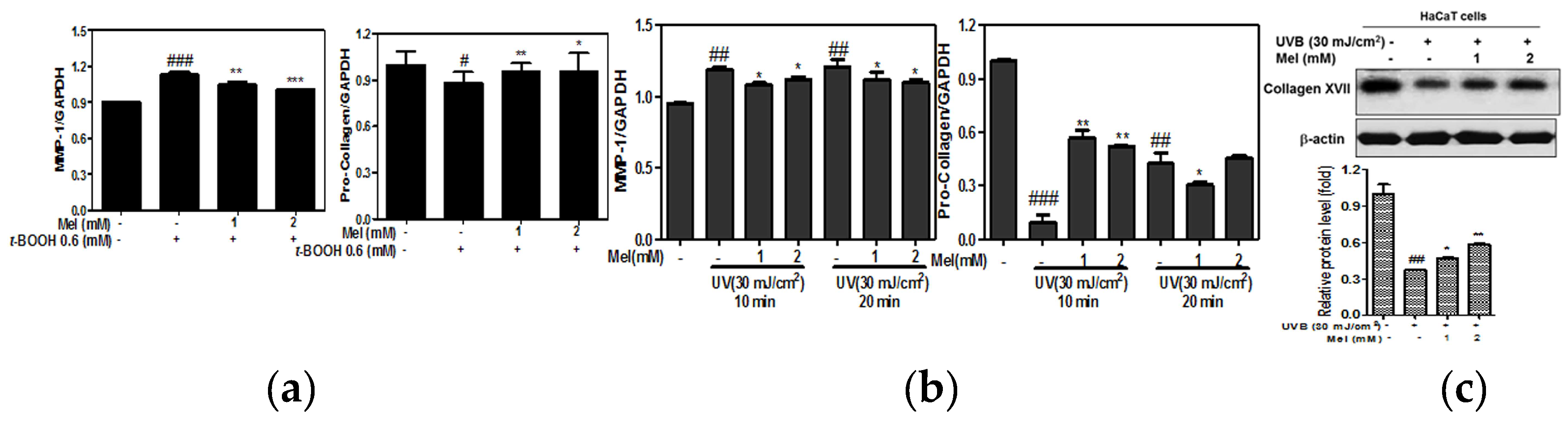
2.2. Melatonin Attenuated the mRNA Expression of MMP-1, Pro-Collagen Degradation in t-BOOH or UVB Induced HaCaT Keratinocytes
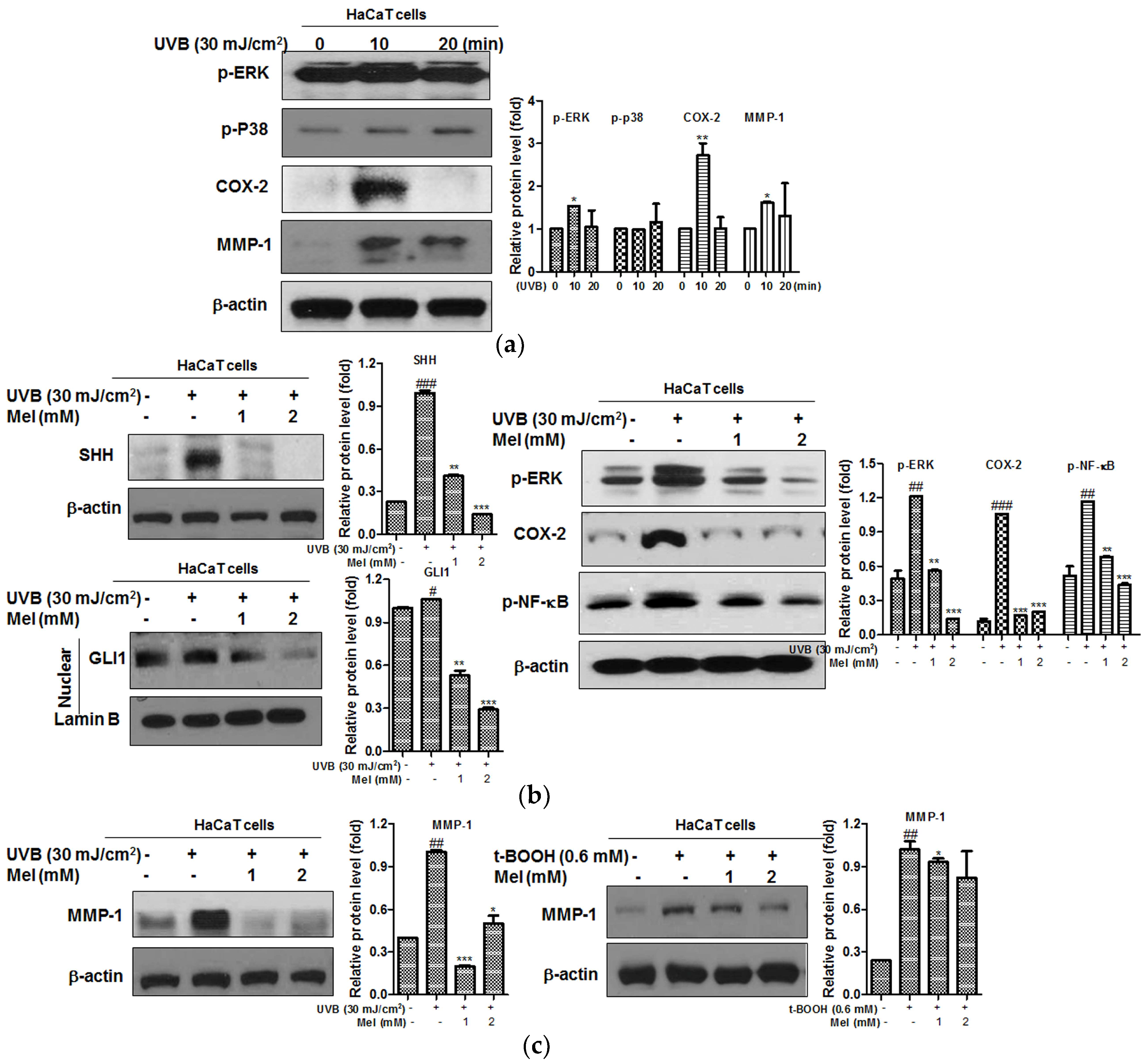
2.3. Melatonin Effectively Suppressed the UVB-Induced Hedgehog Signaling and MMP-1 Related Proteins in HaCaT Keratinocytes
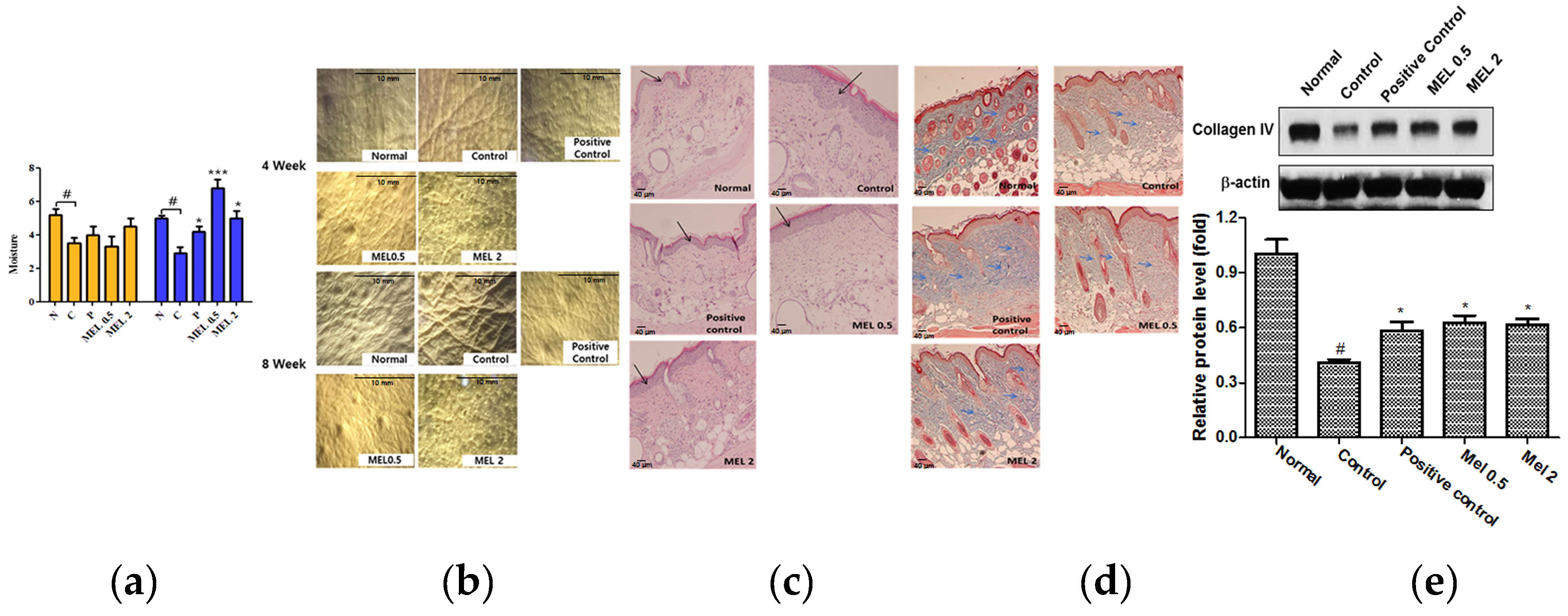
2.4. Melatonin Prevented Against Loss of Water on the Dorsal skin of SKH1 Hairless Mice Exposed to UVB
2.5. Melatonin Suppressed the Degree of Wrinkles and Epidermal Thickness Induced by UVB Irradiation on the Dorsal skin of SKH1 Hairless Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. UVB Irradiation
4.4. Cell Viability Assay
4.5. RT-qPCR Assay
4.6. Western Blotting
4.7. Nuclear Fraction for GLI1
4.8. Measurement of Reactive Oxygen Species (ROS) Generation
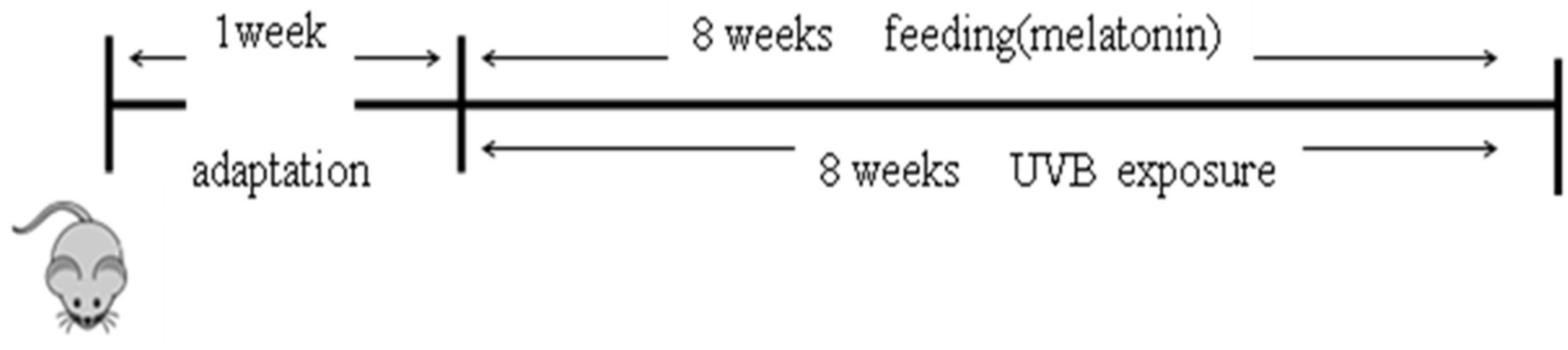
4.9. Animals and Treatments
- (1)
- 0 week: 60 mJ/cm2 for 200 s, three times a week (1 minimal erythema dose; M.E.D)
- (2)
- 1 week: 120 mJ/cm2 for 400 s, three times a week (2 M.E.D)
- (3)
- 2~3 weeks: 180 mJ/cm2 for 600 s 1 week: 120 mJ/cm2 for 400 s, three times a week (3 M.E.D)
- (4)
- 4~5 weeks: 240 mJ/cm2 for 800 s, three times a week (4 M.E.D)
4.10. Observation of Skin Wrinkle Formation
4.11. Measurement of Skin Moisture
4.12. Histological Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UVB | Ultraviolet B |
| t-BOOH | tert-Butyl hydroperoxide |
| ROS | Reactive Oxygen Species |
| MMP-1 | Matrix metalloprotease 1 |
| mRNA | messenger RNA |
| PCR | Polymerase Chain Reaction |
| SHH | Sonic hedgehog |
| p-ERK | Phospho-Extracellular Signal-regulated Kinase-1 |
| p-P38 | Phospho -P38 mitogen-activated protein kinases |
| COX-2 | Cyclooxygenase-2 |
| i.p | intraperitoneal |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
References
- Hwang, K.A.; Yi, B.R.; Choi, K.C. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J. Investig. Dermatol. Symp. Proc. 2009, 14, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed]
- Bossi, O.; Gartsbein, M.; Leitges, M.; Kuroki, T.; Grossman, S.; Tennenbaum, T. UV irradiation increases ROS production via PKCdelta signaling in primary murine fibroblasts. J. Cell. Biochem. 2008, 105, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Avila Rodriguez, M.I.; Rodriguez Barroso, L.G.; Sanchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Contet-Audonneau, J.L.; Jeanmaire, C.; Pauly, G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles and sun-protected areas. Br. J. Dermatol. 1999, 140, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Tang, X.; Lee, J.L.; Kopelovich, L.; Kim, A.L. Hedgehog signalling in skin development and cancer. Exp. Dermatol. 2006, 15, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chi, S.; Xie, J. Hedgehog signaling in skin cancers. Cell. Signal. 2011, 23, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Song, J.H.; Lee, M.Y.; Chae, S. Magnolol reduces UVB-induced photodamage by regulating matrix metalloproteinase activity. Environ. Toxicol. Pharmacol. 2015, 39, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Jung, Y.; Youm, J.K.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Abietic acid inhibits UVB-induced MMP-1 expression in human dermal fibroblast cells through PPARalpha/gamma dual activation. Exp. Dermatol. 2015, 24, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Hwang, E.; Lee, H.J.; Lee, T.Y.; Song, H.G.; Park, S.Y.; Shin, H.S.; Lee, D.G.; Yi, T.H. Effects of Galla chinensis extracts on UVB-irradiated MMP-1 production in hairless mice. J. Nat. Med. 2015, 69, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular Mechanisms of Green Tea Polyphenols with Protective Effects against Skin Photoaging. Crit. Rev. Food Sci. Nutr. 2015, 57, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Cesquini, M.; Torsoni, M.A.; Stoppa, G.R.; Ogo, S.H. t-BOOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed. Pharmacother. 2003, 57, 124–129. [Google Scholar] [CrossRef]
- Wang, X.; Bi, Z.; Chu, W.; Wan, Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int. J. Mol. Med. 2005, 16, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Murakami, Y.; Tanaka, H.; Nakata, S. Increase of stratifin triggered by ultraviolet irradiation is possibly related to premature aging of human skin. Exp. Dermatol. 2014, 23, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Gera, J.; Ghosh, S.; Mandal, L.; Mandal, S. Noncanonical Decapentaplegic Signaling Activates Matrix Metalloproteinase 1 To Restrict Hedgehog Activity and Limit Ectopic Eye Differentiation in Drosophila. Genetics 2017, 207, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.T.; Lee, D.; Koo, K.; Lee, J.; Yoon, H.S.; Choi, Y.M.; Kwon, T.R.; Kim, B.J. Superoxide dismutase 1 inhibits alpha-melanocyte stimulating hormone and ultraviolet B-induced melanogenesis in murine skin. Ann. Dermatol. 2014, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Duffy, J.F. Circadian Rhythm Sleep-Wake Disorders in Older Adults. Sleep Med. Clin. 2018, 13, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Leeboonngam, T.; Pramong, R.; Sae-Ung, K.; Govitrapong, P.; Phansuwan-Pujito, P. Neuroprotective effects of melatonin on amphetamine-induced dopaminergic fiber degeneration in the hippocampus of postnatal rats. J. Pineal Res. 2018, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Q.; Sun, Y.; Jin, Y.; Zhang, J.; Wu, J. Melatonin induces anti-inflammatory effects via endoplasmic reticulum stress in RAW264.7 macrophages. Mol. Med. Rep. 2018, 17, 6122–6129. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2018, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Scholz, G.; Knoll, B.; Hipler, U.C.; Elsner, P. Melatonin reduces UV-induced reactive oxygen species in a dose-dependent manner in IL-3-stimulated leukocytes. J. Pineal Res. 2001, 31, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bardak, Y.; Ozerturk, Y.; Ozguner, F.; Durmus, M.; Delibas, N. Effect of melatonin against oxidative stress in ultraviolet-B exposed rat lens. Curr. Eye Res. 2000, 20, 225–230. [Google Scholar] [CrossRef]
- Fischer, T.W.; Zbytek, B.; Sayre, R.M.; Apostolov, E.O.; Basnakian, A.G.; Sweatman, T.W.; Wortsman, J.; Elsner, P.; Slominski, A. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J. Pineal Res. 2006, 40, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Baek, B.; Lee, S.H.; Kim, K.; Lim, H.W.; Lim, C.J. Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J. Physiol. Pharmacol. 2016, 20, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxidative Med. Cell Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Dlugosz, A.A.; Cheng, C.; Denning, M.F.; Dempsey, P.J.; Coffey, R.J.J.; Yuspa, S.H. Keratinocyte growth factor receptor ligands induce transforming growth factor alpha expression and activate the epidermal growth factor receptor signaling pathway in cultured epidermal keratinocytes. Cell Growth Differ. 1994, 5, 1283–1292. [Google Scholar] [PubMed]
- Lewis, D.A.; Spandau, D.F. UVB-induced activation of NF-kappaB is regulated by the IGF-1R and dependent on p38 MAPK. J. Invest. Dermatol. 2008, 128, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Syed, D.N.; Pal, H.C.; Mukhtar, H.; Afaq, F. Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-kappaB and MAPK signaling pathways in mouse skin. Photochem. Photobiol. 2012, 88, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Noh, E.M.; Jeong, E.Y.; Yun, S.K.; Jeong, Y.J.; Kim, J.H.; Kwon, K.B.; Kim, B.S.; Lee, S.H.; Park, C.S.; et al. Cordycepin inhibits UVB-induced matrix metalloproteinase expression by suppressing the NF-kappaB pathway in human dermal fibroblasts. Exp. Mol. Med. 2009, 41, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Mohana, S.; Srithar, G. 7-Hydroxycoumarin prevents UVB-induced activation of NF-kappaB and subsequent overexpression of matrix metalloproteinases and inflammatory markers in human dermal fibroblast cells. J. Photochem. Photobiol. B 2016, 161, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.Y.; Shin, J.C.; Park, S.M.; Kim, N.R.; Kwak, W.; Choi, B.H. Pinus densiflora extract protects human skin fibroblasts against UVB-induced photoaging by inhibiting the expression of MMPs and increasing type I procollagen expression. Toxicol. Rep. 2014, 1, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lee, E.H.; Lee, T.H.; Cho, M.H. The Methoxyflavonoid Isosakuranetin Suppresses UV-B-Induced Matrix Metalloproteinase-1 Expression and Collagen Degradation Relevant for Skin Photoaging. Int. J. Mol. Sci. 2016, 17, 1449. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tanaka, N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis and Cancer. J. Dev. Biol. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Song, J.H.; Youn, U.J.; Hyun, J.W.; Jeong, W.S.; Lee, M.Y.; Choi, H.J.; Lee, H.K.; Chae, S. Inhibition of UVB-induced wrinkle formation and MMP-9 expression by mangiferin isolated from Anemarrhena asphodeloides. Eur. J. Pharmacol. 2012, 689, 38–44. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.K.; Lee, H.-J.; Lee, H.; Kim, J.-H.; Hwang, J.; Koo, J.I.; Kim, S.-H. The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins. Int. J. Mol. Sci. 2018, 19, 1995. https://doi.org/10.3390/ijms19071995
Park EK, Lee H-J, Lee H, Kim J-H, Hwang J, Koo JI, Kim S-H. The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins. International Journal of Molecular Sciences. 2018; 19(7):1995. https://doi.org/10.3390/ijms19071995
Chicago/Turabian StylePark, Eun Kyung, Hyo-Jung Lee, Hyemin Lee, Ju-Ha Kim, Jisung Hwang, Ja Il Koo, and Sung-Hoon Kim. 2018. "The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins" International Journal of Molecular Sciences 19, no. 7: 1995. https://doi.org/10.3390/ijms19071995
APA StylePark, E. K., Lee, H.-J., Lee, H., Kim, J.-H., Hwang, J., Koo, J. I., & Kim, S.-H. (2018). The Anti-Wrinkle Mechanism of Melatonin in UVB Treated HaCaT Keratinocytes and Hairless Mice via Inhibition of ROS and Sonic Hedgehog Mediated Inflammatory Proteins. International Journal of Molecular Sciences, 19(7), 1995. https://doi.org/10.3390/ijms19071995





