Cecropin A Modulates Tight Junction-Related Protein Expression and Enhances the Barrier Function of Porcine Intestinal Epithelial Cells by Suppressing the MEK/ERK Pathway
Abstract
1. Introduction
2. Results
2.1. The Antibacterial Activity of Different AMPs
2.2. Cytotoxicity to IPEC-J2 Cells
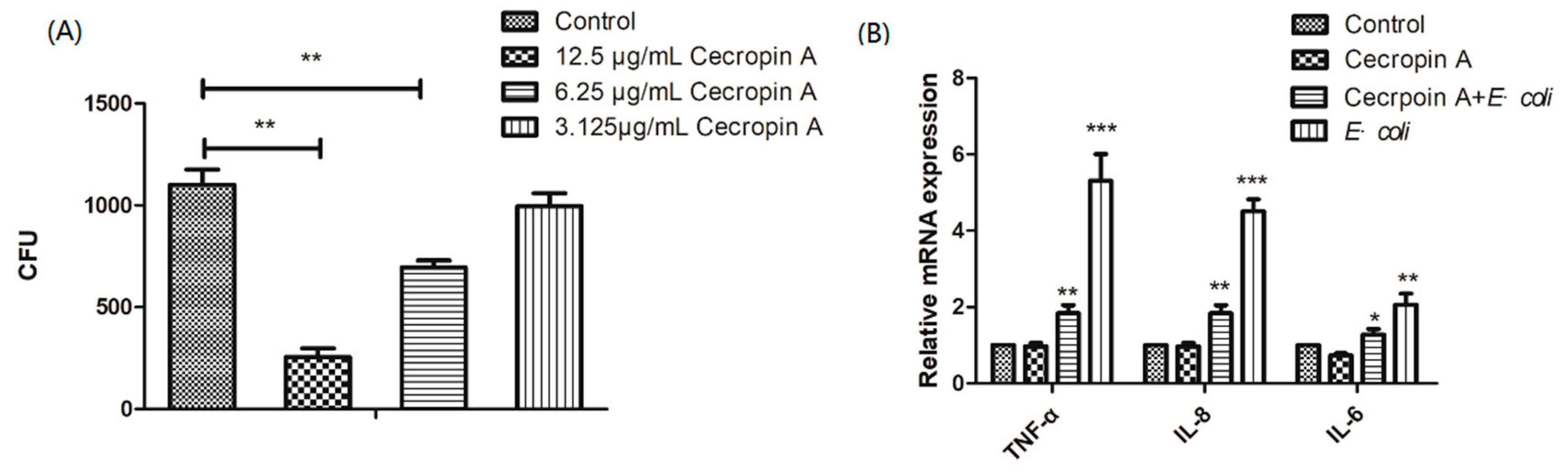
2.3. Cecropin A Inhibits E. coli Adherence and Ameliorates Inflammation
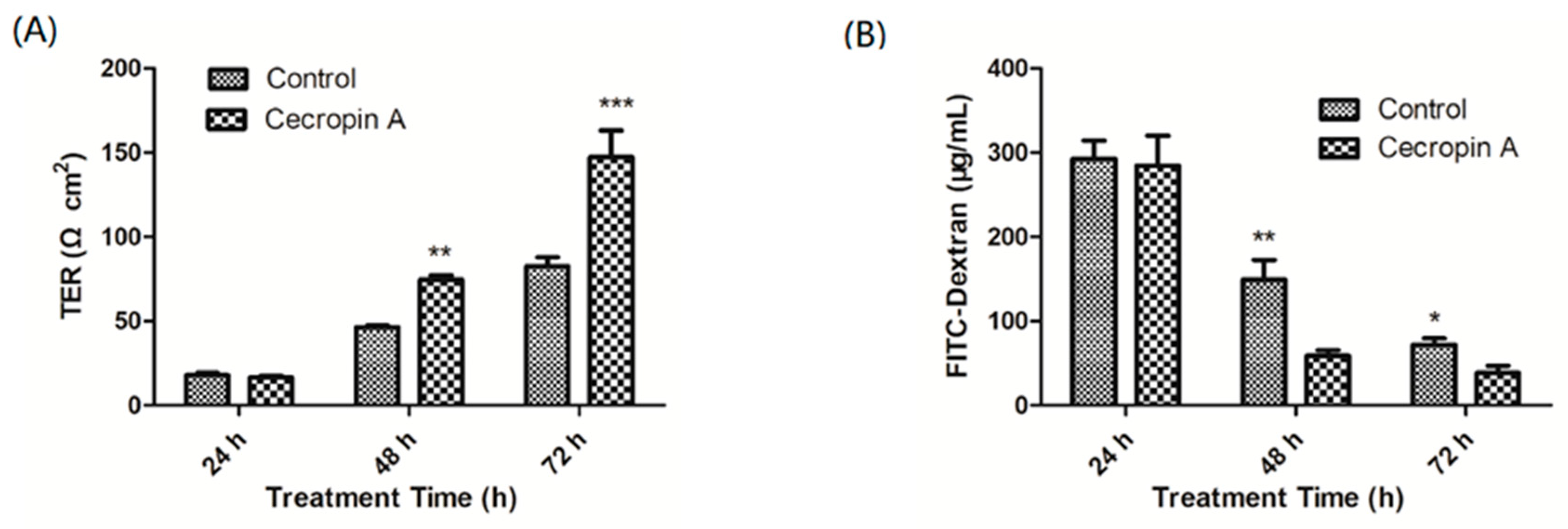
2.4. Cecropin A Increases the TER and Decreases the Paracellular Diffusion of FITC-Dextran through the IPEC-J2 Monolayer
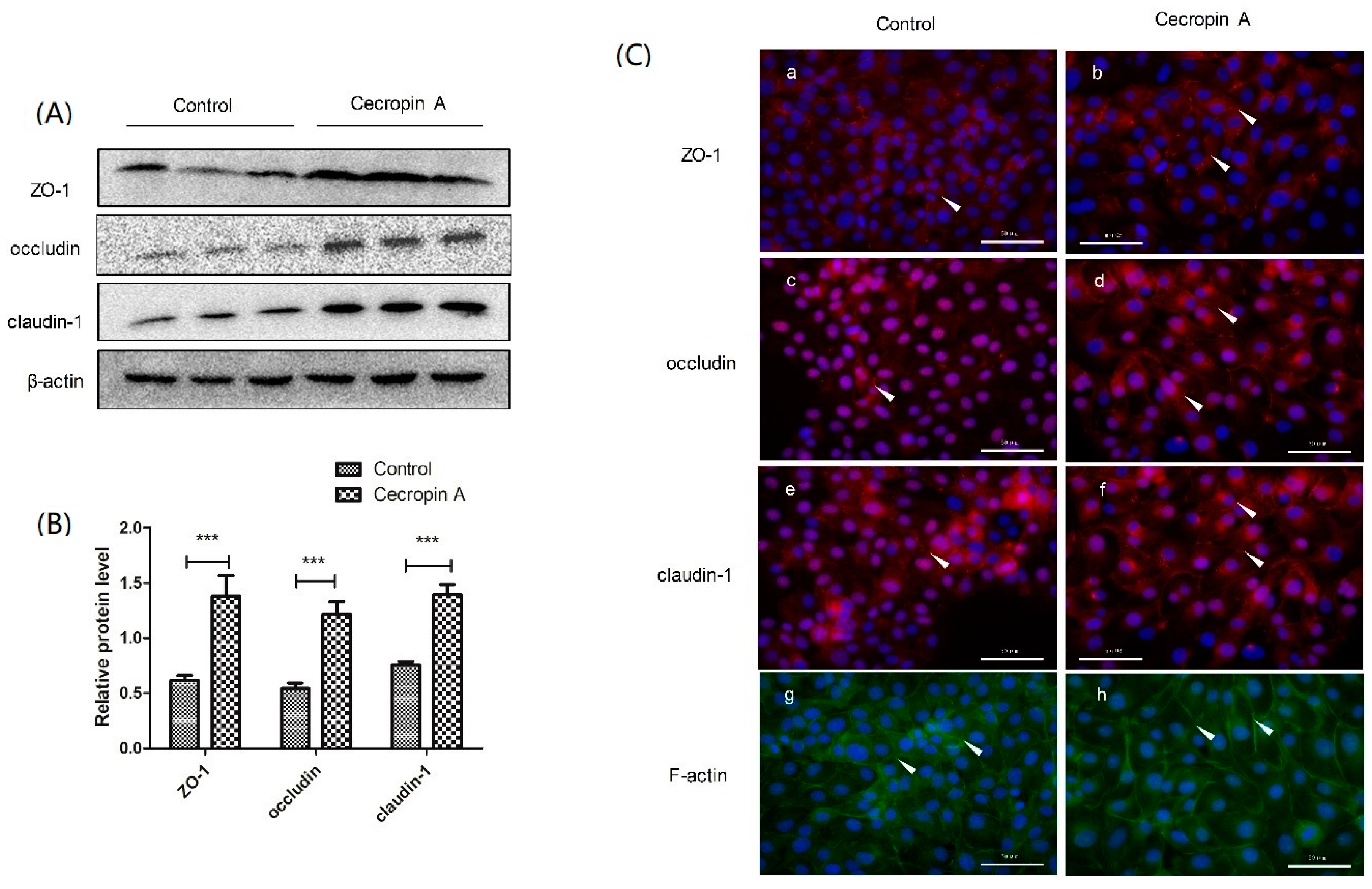
2.5. Cecropin A Regulates TJ Protein Expression Levels, Membrane Distribution and F-Actin Polymerization
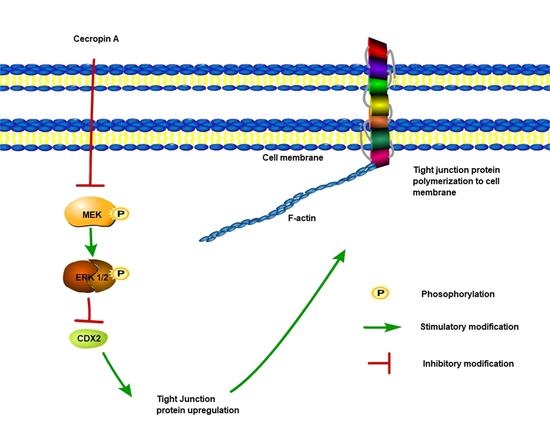
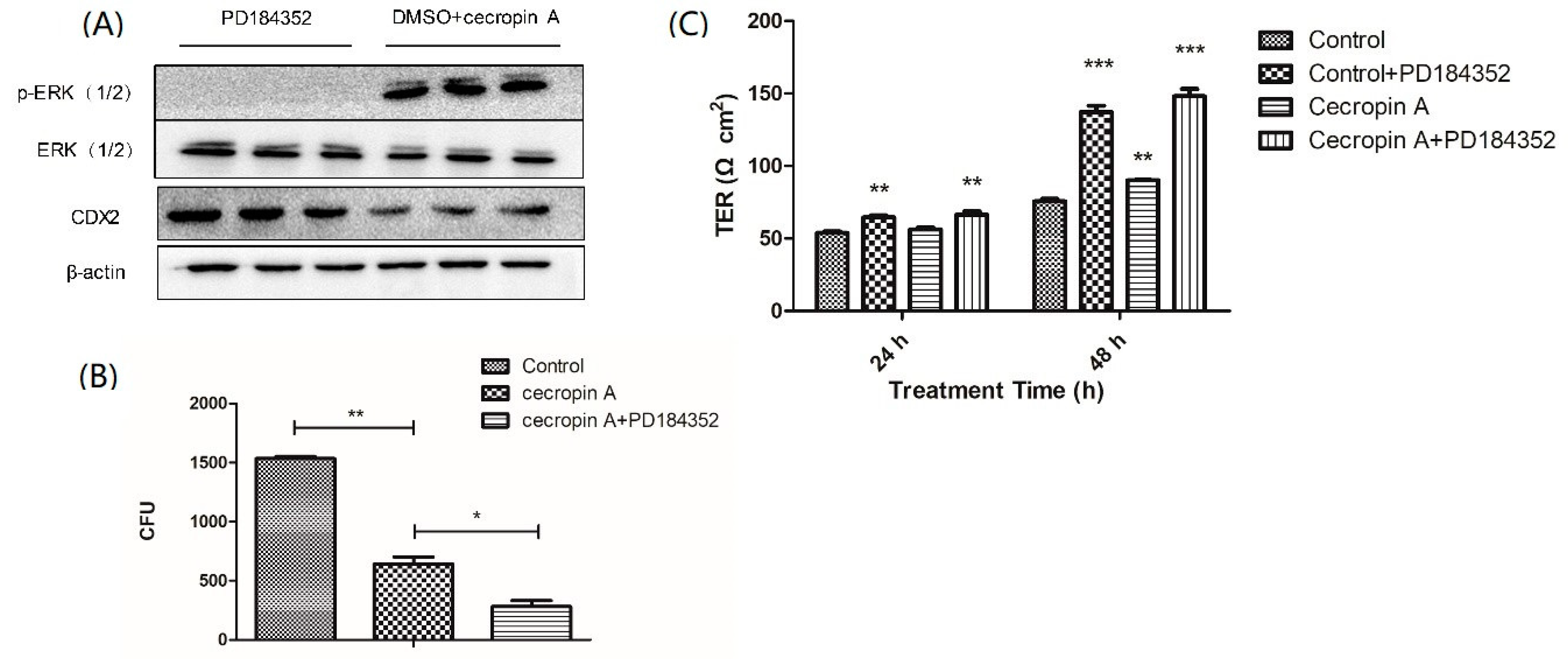
2.6. Cecropin A Regulates the Intestinal Barrier by Downregulating the MEK/ERK Pathways
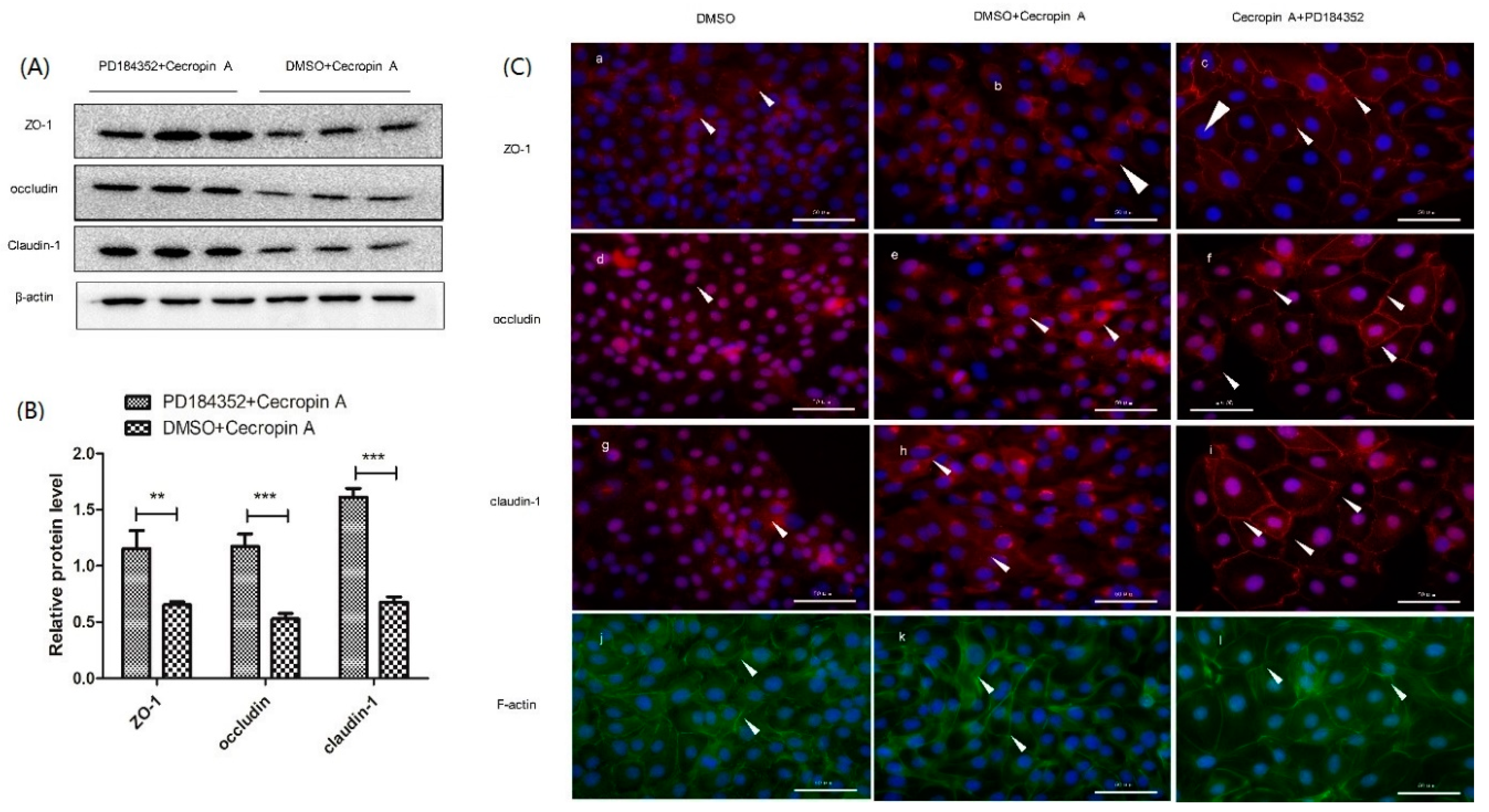
2.7. Inhibition of the MEK/ERK Pathway Increases TER, TJ Expression, Membrane Distribution and F-Actin Polymerization
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Peptide Synthesis
4.3. Cell Culture
4.4. MIC/MBC Test
4.5. Cell Vitality Assay
4.6. Quantifying Adhe7rent Bacteria
4.7. TER and Permeability Measurement
4.8. qPCR
4.9. Western Blotting
4.10. Cell Immunofluorescence Aassay
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IBD | Inflammatory bowel disease |
| AMP | Antimicrobial peptides |
| TJ | Tight junction |
| E. coli | Escherichia coli |
| IPEC-J2 | Porcine jejunum epithelial cells |
| TER | Trans-epithelial electrical resistance |
| MEK | Mitogen-activated protein kinase kinase |
| ERK | Extracellular signal-regulated kinase |
| AA | Amino acid |
| JAMs | Junctional adhesion molecules |
| ZO | Zonula occludens |
| MAPK | Mitogen-activated protein kinase |
References
- Sheehan, D.; Shanahan, F. The Gut Microbiota in Inflammatory Bowel Disease. Gastroenterol. Clin. 2017, 46, 143. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-Resistant Gram-Negative Bacterial Infections in Patients with Cancer. Clin. Infect. Dis. 2014, 59, S335–S339. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Rangarajan, N.; Weisshaar, J.C. Lights, Camera, Action! Antimicrobial Peptide Mechanisms Imaged in Space and Time. Trends Microbiol. 2016, 24, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, Z.L.; Tang, J.; Lv, Q.M.; Rong, M.Q.; Lai, R. A short peptide from frog skin accelerates diabetic wound healing. FEBS J. 2014, 281, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Xia, X.; Han, F.F.; Jiang, Q.; Rong, Y.L.; Song, D.G.; Wang, Y.Z. Cathelicidin-BF, a Novel Antimicrobial Peptide from Bungarus fasciatus, Attenuates Disease in a Dextran Sulfate Sodium Model of Colitis. Mol. Pharm. 2015, 12, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, N.; Bakshi, S.; Weisshaar, J.C. Localized Permeabilization of E. coli Membranes by the Antimicrobial Peptide Cecropin A. Biochemistry 2013, 52, 6584–6594. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.M.; Bjorstad, A.; Dahlgren, C.; Bylund, J. A bactericidal cecropin-A peptide with a stabilized alpha-helical structure possess an increased killing capacity but no proinflammatory activity. Inflammation 2004, 28, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Acharya, S.; Gurley, C.; Park, Y.; Armstrong, C.A.; Song, P.I. Antimicrobial and anti-inflammatory effects of newly designed synthetic Cecropin A (1–8)-Magainin 2 (1–12) hybrid peptide CA-MA analogue P5 against Malassezia furfur. J. Investig. Dermatol. 2010, 130, S123. [Google Scholar]
- Van der Flier, L.G.; Clevers, H. Stem Cells, Self-Renewal, and Differentiation in the Intestinal Epithelium. Annu. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Miner-Williams, W.M.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Tumer, J.R. Tight, Junction Pore and Leak Pathways: A Dynamic Duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.J.; Wang, A.N.; Zeng, X.F.; Hou, C.L.; Liu, H.; Qiao, S.Y. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Gunzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Choi, W.; Buckley, A.; Shashikanth, N.; Joseph, N.E.; Wang, Y.; Warren, M.H.; Buschmann, M.M.; Pavlyuk, R.; Hildebrand, J.; et al. ZO-1 interactions with F-actin and occludin direct epithelial polarization and single lumen specification in 3D culture. J. Cell Sci. 2017, 130, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Osaki, L.H.; Gama, P. MAPKs and Signal Transduction in the Control of Gastrointestinal Epithelial Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2013, 14, 10143–10161. [Google Scholar] [CrossRef] [PubMed]
- Piegholdt, S.; Pallauf, K.; Esatbeyoglu, T.; Speck, N.; Reiss, K.; Ruddigkeit, L.; Stocker, A.; Huebbe, P.; Rimbach, G. Biochanin A and prunetin improve epithelial barrier function in intestinal CaCo-2 cells via downregulation of ERK, NF-kappa B, and tyrosine phosphorylation. Free Radic. Biol. Med. 2014, 70, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jin, X.L.; Chen, Y.F.; Song, Z.H.; Jiang, X.S.; Hu, F.L.; Conlon, M.A.; Topping, D.L. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients 2016, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Kubicek-Sutherland, J.Z.; Lofton, H.; Vestergaard, M.; Hjort, K.; Ingmer, H.; Andersson, D.I. Antimicrobial peptide exposure selects for Staphylococcus aureus resistance to human defence peptides. J. Antimicrob. Chemother. 2017, 72, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Fu, C.I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. B 2016, 371, 20150292. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pinto, S.; Evangelista, M.B.; Gil, H.; Kallip, S.; Ferreira, M.G.S.; Ferreira, L. High-density antimicrobial peptide coating with broad activity and low cytotoxicity against human cells. Acta Biomater. 2016, 33, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, N.; Brandner, J.M. Barriers and more: Functions of tight junction proteins in the skin. Ann. N. Y. Acad. Sci. 2012, 1257, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.F.; Yang, W.; Shen, L.; Turner, J.R.; Coyne, C.B.; Wang, T.Y. Tight Junction Proteins Claudin-1 and Occludin Control Hepatitis C Virus Entry and Are Downregulated during Infection to Prevent Superinfection. J. Virol. 2009, 83, 2011–2014. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Niyonsaba, F.; Kiatsurayanon, C.; Nguyen, T.T.; Ushio, H.; Fujimura, T.; Ueno, T.; Okumura, K.; Ogawa, H.; Ikeda, S. The Human Cathelicidin LL-37 Host Defense Peptide Upregulates Tight Junction-Related Proteins and Increases Human Epidermal Keratinocyte Barrier Function. J. Innate Immun. 2014, 6, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Krueger, F.; Madeja, Z.; Hemberger, M.; McMahon, M.; Cook, S.J.; Gaunt, S.J. Down-regulation of Cdx2 in colorectal carcinoma cells by the Raf-MEK-ERK 1/2 pathway. Cell. Signal. 2009, 21, 1846–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.W.; Wang, C.Y.; Zhao, Y.; Zhang, H.; Tan, Z.J.; Song, Z.H.; Ding, M.X.; Deng, H.K. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation 2007, 75, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Li, X.; Gao, Y.B.; Wang, S.M.; Zhao, L.; Dong, J.; Yao, B.W.; Xu, X.P.; Chang, G.M.; Zhou, H.M.; et al. Activation of VEGF/Flk-1-ERK Pathway Induced Blood-Brain Barrier Injury after Microwave Exposure. Mol. Neurobiol. 2015, 52, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.J.; Suh, E.R.; Lynch, J.P. The role of Cdx proteins in intestinal development and cancer. Cancer Biol. Ther. 2004, 3, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Troelsen, J.T.; Nielsen, O.H. The role of CDX2 in intestinal homeostasis and inflammation. BBA Mol. Basis Dis. 2011, 1812, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, E.; Boucher, M.J.; Mongrain, S.; Boudreau, F.; Asselin, C.; Rivard, N. Constitutive activation of the MEK/ERK pathway inhibits intestinal epithelial cell differentiation. Am. J. Physiol. Gastrointest. Liv. Physiol. 2011, 301, G719–G730. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) Regulates Claudin-2 Expression and Tight Junction Permeability in Intestinal Epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [PubMed]
- Kiatsurayanon, C.; Niyonsaba, F.; Smithrithee, R.; Akiyama, T.; Ushio, H.; Hara, M.; Okumura, K.; Ikeda, S.; Ogawa, H. Host Defense (Antimicrobial) Peptide, Human beta-Defensin-3, Improves the Function of the Epithelial Tight-Junction Barrier in Human Keratinocytes. J. Investig. Dermatol. 2014, 134, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.Z.; Peng, Y.Y.; Li, T.J.; Zeng, B.H.; et al. Intestinal Microbiota-Derived GABA Mediates Interleukin-17 Expression during Enterotoxigenic Escherichia coli Infection. Front. Immunol. 2017, 7, 685. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Ni, X.; Jin, C.; Ren, W.; Li, J.; Deng, J.; Deng, B.; Yin, Y. Cecropin A Modulates Tight Junction-Related Protein Expression and Enhances the Barrier Function of Porcine Intestinal Epithelial Cells by Suppressing the MEK/ERK Pathway. Int. J. Mol. Sci. 2018, 19, 1941. https://doi.org/10.3390/ijms19071941
Zhai Z, Ni X, Jin C, Ren W, Li J, Deng J, Deng B, Yin Y. Cecropin A Modulates Tight Junction-Related Protein Expression and Enhances the Barrier Function of Porcine Intestinal Epithelial Cells by Suppressing the MEK/ERK Pathway. International Journal of Molecular Sciences. 2018; 19(7):1941. https://doi.org/10.3390/ijms19071941
Chicago/Turabian StyleZhai, Zhenya, Xiaojun Ni, Chenglong Jin, Wenkai Ren, Jie Li, Jinping Deng, Baichuan Deng, and Yulong Yin. 2018. "Cecropin A Modulates Tight Junction-Related Protein Expression and Enhances the Barrier Function of Porcine Intestinal Epithelial Cells by Suppressing the MEK/ERK Pathway" International Journal of Molecular Sciences 19, no. 7: 1941. https://doi.org/10.3390/ijms19071941
APA StyleZhai, Z., Ni, X., Jin, C., Ren, W., Li, J., Deng, J., Deng, B., & Yin, Y. (2018). Cecropin A Modulates Tight Junction-Related Protein Expression and Enhances the Barrier Function of Porcine Intestinal Epithelial Cells by Suppressing the MEK/ERK Pathway. International Journal of Molecular Sciences, 19(7), 1941. https://doi.org/10.3390/ijms19071941