Keratinocyte Motility Is Affected by UVA Radiation—A Comparison between Normal and Dysplastic Cells
Abstract
:1. Introduction
2. Results
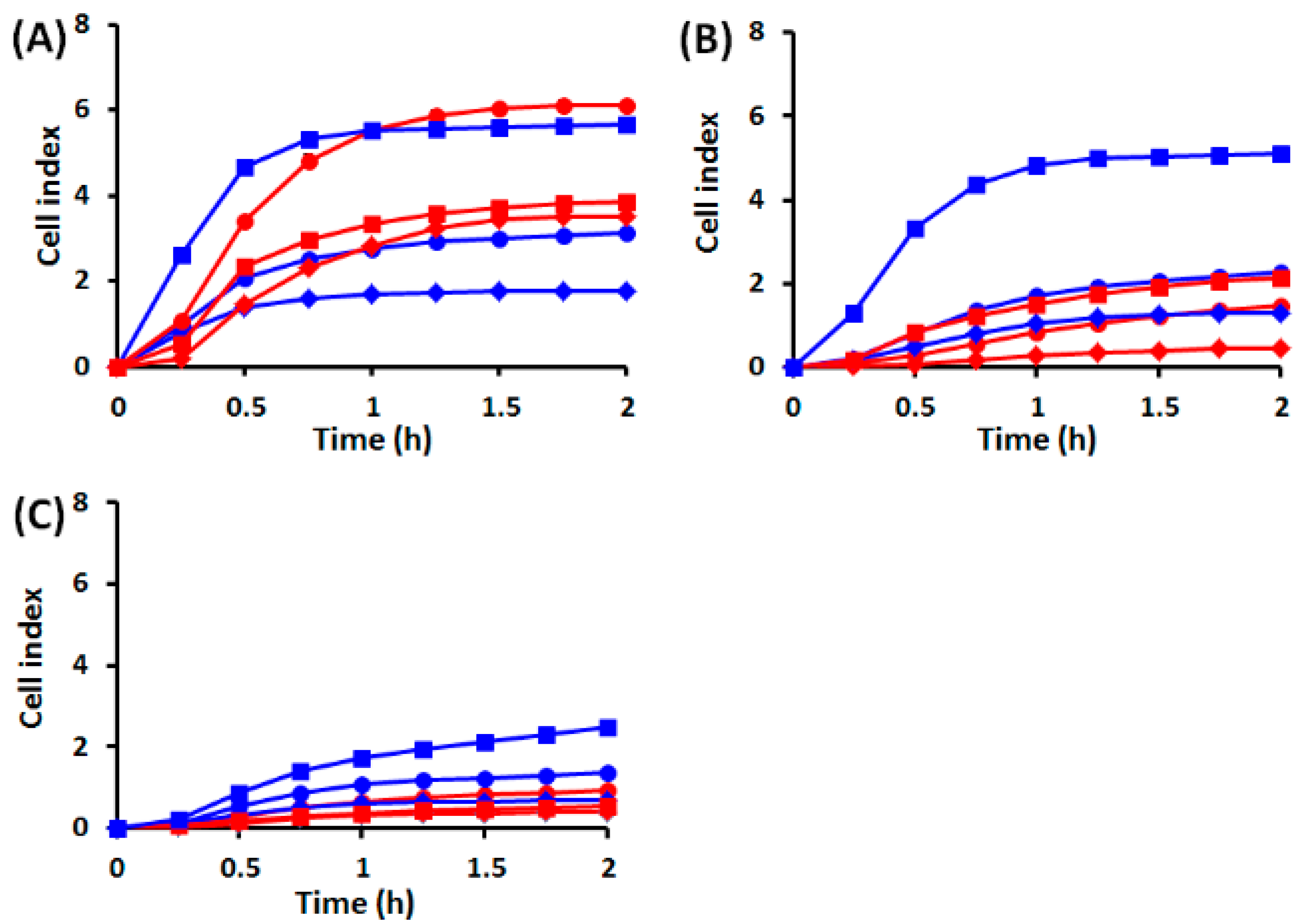
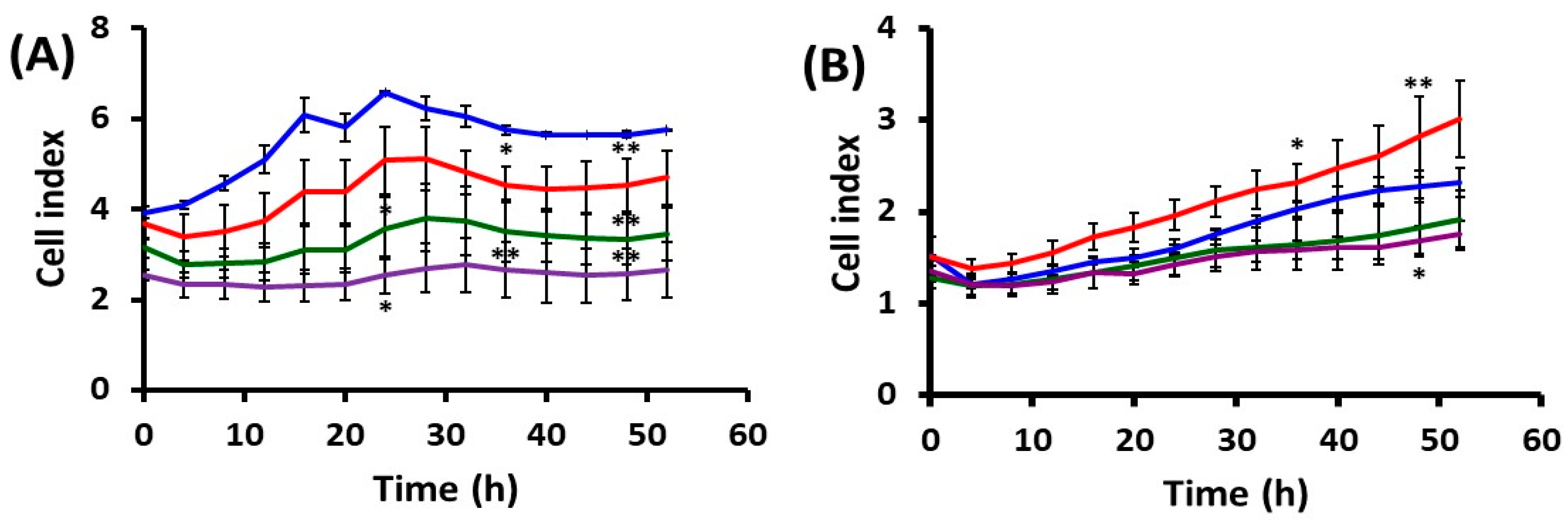
2.1. Effects of UVA Radiation on Cell Attachment, Spreading and Proliferation
2.1.1. Cell Behavior before UVA Exposure
2.1.2. Cell Behavior after UVA Exposure
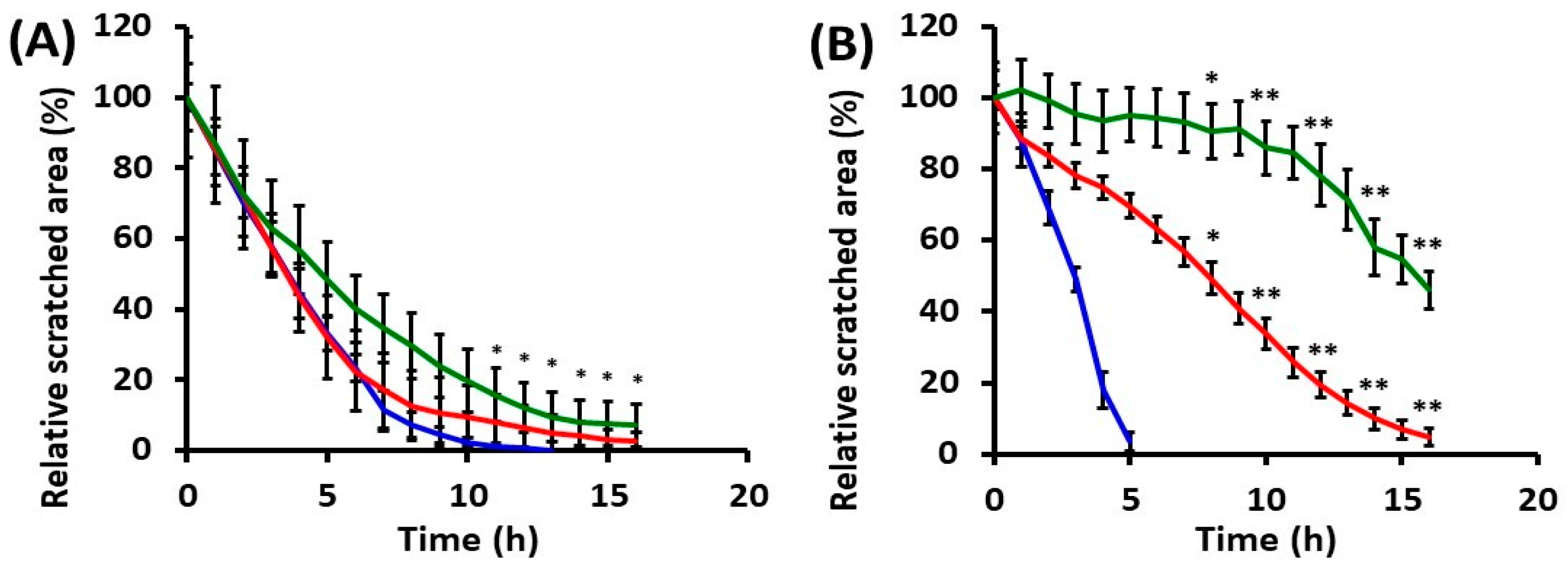
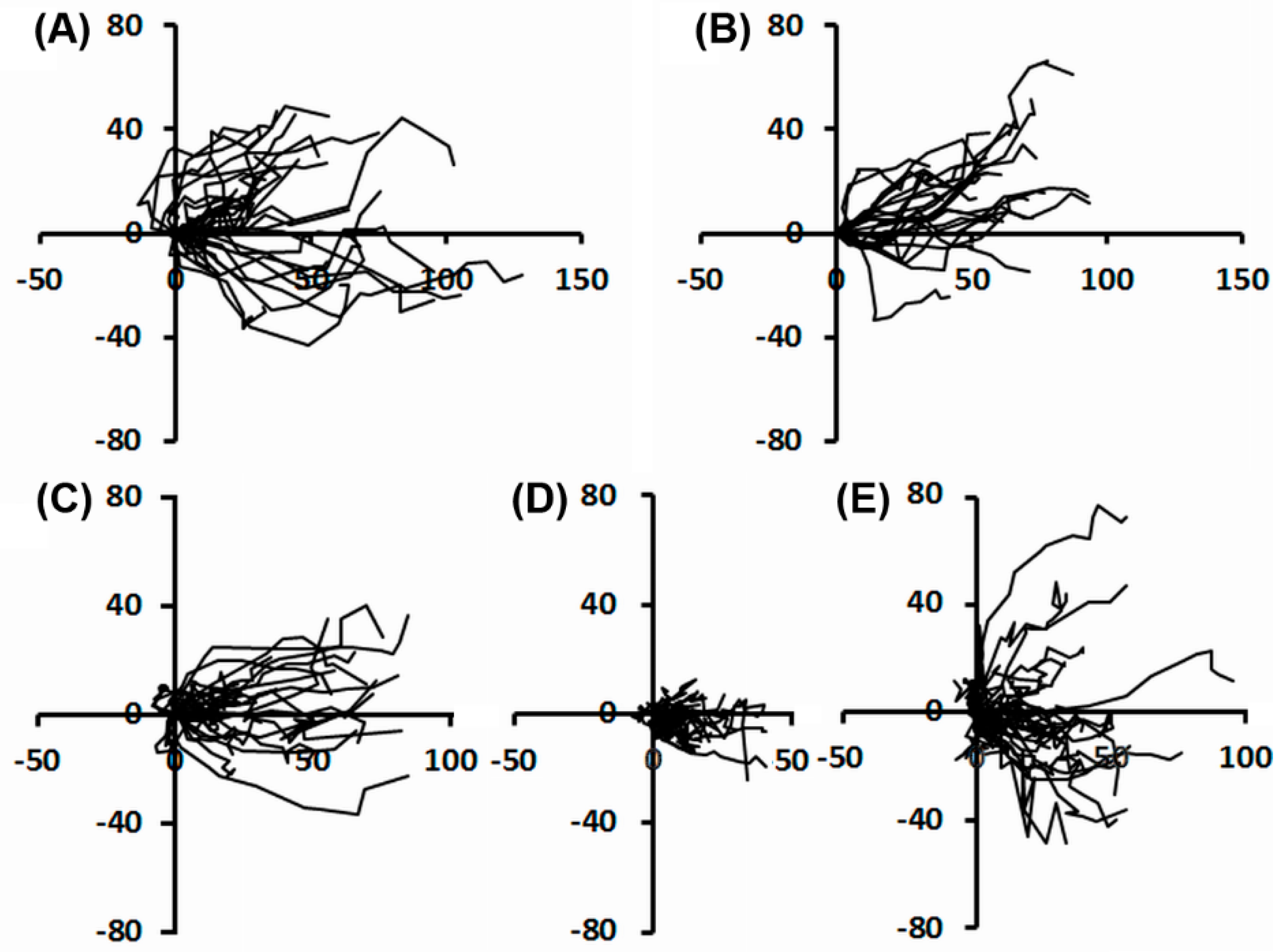
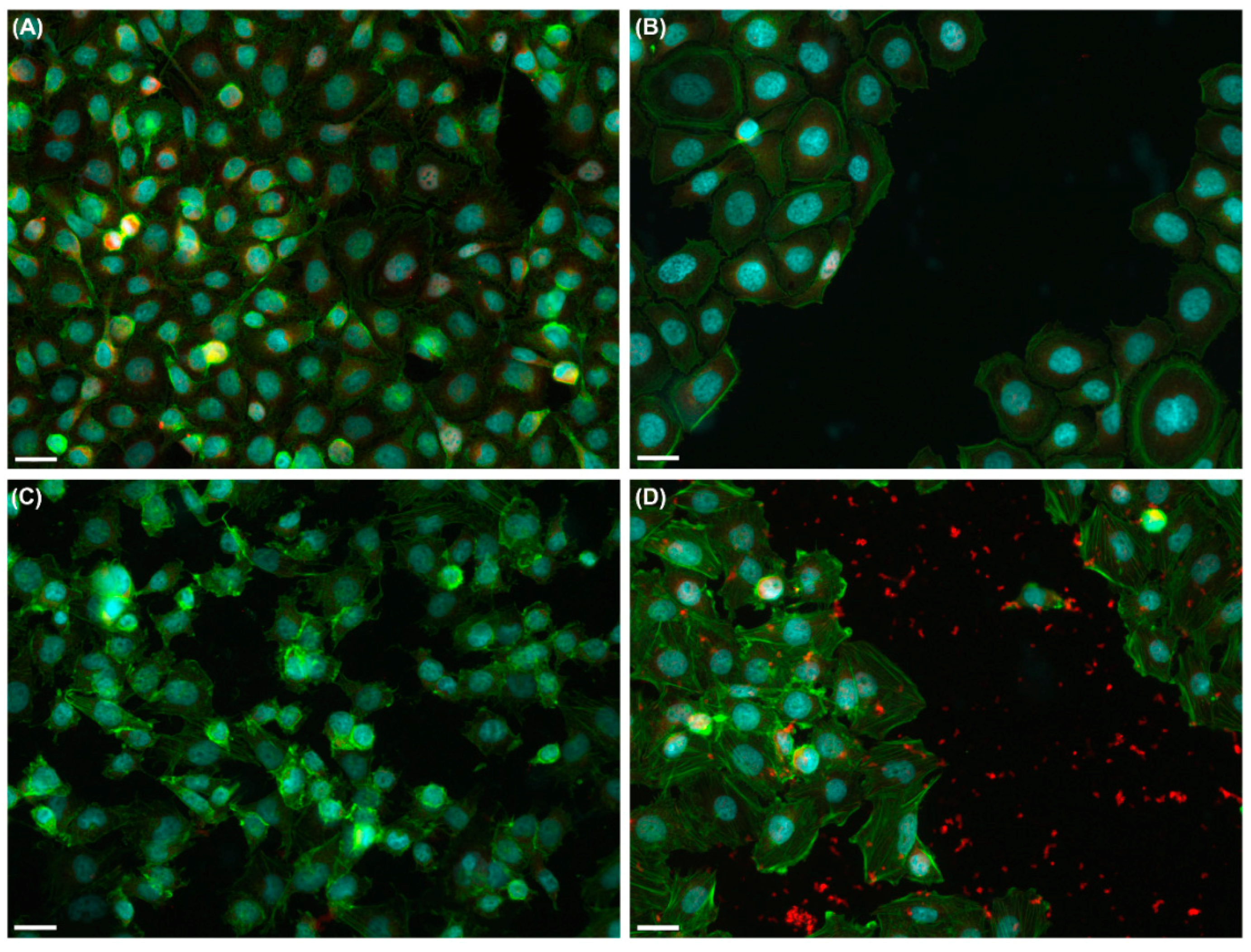
2.2. Effects of UVA Radiation on Cell Motility during In Vitro Wound Healing
2.2.1. Collective Cell Migration
2.2.2. Individual Cell Trajectories
2.3. Effects of UVA Radiation on the Actin Cytoskeleton and Focal Contacts
3. Discussion
3.1. Cell Adhesion, Spreading, and Proliferation Are Affected by UVA Radiation
3.2. UVA Exposure Affects the Directionality of the Cell Movement and Might Disturb Wound Healing
3.3. UVA Radiation Induces the Remodeling of Cytoskeleton and Adhesion Structures
4. Materials and Methods
4.1. Cell Cultures
4.2. UVA Source and Irradiation Conditions
4.3. Cell Impedance Monitoring
4.4. Time-Lapse Videomicroscopy
4.5. Immunofluorescence
4.6. Data Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DOK | dysplastic oral keratinocyte |
| FAK | focal adhesion kinase |
| FBS | fetal bovine serum |
| PBS | phosphate buffered saline |
| RTCA DP | real-time cell analyzer dual plate |
| UVA | ultraviolet A |
References
- Karran, P.; Brem, R. Protein oxidation, UVA and human DNA repair. DNA Repair 2016, 44, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Radack, K.P.; Farhangian, M.E.; Anderson, K.L.; Feldman, S.R. A review of the use of tanning beds as a dermatological treatment. Dermatol. Ther. 2015, 5, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, L.T.; Hannevik, M.; Veierød, M.B. Ultraviolet exposure from indoor tanning devices: A systematic review. Br. J. Dermatol. 2016, 174, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Hien, T.T.; Lim, S.C.; Kang, K.W. Role of Pin1 in UVA-induced cell proliferation and malignant transformation in epidermal cells. Biochem. Biophys. Res. Commun. 2011, 410, 68–74. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Huang, J.L.; Sik, R.H.; Liu, J.; Waalkes, M.P.; Chignell, C.F. Expression profiling of human keratinocyte response to ultraviolet A: Implications in apoptosis. J. Investig. Dermatol. 2004, 122, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Narayanapillai, S.; Agarwal, C.; Tilley, C.; Agarwal, R. Silibinin is a potent sensitizer of UVA radiation-induced oxidative stress and apoptosis in human keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Afaq, F.; Mukhtar, H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem. Photobiol. 2012, 88, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Battie, C.; Jitsukawa, S.; Bernerd, F.; del Bino, S.; Marionnet, C.; Verschoore, M. New insights in photoaging, UVA induced damage and skin types. Exp. Dermatol. 2014, 23, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grether-Beck, S.; Olaizola-Horn, S.; Schmitt, H.; Grewe, M.; Jahnke, A.; Johnson, J.; Briviba, K.; Sies, H.; Krutmann, J. Activation of transcription factor AP-2 mediates UVA radiation- and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14586–14591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, N.; Moreau, M.; Leturque, A.; Nizard, C. Ultraviolet A radiation transiently disrupts gap junctional communication in human keratinocytes. Am. J. Physiol. Cell Physiol. 2003, 28, C51–C59. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.F. Wound repair: Overview and general considerations. In The Molecular and Cellular Biology of Wound Repair; Clark, R.A.F., Ed.; Plenum Press: New York, NY, USA, 1996; pp. 3–35. [Google Scholar]
- Wells, A.; Grahovac, J.; Wheeler, S.; Ma, B.; Lauffenburger, D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol. Sci. 2013, 34, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Q.; Sun, J.; Ju, Y.; Morita, Y.; Song, G. Chromatin organization regulated by EZH2-mediated H3K27me3 is required for OPN-induced migration of bone marrow-derived mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2018, 96, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamir, E.R.; Ewald, A.J. Adhesion in mammary development: Novel roles for E-cadherin in individual and collective cell migration. Curr. Top. Dev. Biol. 2015, 112, 353–382. [Google Scholar] [PubMed]
- Hurd, T.R.; DeGennaro, M.; Lehmann, R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012, 22, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, M.L.; Almeida, M.E.S.; Vicente-Manzanares, M.; Horwitz, A.F.; Santos, M.F. High glucose-mediated oxidative stress impairs cell migration. PLoS ONE 2011, 6, e22865. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.C.; Liu, I.H.; Fang, A.H.; Wen, C.H.; Wu, C.S. Hyperglycaemic conditions decrease cultured keratinocyte mobility: Implications for impaired wound healing in patients with diabetes. Br. J. Dermatol. 2008, 159, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, W.; Du, J.; Reid, B.; Deng, X.; Liu, Z.; Zong, Z.; Wang, H.; Yao, B.; Yang, C.; et al. Electric fields guide migration of epidermal stem cells and promote skin wound healing. Wound Repair Regen. 2012, 20, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Mingle, L.; van de Water, L.; Liu, G. Control of cell migration through mRNA localization and local translation. Wiley Interdiscip. Rev. RNA 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; McCaig, C.D.; Cao, L.; Zhao, Z.; Segall, J.E.; Zhao, M. EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J. Cell Sci. 2007, 120, 3395–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.E.; Foster, S.; Betts, D.; Marnock, W.E. DOK, a cell line established from human dysplastic oral mucosa, shows a partially transformed non-malignant phenotype. Int. J. Cancer 1992, 52, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Paul, N.R.; Humphries, M.J.; Morgan, M.R. Emerging properties of adhesion complexes: What are they and what do they do? Trends Cell Biol. 2015, 25, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.M.; Crameri, F.M.; Olivry, T.; Mueller, E.; von Tscharner, C.; Jensen, P.J. Keratinocyte biology and pathology. Vet. Dermatol. 1997, 8, 67–100. [Google Scholar] [CrossRef]
- Pastila, R.; Leszczynski, D. Ultraviolet A exposure alters adhesive properties of mouse melanoma cells. Photodermatol. Photoimmunol. Photomed. 2005, 21, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, B.; Niethammer, P. Mechanisms of epithelial wound detection. Trends Cell Biol. 2015, 25, 398–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notbohm, J.; Banerjee, S.; Utuje, K.J.; Gweon, B.; Jang, H.; Park, Y.; Shin, J.; Butler, J.P.; Fredberg, J.J.; Marchetti, M.C. Cellular contraction and polarization drive collective cellular motion. Biophys. J. 2016, 110, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Petrie, R.J.; Doyle, A.D.; Yamada, K.M. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 538–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadea, G.; de Toledo, M.; Anguille, C.; Roux, P. Loss of p53 promotes Rho-ROCK-dependent cell migration and invasion in 3D matrices. J. Cell Biol. 2007, 178, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Caswell, P.T.; Doyle, B.; Iwanicki, M.P.; Tan, E.H.; Karim, S.; Lukashchuk, N.; Gillespie, D.A.; Ludwig, R.L.; Gosselin, P.; et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009, 139, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.E.; Clark, L.J.; Yeudall, W.A.; Mitchell, R.; Mackenzie, K.; Chang, S.E.; Parkinson, E.K. The p53 status of cultured human premalignant oral keratinocytes. Br. J. Cancer 1994, 70, 591–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeudall, W.A.; Wrighton, K.H.; Deb, S. Mutant p53 in cell adhesion and motility. Methods Mol. Biol. 2013, 962, 135–146. [Google Scholar] [PubMed]
- Roussos, E.T.; Condeelis, J.S.; Patsialou, A. Chemotaxis in cancer. Nat. Rev. Cancer 2011, 11, 573–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.P.; Beaty, B.T.; Patsialou, A.; Liu, H.; Clarke, M.; Cox, D.; Condeelis, J.S.; Eddy, R.J. Reconstitution of in vivo macrophage-tumor cell pairing and streaming motility on one-dimensional micro-patterned substrates. Intravital 2012, 1, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitta, H.; Wada, Y.; Kawano, Y.; Murakami, Y.; Irie, A.; Taniguchi, K.; Kikuchi, K.; Yamada, G.; Suzuki, K.; Honda, J.; et al. Enhancement of human cancer cell motility and invasiveness by anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88). Clin. Cancer Res. 2013, 19, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, M.T.; Niculiţe, C.M.; Urs, A.O.; Regalia, T.; Mocanu, M.; Popescu, A.; Manda, G.; Dinu, D.; Leabu, M. UVA irradiation of dysplastic keratinocytes: Oxidative damage versus antioxidant defense. Int. J. Mol. Sci. 2012, 13, 16718–16736. [Google Scholar] [CrossRef] [PubMed]
- Schiller, H.B.; Fässler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013, 14, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M. Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. J. Cell Sci. 2010, 123, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. How actin/myosin crosstalks guide the adhesion, locomotion and polarization of cells. Biochim. Biophys. Acta 2015, 1853, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Donais, K.; Whitmore, L.A.; Thomas, S.M.; Turner, C.E.; Parsons, J.T.; Horwitz, A.F. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 2004, 6, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanicki, M.P.; Vomastek, T.; Tilghman, R.W.; Martin, K.H.; Banerjee, J.; Wedegaertner, P.B.; Parsons, J.T. FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J. Cell Sci. 2008, 121, 895–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Chen, H.; Yang, H.; Liang, J.; Li, X. Low-dose UVA radiation-induced adaptive response in cultured human fibroblasts. Int. J. Photoenergy 2012, 2012, 167425. [Google Scholar] [CrossRef]
- Leabu, M.; Uniyal, S.; Xie, J.; Xu, Y.Q.; Vladau, C.; Morris, V.L.; Chan, B.M. Integrin α2β1 modulates EGF stimulation of Rho GTPase-dependent morphological changes in adherent human rhabdomyosarcoma RD cells. J. Cell. Physiol. 2005, 202, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Niculiţe, C.; Leabu, M. Timelapse monitoring of cell behavior as a tool in tissue engineering. In International Conference on Advancements of Medicine and Health Care through Technology, IFMBE Proceedings; Vlad, S., Ciupa, R.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 352–357. [Google Scholar]
- Rosello, C.; Ballet, P.; Planus, E.; Tracqui, P. Model driven quantification of individual and collective cell migration. Acta Biotheor. 2004, 52, 343–363. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculiţe, C.M.; Nechifor, M.T.; Urs, A.O.; Olariu, L.; Ceafalan, L.C.; Leabu, M. Keratinocyte Motility Is Affected by UVA Radiation—A Comparison between Normal and Dysplastic Cells. Int. J. Mol. Sci. 2018, 19, 1700. https://doi.org/10.3390/ijms19061700
Niculiţe CM, Nechifor MT, Urs AO, Olariu L, Ceafalan LC, Leabu M. Keratinocyte Motility Is Affected by UVA Radiation—A Comparison between Normal and Dysplastic Cells. International Journal of Molecular Sciences. 2018; 19(6):1700. https://doi.org/10.3390/ijms19061700
Chicago/Turabian StyleNiculiţe, Cristina M., Marina T. Nechifor, Andreea O. Urs, Laura Olariu, Laura C. Ceafalan, and Mircea Leabu. 2018. "Keratinocyte Motility Is Affected by UVA Radiation—A Comparison between Normal and Dysplastic Cells" International Journal of Molecular Sciences 19, no. 6: 1700. https://doi.org/10.3390/ijms19061700
APA StyleNiculiţe, C. M., Nechifor, M. T., Urs, A. O., Olariu, L., Ceafalan, L. C., & Leabu, M. (2018). Keratinocyte Motility Is Affected by UVA Radiation—A Comparison between Normal and Dysplastic Cells. International Journal of Molecular Sciences, 19(6), 1700. https://doi.org/10.3390/ijms19061700




