Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models
Abstract
1. Introduction
2. Results
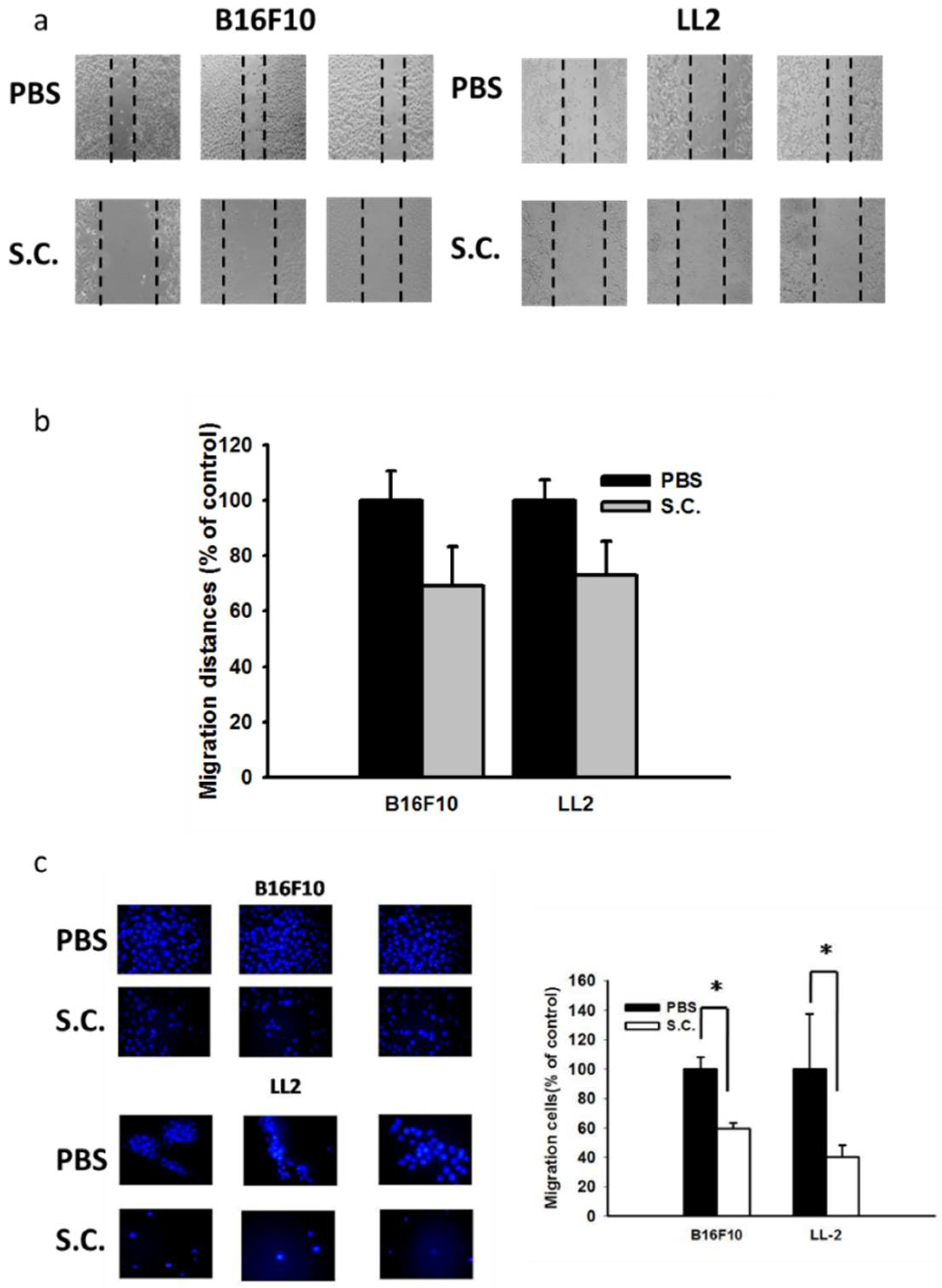
2.1. Salmonella Inhibition of Tumor Cell Migration
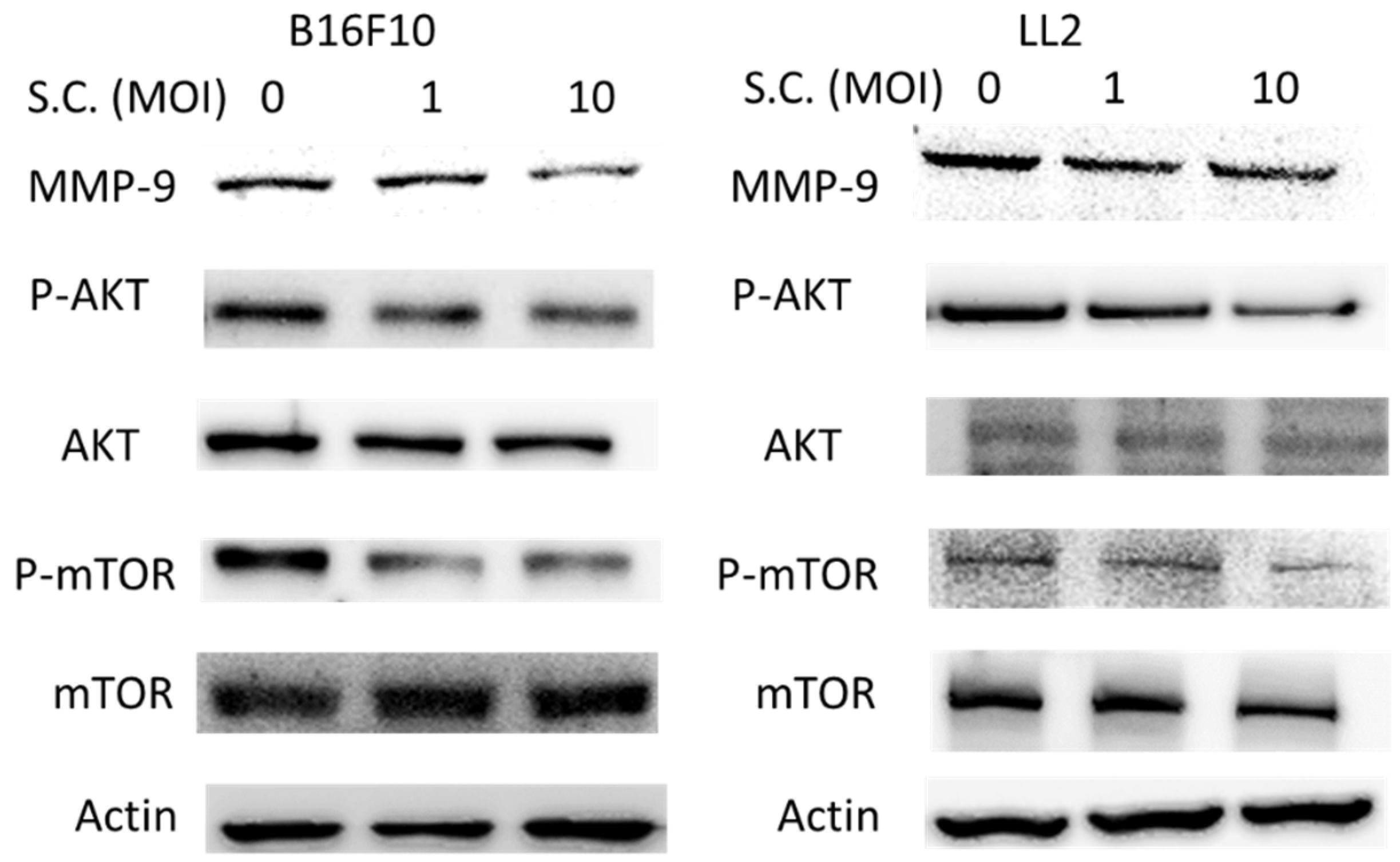
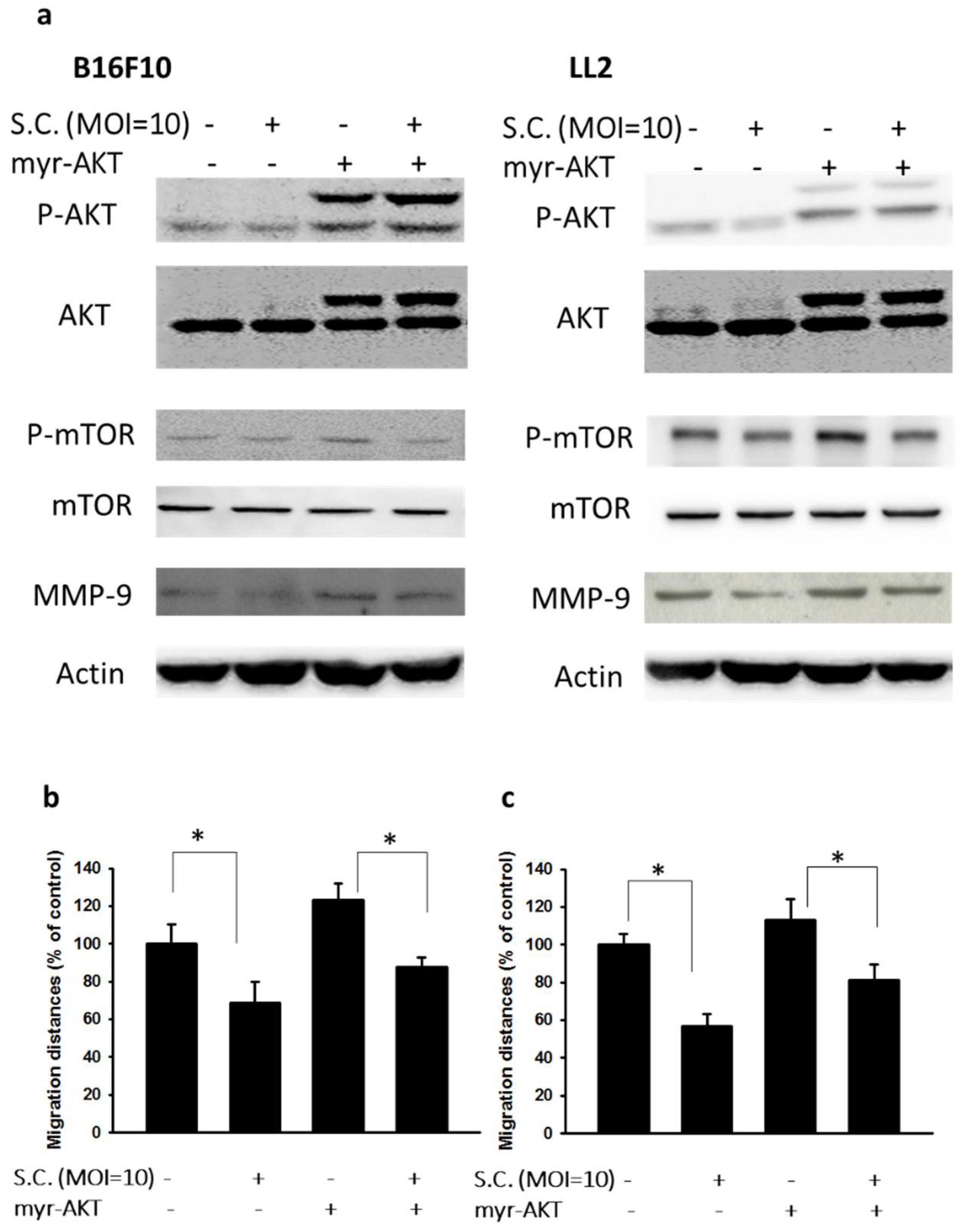
2.2. The Phospho-Protein Kinase B (P-AKT)/Phospho-Mammalian Targets of the Rapamycin (P-mTOR) Pathway as Requirement for Salmonella-Mediated MMP-9 Expression
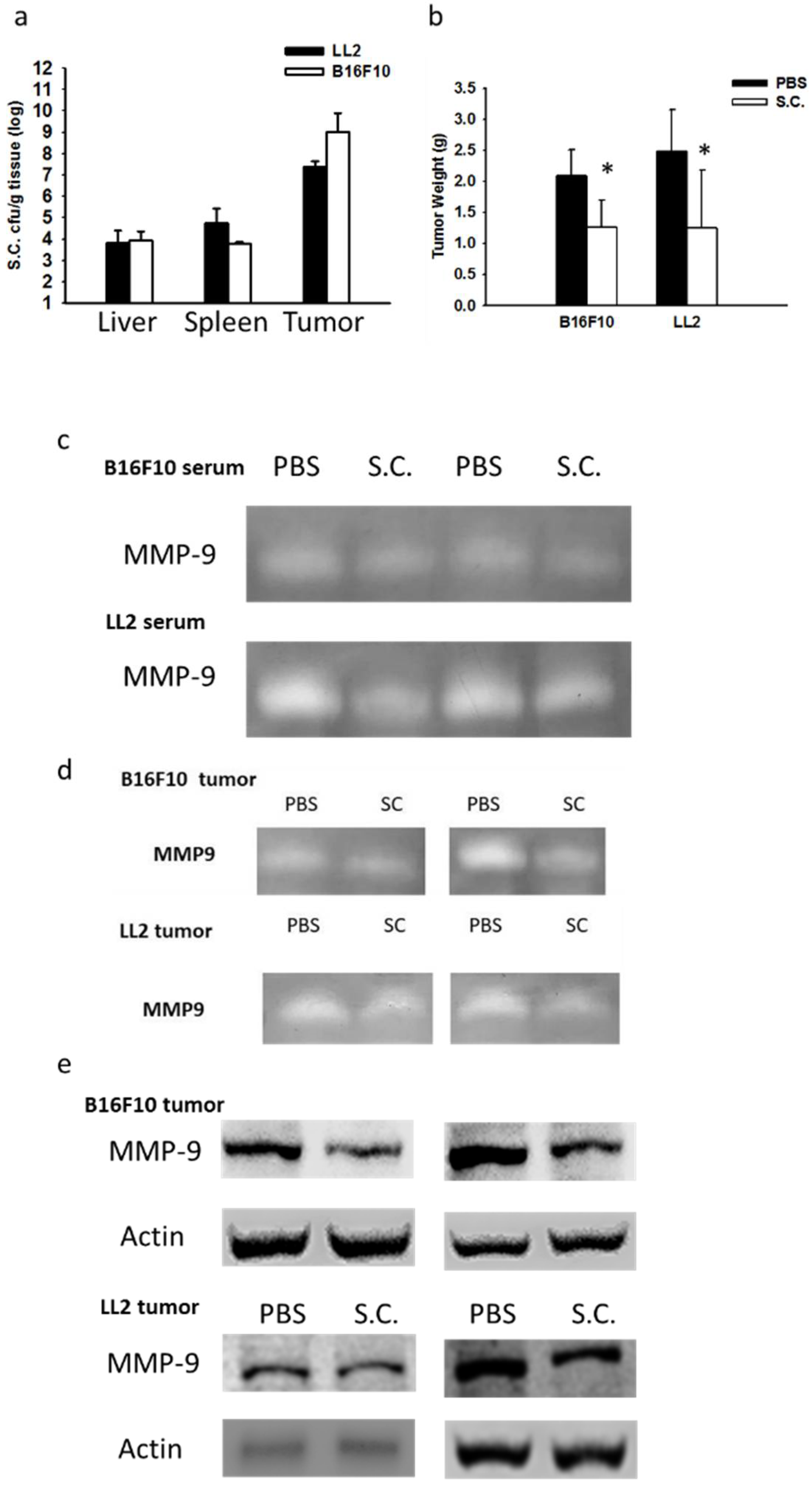
2.3. Salmonella Supresssion of Matrix MetalloProteinase 9 Expression In Vivo
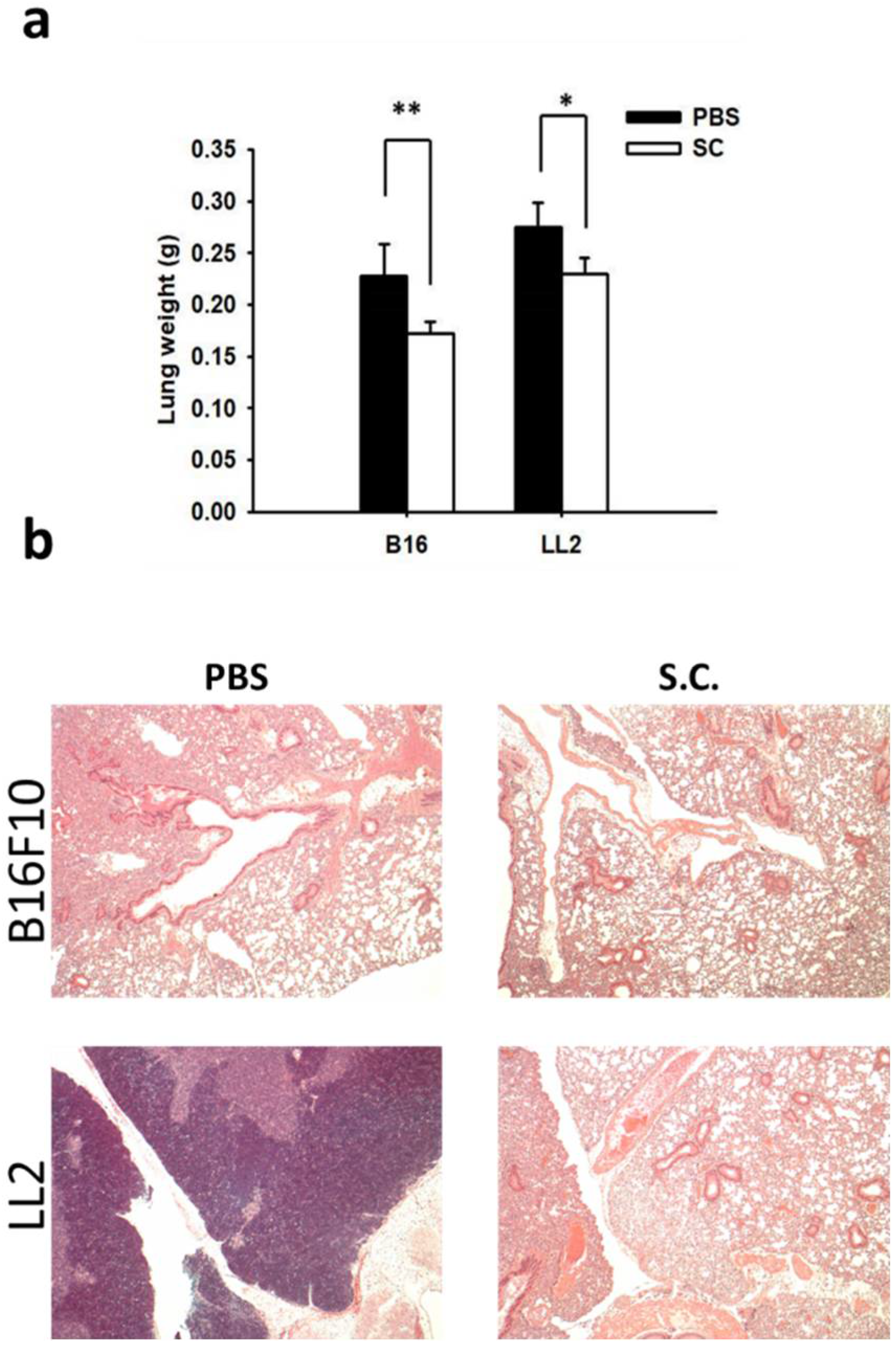
2.4. Salmonella Inhibition of Tumor Metastasis In Vivo
3. Discussion
4. Materials and Methods
4.1. Cells, Reagents, Animal, Bacteria, and Plasmids
4.2. Wound-Healing and Transwell Assay
4.3. Western Blotting, Gelatin Zymography, and Transfection
4.4. Mouse Experiments
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MMP | Matrix metalloproteinases |
| AKT | Protein kinase B |
| mTOR | Mammalian targets of rapamycin |
| PI3K | Phosphatidylinositol 3-kinase |
| S.C. | Salmonella |
| MOI | The multiplicity of infection |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| cfu | Colony-forming units |
References
- Lv, Y.; Zhao, X.; Zhu, L.; Li, S.; Xiao, Q.; He, W.; Yin, L. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics 2018, 8, 2830–2845. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Kim, Y.J.; An, H.; Sung, D.; Cho, T.M.; Farrand, L.; Jang, S.; Seo, J.H.; Kim, J.Y. Flubendazole elicits anti-metastatic effects in triple-negative breast cancer via STAT3 inhibition. Int. J. Cancer 2018. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lan, M.; Chen, X.; Dai, Y.; Zhao, X.; Wang, L.; Zhao, T.; Li, Y.; Zhu, J.; Zhang, X.; et al. Anti-invasion and anti-metastasis effects of Valjatrate E via reduction of matrix metalloproteinases expression and suppression of MAPK/ERK signaling pathway. Biomed. Pharmacother. 2018. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.Y.; Kemppainen, K.; Lassila, T.; Törnquist, K. Sphingosine 1-phosphate attenuates MMP2 and MMP9 in human anaplastic thyroid cancer C643 cells: Importance of S1P2. PLoS ONE 2018, 13, e0196992. [Google Scholar] [CrossRef] [PubMed]
- Kaimala, S.; Al-Sbiei, A.; Cabral-Marques, O.; Fernandez-Cabezudo, M.J.; Al-Ramadi, B.K. Attenuated bacteria as immunotherapeutic tools for cancer treatment. Front. Oncol. 2018, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Chang, W.W.; Lin, S.T.; Chen, M.C.; Lee, C.H. Salmonella overcomes drug resistance in tumor through p-glycoprotein downregulation. Int. J. Med. Sci. 2018, 15, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Singh, A.S.; Eckardt, M.A.; Nelson, S.D.; Russell, T.A.; Dry, S.M.; et al. Tumor-targeting Salmonella typhimurium A1-R is a highly effective general therapeutic for undifferentiated soft tissue sarcoma patient-derived orthotopic xenograft nude-mouse models. Biochem. Biophys. Res. Commun. 2018, 497, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.G.; Masner, M.; Ferreira, F.A.; Hoffman, R.M. Bacterial therapy of cancer: Promises, limitations, and insights for future directions. Front. Microbiol. 2018, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Tai, Y.S.; Shiau, A.L. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Mol. Ther. 2005, 11, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, D.; Zhou, R.; Zhong, W.; Lu, S.; Chai, Y. Geraniin inhibits migration and invasion of human osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2 signaling pathways. Anticancer Drugs 2017, 28, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Zhang, X.; Cao, Y.; Wang, Y.; Zhang, X. Effect of siRNA-silencing of SALL2 gene on growth, migration and invasion of human ovarian carcinoma A2780 cells. BMC Cancer 2017, 17, 838. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chang, W.W.; Kuan, Y.D.; Lin, S.T.; Hsu, H.C.; Lee, C.H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012, 18, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H. Engineering bacteria toward tumor targeting for cancer treatment: Current state and perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.W.; Lee, C.H. Salmonella as an innovative therapeutic antitumor agent. Int. J. Mol. Sci. 2014, 15, 14546–14554. [Google Scholar] [CrossRef] [PubMed]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Heise, U.; Rohde, M.; Zimmermann, K.; Falk, C.; Erhardt, M.; Weiss, S. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology 2017, 7, e1382791. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther. 2005, 12, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Yu, C.C.; Wang, B.Y.; Chang, W.W. Tumorsphere as an effective in vitro platform for screening anticancer stem cell drugs. Oncotarget 2016, 7, 1215–1226. [Google Scholar] [PubMed]
- Wang, S.; Yan, Y.; Cheng, Z.; Hu, Y.; Liu, T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.W.; Kuan, Y.D.; Chen, M.C.; Lin, S.T.; Lee, C.H. Tracking of mouse breast cancer stem-like cells with Salmonella. Exp. Biol. Med. 2012, 237, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Salmonella choleraesuis as an anticancer agent in a syngeneic model of orthotopic hepatocellular carcinoma. Int. J. Cancer 2008, 122, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J. Gene Med. 2004, 6, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, P.Y.; Shiau, A.L. T cell augments the antitumor activity of tumor-targeting Salmonella. Appl. Microbiol. Biotechnol. 2011, 90, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, J.L.; Wu, C.L.; Hsu, H.C.; Shiau, A.L. B cells are required for tumor-targeting Salmonella in host. Appl. Microbiol. Biotechnol. 2011, 92, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.G.; Chang, W.W.; Lin, S.T.; Kuo, C.Y.; Tsao, Y.T.; Lee, C.H. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget 2016, 7, 37513–37523. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.D.; Lee, C.H. Salmonella overcomes tumor immune tolerance by inhibition of tumor indoleamine 2, 3-dioxygenase 1 expression. Oncotarget 2016, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lin, S.T.; Liu, J.J.; Chang, W.W.; Hsieh, J.L.; Wang, W.K. Salmonella induce autophagy in melanoma by the downregulation of AKT/mTOR pathway. Gene Ther. 2014, 21, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G.; et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Z.Y.; Yang, H.Z.; Liu, H.Z.; Mi, S.; Lv, X.X.; Fu, X.M.; Yan, H.M.; Zhang, X.W.; Zhan, Q.M.; et al. Timing is critical for an effective anti-metastatic immunotherapy: The decisive role of IFNγ/STAT1-mediated activation of autophagy. PLoS ONE 2011, 6, e24705. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Philpott, D.J.; Girardin, S.E. The bacterial and cellular determinants controlling the recruitment of mTOR to the Salmonella-containing vacuole. Biol. Open 2012, 1, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Budak, G.; Eren Ozsoy, O.; Aydin Son, Y.; Can, T.; Tuncbag, N. Reconstruction of the temporal signaling network in Salmonella-infected human cells. Front. Microbiol. 2015, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Nishikawa, T.; Kaneda, Y. Salmonella mediated the hemagglutinating virus of Japan-envelope transfer suppresses tumor growth. Oncotarget 2017, 8, 35048–35060. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, Y.-T.; Kuo, C.-Y.; Cheng, S.-P.; Lee, C.-H. Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models. Int. J. Mol. Sci. 2018, 19, 1630. https://doi.org/10.3390/ijms19061630
Tsao Y-T, Kuo C-Y, Cheng S-P, Lee C-H. Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models. International Journal of Molecular Sciences. 2018; 19(6):1630. https://doi.org/10.3390/ijms19061630
Chicago/Turabian StyleTsao, Yu-Tzu, Chun-Yu Kuo, Shun-Ping Cheng, and Che-Hsin Lee. 2018. "Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models" International Journal of Molecular Sciences 19, no. 6: 1630. https://doi.org/10.3390/ijms19061630
APA StyleTsao, Y.-T., Kuo, C.-Y., Cheng, S.-P., & Lee, C.-H. (2018). Downregulations of AKT/mTOR Signaling Pathway for Salmonella-Mediated Suppression of Matrix Metalloproteinases-9 Expression in Mouse Tumor Models. International Journal of Molecular Sciences, 19(6), 1630. https://doi.org/10.3390/ijms19061630





