Ligand Access Channels in Cytochrome P450 Enzymes: A Review
Abstract
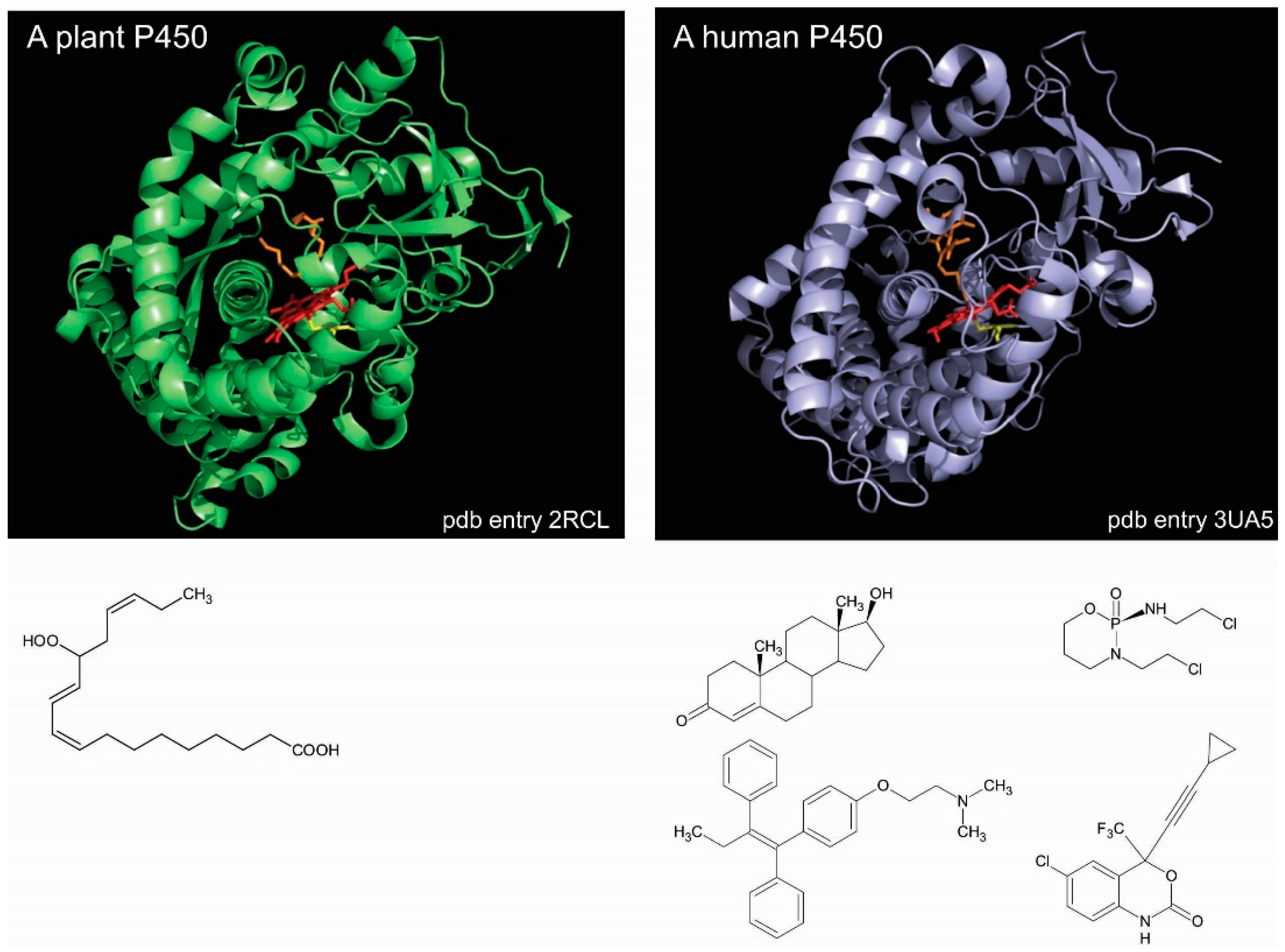
:1. Introduction
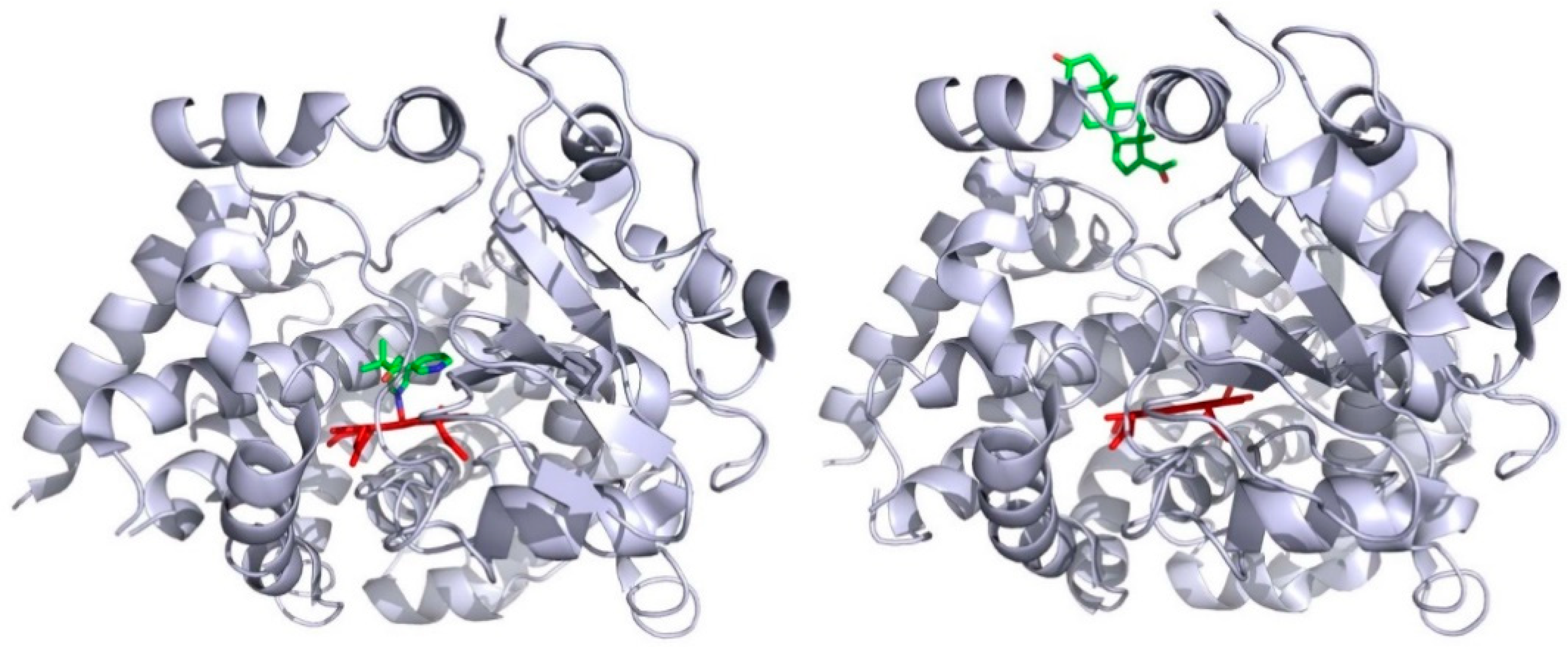
2. Identifying Channels in P450 Enzymes
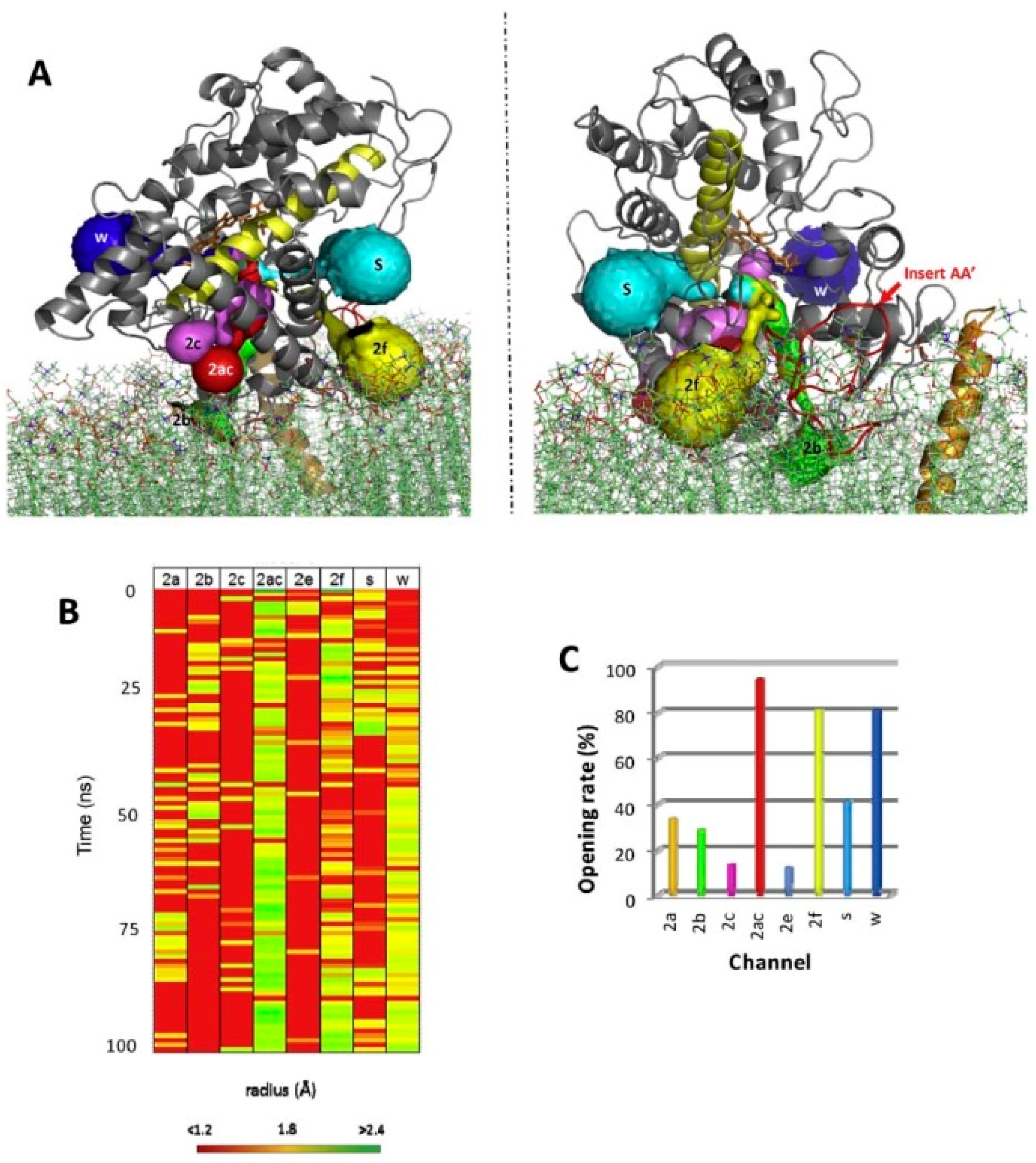
3. Channel Dynamics in Cytochromes P450
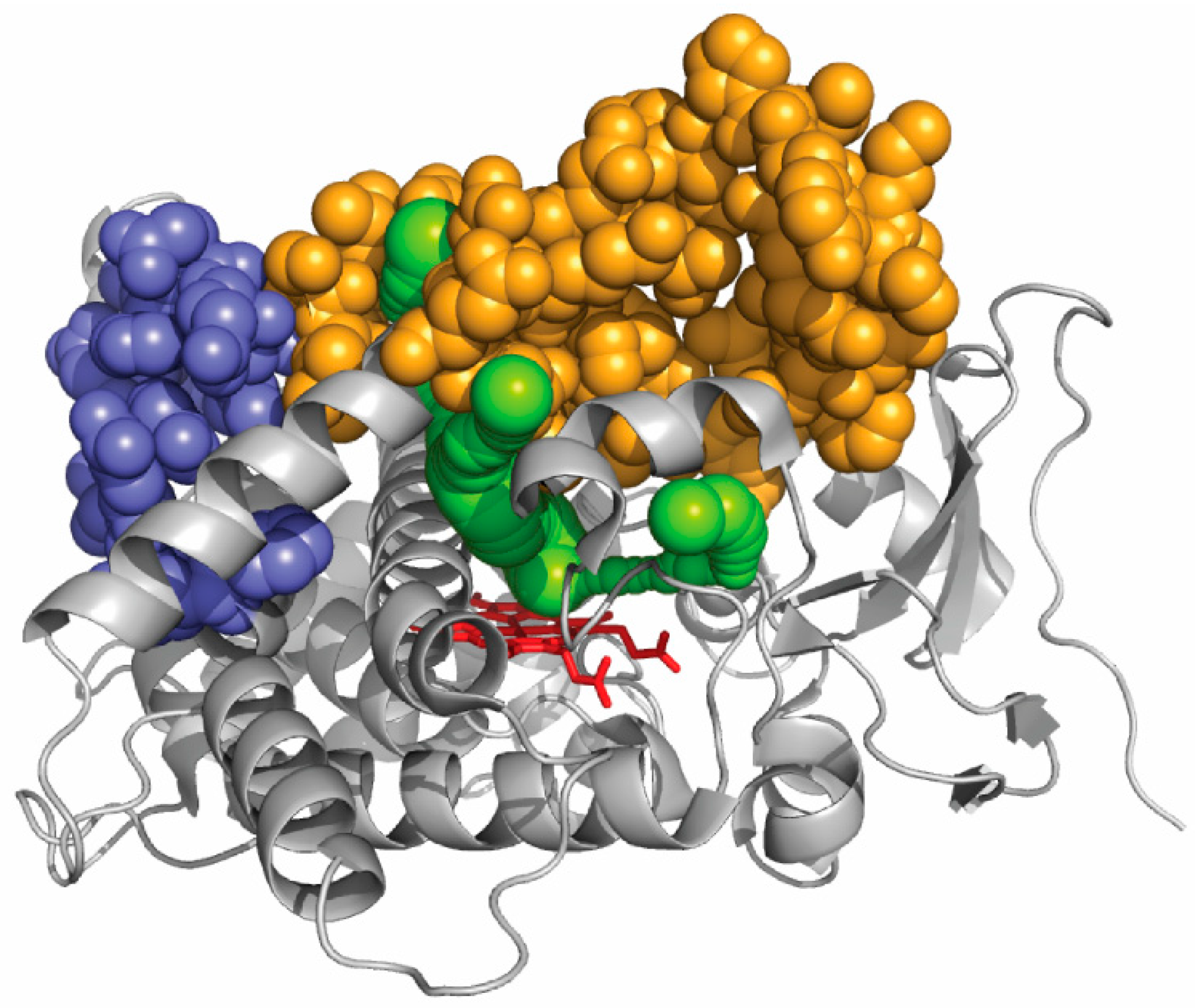
4. The F/G Region and Aromatic Residues
5. The Membrane
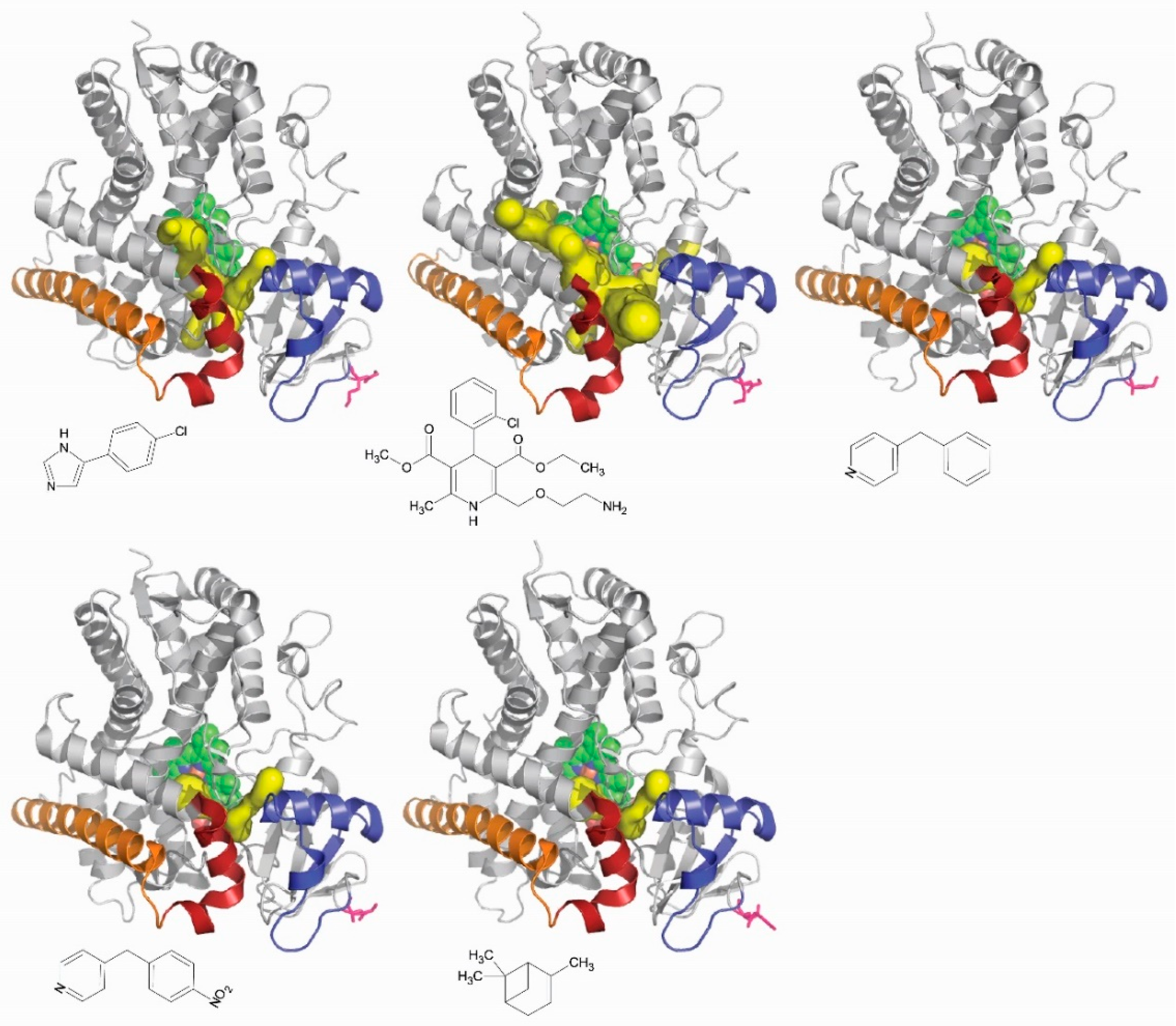
6. Experimental Validation of Channels in Cytochromes P450
7. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| CCCPP | Computing cavities, channels, pores and pockets |
| P450 | Cytochrome P450 |
| QSAR | Quantitative structure-activity relationships |
| RAMD | Random acceleration molecular dynamics |
| REMD | Random expulsion molecular dynamics |
References
- Coon, M.J. Cytochrome P450: Nature’s most versatile biological catalyst. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.H. Probing Structure-Function Relations in Heme-Containing Oxygenases and Peroxidases. Science 1988, 240, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, D.; Zhang, H.; Hollenberg, P. Oxygen activation by cytochrome P450 monooxygenase. Photosynth. Res. 2008, 98, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Green, M.T. C-H bond activation in heme proteins: The role of thiolate ligation in cytochrome P450. Curr. Opin. Chem. Biol. 2009, 13, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018, 1, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Girvan, H.M.; Munro, A.W. Applications of microbial cytochrome P450 enzymes in biotechnology and synthetic biology. Curr. Opin. Chem. Biol. 2016, 31, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Bassard, E.; Hamberger, B.; Werck-Reichhart, D. Cytochrome P450-mediated metabolic engineering: Current progress and future challenges. Curr. Opin. Plant Biol. 2014, 19, 27–34. [Google Scholar]
- Miller, W.L. Steroidogenesis: Unanswered questions. Trends Endocrinol. Metab. 2017, 28, 771–793. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, K.L.; Endo, T.; Darwesh, A.M.; Samokhvalov, V.; Seubert, J.M. Cytochrome P450-derived eicosanoids and heart function. Pharmacol. Ther. 2017, 179, 47–83. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Krest, C.M.; Onderko, E.L.; Yosca, T.H.; Calixto, J.C.; Karp, R.F.; Livada, J.; Rittle, J.; Green, M.T. Reactive intermediates in cytochrome P450 catalysis. J. Biol. Chem. 2013, 288, 17074–17081. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.W.; Girvan, H.M.; Mason, A.E.; Dunford, A.J.; McLean, K.J. What makes a P450 tick? Trends Biochem. Sci. 2013, 38, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.C.; Roberts, A.G.; Halpert, J.R. Structural features of cytochromes P450 and ligands that affect drug metabolism as revealed by X-ray crystallography and NMR. Future Med. Chem. 2010, 2, 1451–1468. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.F.; Stout, C.D. Structural diversity of eukaryotic membrane cytochrome P450s. J. Biol. Chem. 2013, 288, 17082–17090. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry 2009, 70, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Nioche, P.; Hamberg, M.; Raman, C.S. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 2008, 455, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.B.; Wilderman, P.R.; Pascual, J.; Zhang, Q.; Stout, C.D.; Halpert, J.R. Conformational adaptation of human cytochrome P450 2B6 and rabbit cytochrome P450 2B4 revealed upon binding multiple amlodipine molecules. Biochemistry 2012, 51, 7225–7238. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Cytochrome P450: Molecular architecture, mechanism, and prospects for rational inhibitor design. Pharm. Res. 1988, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yosca, T.H.; Ledray, A.P.; Ngo, J.; Green, M.T. A new look at the role of thiolate ligation in cytochrome P450. J. Biol. Inorg. Chem. 2017, 22, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R.; De Voss, J.J. Oxidizing species in the mechanism of cytochrome P450. Nat. Prod. Rep. 2002, 19, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Poulos, T.L. Crystallization of cytochromes P450 and substrate-enzyme interactions. Curr. Top. Med. Chem. 2004, 4, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.J.; Lill, M.A. Substrate tunnels in enzymes: Structure-function relationships and computational methodology. Proteins 2015, 83, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Pravda, L.; Berka, K.; Varekova, R.S.; Sehnal, D.; Banas, P.; Laskowski, R.A.; Koca, J.; Otyepka, M. Anatomy of enzyme channels. BMC Bioinform. 2014, 15, e379. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, B.P.; Watson, H.C.; Kendrew, J.C. inding of xenon to sperm whale myoglobin. Nature 1965, 207, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Tilton, R.F.; Kuntz, I.D.; Petsko, G.A. Cavities in proteins: Structure of a metmyoglobin-xenon complex solved to 1.9 Å. Biochemistry 1984, 23, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Sott, E.E.; Gibson, Q.H. Ligand migration in sperm whale myoglobin. Biochemistry 1997, 36, 11909–11917. [Google Scholar]
- Ho, C.M.; Marshall, G.R. Cavity search: An algorithm for the isolation and display of cavity-like binding regions. J. Comput. Aided Mol. Des. 1990, 4, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Fedyukina, D.V.; Cavagnero, S. Protein folding at the exit tunnel. Annu. Rev. Biophys. 2011, 40, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Ohtsubo, Y.; Tsuda, M. Properties and biotechnological applications of natural and engineered haloalkane dehalogenases. Appl. Microbiol. Biotechnol. 2015, 99, 9865–9881. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.E.; Peterson, J.A. How similar are P450s and what can their differences teach us? Arch. Biochem. Biophys. 1999, 369, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, S.K.; Carugo, O.; Wade, R.C. Substrate access to cytochrome P450cam: A comparison of a thermal motion pathway analysis with molecular dynamics simulation data. J. Mol. Model. 1997, 3, 369–374. [Google Scholar] [CrossRef]
- Lüdemann, S.K.; Lounnas, V.; Wade, R.C. How do substrates enter and products exit the buried active site of cytochrome P450cam? 1. Random expulsion molecular dynamics of ligand access channels and mechanisms. J. Mol. Biol. 2000, 303, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, S.K.; Lounnas, V.; Wade, R.C. How do substrates enter and products exit the buried active site of cytochrome P450cam? 2. Steered molecular dynamics and adiabatic mapping of substrate pathways. J. Mol. Biol. 2000, 303, 813–830. [Google Scholar] [CrossRef] [PubMed]
- Doshi, U.; Hamelberg, D. Towards fast, rigorous and efficient conformational sampling of biomolecules: Advances in accelerated molecular dynamics. Biochim. Biophys. Acta 2015, 1850, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Petřek, M.; Otyepka, M.; Banáš, P.; Košinová, P.; Koča, J.; Damborský, J. CAVER: A New Tool to Explore Routes from Protein Clefts, Pockets and Cavities. BMC Bioinform. 2006, 7, e316. [Google Scholar] [CrossRef] [PubMed]
- Petrek, M.; Kosinová, P.; Koca, J.; Otyepka, M. MOLE: A Voronoi diagram-based explorer of molecular channels, pores, and tunnels. Structure 2007, 15, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.C.; Winn, P.J.; Schlichting, I.; Sudarko. A survey of active site access channels in cytochromes P450. J. Inorg. Biochem. 2004, 98, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.; Winn, P.J.; Wade, R.C. The ins and outs of cytochrome P450s. Biochim. Biophys. Acta 2007, 1770, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Benkaidali, L.; André, F.; Maouche, B.; Siregar, P.; Benyettou, M.; Maurel, F.; Petitjean, M. Computing cavities, channels, pores and pockets in proteins from non-spherical ligands models. Bioinformatics 2014, 30, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Rydzewski, J.; Nowak, W. Memetic algorithms for ligand expulsion from protein cavities. J. Chem. Phys. 2015, 143, e124101. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Finnigan, J.D.; Cook, K.; Black, G.W.; Charnock, S.J. Cytochromes P450: History, Classes, Catalytic Mechanism, and Industrial Application. Adv. Protein Chem. Struct. Biol. 2016, 105, 105–126. [Google Scholar] [PubMed]
- Katagiri, M.; Ganguli, B.N.; Gunsalus, I. A soluble cytochrome P-450 functional in methylene hydroxylation. J. Biol. Chem. 1968, 243, 3543–3546. [Google Scholar] [PubMed]
- Grogan, G.; Roberts, S.; Wan, P.; Willetts, A. Camphor-grown Pseudomonas putida, a multifunctional biocatalyst for undertaking Baeyer-Villiger monooxygenase dependent biotransformations. Biotechnol. Lett. 1993, 15, 913–918. [Google Scholar] [CrossRef]
- Poulos, T.L.; Finzel, B.C.; Howard, A.J. Crystal structure of substrate-free Pseudomonas putida cytochrome P-450. Biochemistry 1986, 25, 5314–5322. [Google Scholar] [CrossRef] [PubMed]
- Hays, A.M.; Dunn, A.R.; Chiu, R.; Gray, H.B.; Stout, C.D.; Goodin, D.B. Conformational states of cytochrome P450cam revealed by trapping of synthetic molecular wires. J. Mol. Biol. 2004, 344, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Wilson, R.F.; Rupniewski, I.; Goodin, D.B. P450cam visits an open conformation in the absence of substrate. Biochemistry 2010, 49, 3412–3419. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Glazer, E.C.; Wilson, R.F.; Stout, C.D.; Goodin, D.B. Three clusters of conformational states in P450cam reveal a multistep pathway for closing of the substrate access channel. Biochemistry 2011, 50, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Sligar, S.G. A novel type of allosteric regulation: Functional cooperativity in monomeric proteins. Arch. Biochem. Biophys. 2012, 519, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Szklarz, G.D.; Halpert, J.R. Molecular basis of P450 inhibition and activation: Implications for drug development and drug therapy. Drug Metab. Dispos. 1998, 26, 1179–1184. [Google Scholar] [PubMed]
- Williams, P.A.; Cosme, J.; Vinkovic, D.M.; Ward, A.; Angove, H.C.; Day, P.J.; Vonrhein, C.; Tickle, I.J.; Jhoti, H. Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 2004, 305, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Davydov, D.R.; Davydova, N.Y.; Sineva, E.V.; Halpert, J.R. Interactions among cytochromes P450 in microsomal membranes: Oligomerization of cytochromes P450 3A4, 3A5, and 2E1 and its functional consequences. J. Biol. Chem. 2015, 290, 3850–3864. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Baylon, J.L.; Grinkova, Y.V.; Tajkhorshid, E.; Sligar, S.G. Drug-drug interactions between atorvastatin and dronedarone mediated by monomeric CYP3A4. Biochemistry 2018, 57, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; McCullough, C.R.; Costache, A.D.; Pullela, P.K.; Sem, D.S. Structural evidence for a functionally relevant second camphor binding site in P450cam: Model for substrate entry into a P450 active site. Proteins 2007, 69, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bell, S.G.; Wang, H.; Zhou, W.; Bartlam, M.; Wong, L.L.; Rao, Z. The structure of CYP101D2 unveils a potential path for substrate entry into the active site. Biochem. J. 2011, 433, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.G.; Boddupalli, S.S.; Hasemann, C.A.; Peterson, J.A.; Deisenhofer, J. Crystal structure of hemoprotein domain of P450BM-3, a prototype for microsomal P450s. Science 1993, 261, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Sutcliffe, M.J.; Primrose, W.U.; Lian, L.Y.; Roberts, G.C.K. The catalytic mechanism of cytochrome P450 BM3 involves a 6 Å movement of the bound substrate on reduction. Nat. Struct. Biol. 1996, 3, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Davydov, D.R.; Davydova, N.Y.; Halpert, J.R. Allosteric transitions in cytochrome P450eryF explored with pressure-perturbation spectroscopy, lifetime FRET, and a novel fluorescent substrate, Fluorol-7GA. Biochemistry 2008, 47, 11348–11359. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, M.D.; Ornstein, R.L. Dramatic differences in the motions of the mouth of open and closed cytochrome P450BM-3 by molecular dynamics simulations. Proteins 1995, 21, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Otyepka, M.; Berka, K.; Anzenbacher, P. Is there a relationship between the substrate preferences and structural flexibility of cytochromes P450? Curr. Drug Metab. 2012, 13, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Ducassou, L.; Dhers, L.; Jonasson, G.; Pietrancosta, N.; Boucher, J.L.; Mansuy, D.; Andre, F. Membrane-bound human orphan cytochrome P450 2U1: Sequence singularities, construction of a full 3D model, and substrate docking. Biochimie 2017, 140, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Behera, R.K.; Mazumdar, S. Roles of two surface residues near the access channel in the substrate recognition by cytochrome P450cam. Biophys. Chem. 2008, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rabovsky, J.; Judy, D.J. The non-linear dependence of cytochrome P450 activity at low microsome concentration. Biochem. Int. 1987, 15, 1033–1041. [Google Scholar] [PubMed]
- Brignac-Huber, L.M.; Park, J.W.; Reed, J.R.; Backes, W.L. Cytochrome P450 Organization and Function Are Modulated by Endoplasmic Reticulum Phospholipid Heterogeneity. Drug Metab. Dispos. 2016, 44, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Hughes, J.M.X.; Hay, S.; Scrutton, N.S. Liver microsomal lipid enhances the activity and redox coupling of colocalized cytochrome P450 reductase-cytochrome P450 3A4 in nanodiscs. FEBS J. 2017, 284, 2302–2319. [Google Scholar] [CrossRef] [PubMed]
- Barnaba, C.; Gentry, K.; Sumangala, N.; Ramamoorthy, A. The catalytic function of cytochrome P450 is entwined with its membrane-bound nature. F1000 Res. 2017, 6, e662. [Google Scholar] [CrossRef] [PubMed]
- Jerabek, P.; Florian, J.; Martínek, V. Lipid molecules can induce an opening of membrane-facing tunnels in cytochrome P450 1A2. Phys. Chem. Chem. Phys. 2016, 18, 30344–30356. [Google Scholar] [CrossRef] [PubMed]
- Paloncyova, M.; Navratilova, V.; Berka, K.; Laio, A.; Otyepka, M. Role of Enzyme Flexibility in Ligand Access and Egress to Active Site: Bias-Exchange Metadynamics Study of 1,3,7-Trimethyluric Acid in Cytochrome P450 3A4. J. Chem. Theory Comput. 2016, 12, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; White, M.A.; Muralidhara, B.K.; Sun, L.; Halpert, J.R.; Stout, C.D. Structure of microsomal cytochrome P450 2B4 complexed with the antifungal drug bifonazole: Insight into P450 conformational plasticity and membrane interaction. J. Biol. Chem. 2006, 281, 5976–5981. [Google Scholar] [CrossRef] [PubMed]
- Skopalik, J.; Anzenbacher, P.; Otyepka, M. Flexibility of human cytochromes P450: Molecular dynamics reveals differences between CYPs 3A4, 2C9, and 2A6, which correlate with their substrate preferences. J. Phys. Chem. B 2008, 112, 8165–8173. [Google Scholar] [CrossRef] [PubMed]
- Ekroos, M.; Sjögren, T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc. Natl. Acad. Sci. USA 2006, 103, 13682–13687. [Google Scholar] [CrossRef] [PubMed]
- Benkaidali, L.; Andre, F.; Moroy, G.; Tangour, B.; Maurel, F.; Petitjean, M. The cytochrome P450 3A4 has three major conformations: New clues to drug recognition by this promiscuous enzyme. Mol. Inf. 2017, 36, e1700044. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Grinkova, Y.V.; Baylon, J.L.; Tajkhorshid, E.; Sligar, S.G. Mechanism of drug-drug interactions mediated by human cytochrome P450 CYP3A4 monomer. Biochemistry 2015, 54, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
- Fishelovitch, D.; Shaik, S.; Wolfson, H.J.; Nussinov, R. Theoretical characterization of substrate access/exit channels in the human cytochrome P450 3A4 enzyme: Involvement of phenylalanine residues in the gating mechanism. J. Phys. Chem. B 2009, 113, 13018–13025. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Zhang, L.; Zhan, T.; Lu, L.; Zhao, L.; Wang, H.; Morrone, J.A.; Liu, W.; Zhou, R. Binding specificity determines the cytochrome P450 3A4 mediated enantioselective Metabolism of metconazole. J. Phys. Chem. B 2018, 122, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Tarr, G.E.; Black, S.D.; Fujita, V.S.; Coon, M.J. Complete amino acid sequence and predicted membrane topology of phenobarbital-induced cytochrome P-450 (isozyme 2) from rabbit liver microsomes. Proc. Natl. Acad. Sci. USA 1983, 80, 6552–6556. [Google Scholar] [CrossRef] [PubMed]
- Monier, S.; Van Luc, P.; Kreibich, G.; Sabatini, D.D.; Adesnik, M. Signals for the incorporation and orientation of cytochrome P450 in the endoplasmic reticulum membrane. J. Cell Biol. 1988, 107, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.J.; Murray, B.P.; Singleton, A.M.; Boobis, A.R. Orientation of cytochromes P450 in the endoplasmic reticulum. Biochemistry 1991, 30, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Griswold, J.; Erman, M.; Pangborn, W. X-ray structure of human aromatase reveals an androgen-specific active site. J. Steroid Biochem. Mol. Biol. 2010, 118, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, J.D.A.; Sabherwal, M.; Sagatova, A.A.; Keniya, M.V.; Negroni, J.; Wilso, R.K.; Wood, M.A.; Tietjen, K.; Monk, B.C. Saccharomyces cerevisiae CYP51 complexed with the plant pathogen inhibitor S-tebuconazole. PLoS ONE 2016, 11, e0167485. [Google Scholar]
- Srejber, M.; Navratilova, V.; Paloncyova, M.; Bazgier, V.; Berka, K.; Anzenbacher, P.; Otyepka, M. Membrane-attached mammalian cytochromes P450: An overview of the membrane’s effects on structure, drug binding, and interactions with redox partners. J. Inorg. Biochem. 2018, 183, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.; Balali-Mood, K.; Sansom, M.S.; Wade, R.C. Structure and dynamics of the membrane-bound cytochrome P450 2C9. PLoS Comput. Biol. 2011, 7, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Berka, K.; Paloncyova, M.; Anzenbacher, P.; Otyepka, M. Behavior of Human Cytochromes P450 on Lipid Membranes. J. Phys. Chem. B 2013, 117, 11556–11564. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, V.; Paloncyova, M.; Berka, K.; Otyepka, M. Effect of Lipid Charge on Membrane Immersion of Cytochrome P450 3A4. J. Phys. Chem. B 2016, 120, 11205–11213. [Google Scholar] [CrossRef] [PubMed]
- Wester, M.R.; Johnson, E.F.; Marques-Soares, C.; Dansette, P.M.; Mansuy, D.; Stout, C.D. Structure of a substrate complex of mammalian cytochrome P450 2C5 at 2.3 A resolution: Evidence for multiple substrate binding modes. Biochemistry 2003, 42, 6370–6379. [Google Scholar] [CrossRef] [PubMed]
- Schleinkofer, K.; Sudarko Winn, P.J.; Lüdemann, S.K.; Wade, R.C. Do mammalian cytochrome P450s show multiple ligand access pathways and ligand channelling? EMBO Rep. 2005, 6, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Cosme, J.; Ward, A.; Angove, H.C.; Matak Vinkovic, D.; Jhoti, H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature 2003, 424, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Berka, K.; Hendrychova, T.; Anzenbacher, P.; Otyepka, M. Membrane Position of Ibuprofen Agrees with Suggested Access Path Entrance to Cytochrome P450 2C9 Active Site. J. Phys. Chem. A 2011, 115, 11248–11255. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.F. Soft Matter in Lipid-Protein Interactions. Annu. Rev. Biophys. 2017, 46, 379–410. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Simon-Plas, F.; Perraki, A.; Bayer, E.; Gerbeau-Pissot, P.; Mongrand, S. An update on plant membrane rafts. Curr. Opin. Plant Biol. 2011, 14, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.E.; Wolf, C.R.; Otyepka, M.; Humphreys, S.C.; Reed, J.R.; Henderson, C.J.; McLaughlin, L.A.; Paloncýová, M.; Navrátilová, V.; Berka, K.; et al. The Role of Protein-Protein and Protein-Membrane Interactions on P450 Function. Drug Metab. Dispos. 2016, 44, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, V.; Paloncyova, M.; Kajsova, M.; Berka, K.; Otyepka, M. Effect of cholesterol on the structure of membrane-attached cytochrome P450 3A4. J. Chem. Inf. Model. 2015, 55, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Janin, J.; Rodier, F. Protein-protein interaction at crystal contacts. Proteins 1995, 23, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Baudry, J.; Berenbaum, M.R.; Schuler, M.A. Ile115Leu mutation in the SRS1 region of an inset cytochrome P450 CYP6B1 compromises substrate turnovers via changes in a predicted product release channel. Protein Eng. Des. Sel. 2005, 18, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kenaan, C.; Hamdane, D.; Hoa, G.H.; Hollenberg, P.F. Effect of conformational dynamics on substrate recognition and specificity as probed by the introduction of a de novo disulfide bond into cytochrome P450 2B1. J. Biol. Chem. 2009, 284, 25678–25686. [Google Scholar] [CrossRef] [PubMed]
- Winn, P.J.; Lüdemann, S.K.; Gauges, R.; Lounnas, V.; Wade, R.C. Comparison of the dynamics of substrate access channels in three cytochrome P450s reveals different opening mechanisms and a novel functional role for a buried arginine. Proc. Natl. Acad. Sci. USA 2002, 99, 5361–5366. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.A.; Szklarz, G.D.; Scott, E.E. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 2013, 288, 12932–12943. [Google Scholar] [CrossRef] [PubMed]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 2007, 282, 14348–14355. [Google Scholar] [CrossRef] [PubMed]
- Shou, M.; Gonzalez, F.J.; Gelboin, H.V. Stereoselective epoxidation and hydration at the K-region of polycyclic aromatic hydrocarbons by cDNA-expressed cytochromes P450 1A1, 1A2, and epoxide hydrolase. Biochemistry 1996, 35, 15807–15813. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Truan, G.; Pompon, D. Access channels to the buried active site control substrate specificity in CYP1A P450 enzymes. Biochim. Biophys. Acta 2015, 1850, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Navratilova, V.; Paloncyova, M.; Berka, K.; Mise, S.; Haga, Y.; Matsumura, C.; Sakaki, T.; Inui, H.; Otyepka, M. Molecular insights into the role of a distal F240A mutation that alters CYP1A1 activity towards persistent organic pollutants. Biochim. Biophys. Acta 2017, 1861, 2852–2860. [Google Scholar] [CrossRef] [PubMed]
- Murayama, N.; Soyama, A.; Saito, Y.; Nakajima, Y.; Komamura, K.; Ueno, K.; Kamakura, S.; Kitakaze, M.; Kimura, H.; Goto, Y.; et al. Six novel nonsynonymous CYP1A2 gene polymorphisms: Catalytic activities of the naturally occurring variant enzymes. J. Pharmacol. Exp. Ther. 2004, 308, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, L.A.; Lewis, D.F.; Wei, D.Q. Long-range effects of a peripheral mutation on the enzymatic activity of cytochrome P450 1A2. J. Chem. Inf. Model. 2011, 51, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Lautier, T.; Urban, P.; Loeper, J.; Jezequel, L.; Pompon, D.; Truan, G. Ordered chimerogenesis applied to CYP2B P450 enzymes. Biochim. Biophys. Acta 2016, 1860, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Tychopoulos, M.; Bichat, F.; Zimmermann, C.; Flinois, J.P.; Diry, M.; Ahlberg, E.; Delaforge, M.; Corcos, L.; Beaune, P.; et al. Improvement of cyclophosphamide activation by CYP2B6 mutants: From in silico to ex vivo. Mol. Pharmacol. 2008, 73, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Wilderman, P.R.; Shah, M.B.; Jang, H.H.; Stout, C.D.; Halpert, J.R. Structural and thermodynamic basis of (+)-α-pinene binding to human cytochrome P450 2B6. J. Am. Chem. Soc. 2013, 135, 10433–10440. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.; Klvana, M.; Prokop, Z.; Chaloupkova, R.; Banas, P.; Otyepka, M.; Wade, R.C.; Tsuda, M.; Nagata, Y.; Damborsky, J. Redesigning dehalogenase access tunnels as a strategy for degrading an anthropogenic substrate. Nat. Chem. Biol. 2009, 5, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L.; Finzel, B.C.; Gunsalus, I.C.; Wagner, G.C.; Kraut, J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P450. J. Biol. Chem. 1985, 260, 16122–16130. [Google Scholar] [PubMed]
- Poulos, T.L.; Finzel, B.C.; Howard, A.J. High-resolution crystal structure of cytochrome P450cam. J. Mol. Biol. 1987, 195, 687–700. [Google Scholar] [CrossRef]
- Poulos, T.L. Cytochrome P450 flexibility. Proc. Natl. Acad. Sci. USA 2003, 100, 13121–13122. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, D.; Richards, L.S.; Poulos, T.L. Effect of redox partner binding on cytochrome P450 conformational dynamics. J. Am. Chem. Soc. 2017, 139, 13193–13199. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Truan, G.; Pompon, D. High-throughput functional screening of steroid substrates with wild-type and chimeric P450 enzymes. Biomed. Res. Int. 2014, 2014, e764102. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urban, P.; Lautier, T.; Pompon, D.; Truan, G. Ligand Access Channels in Cytochrome P450 Enzymes: A Review. Int. J. Mol. Sci. 2018, 19, 1617. https://doi.org/10.3390/ijms19061617
Urban P, Lautier T, Pompon D, Truan G. Ligand Access Channels in Cytochrome P450 Enzymes: A Review. International Journal of Molecular Sciences. 2018; 19(6):1617. https://doi.org/10.3390/ijms19061617
Chicago/Turabian StyleUrban, Philippe, Thomas Lautier, Denis Pompon, and Gilles Truan. 2018. "Ligand Access Channels in Cytochrome P450 Enzymes: A Review" International Journal of Molecular Sciences 19, no. 6: 1617. https://doi.org/10.3390/ijms19061617
APA StyleUrban, P., Lautier, T., Pompon, D., & Truan, G. (2018). Ligand Access Channels in Cytochrome P450 Enzymes: A Review. International Journal of Molecular Sciences, 19(6), 1617. https://doi.org/10.3390/ijms19061617





