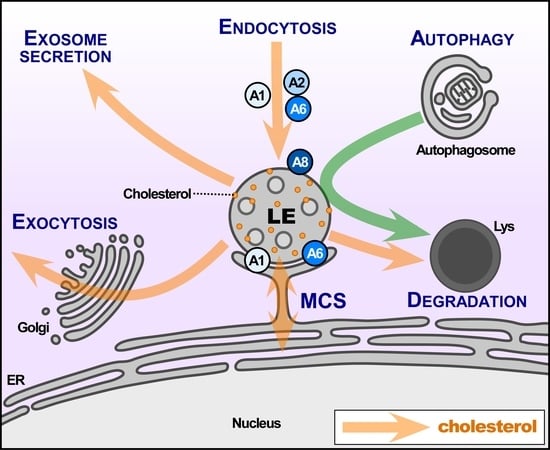
Annexins—Coordinators of Cholesterol Homeostasis in Endocytic Pathways
Abstract
1. Introduction
2. Late Endocytic Compartments
3. Annexins in Late Endocytic Compartments
4. Annexins and Cholesterol Homeostasis
5. Annexins and Signalling in Late Endocytic Compartments
5.1. Regulation of Endosomal Fate: Rab Proteins
5.2. The Coordination of EGFR Signalling and Trafficking
5.3. mTORC1 Signalling from the LE/Lys Compartment
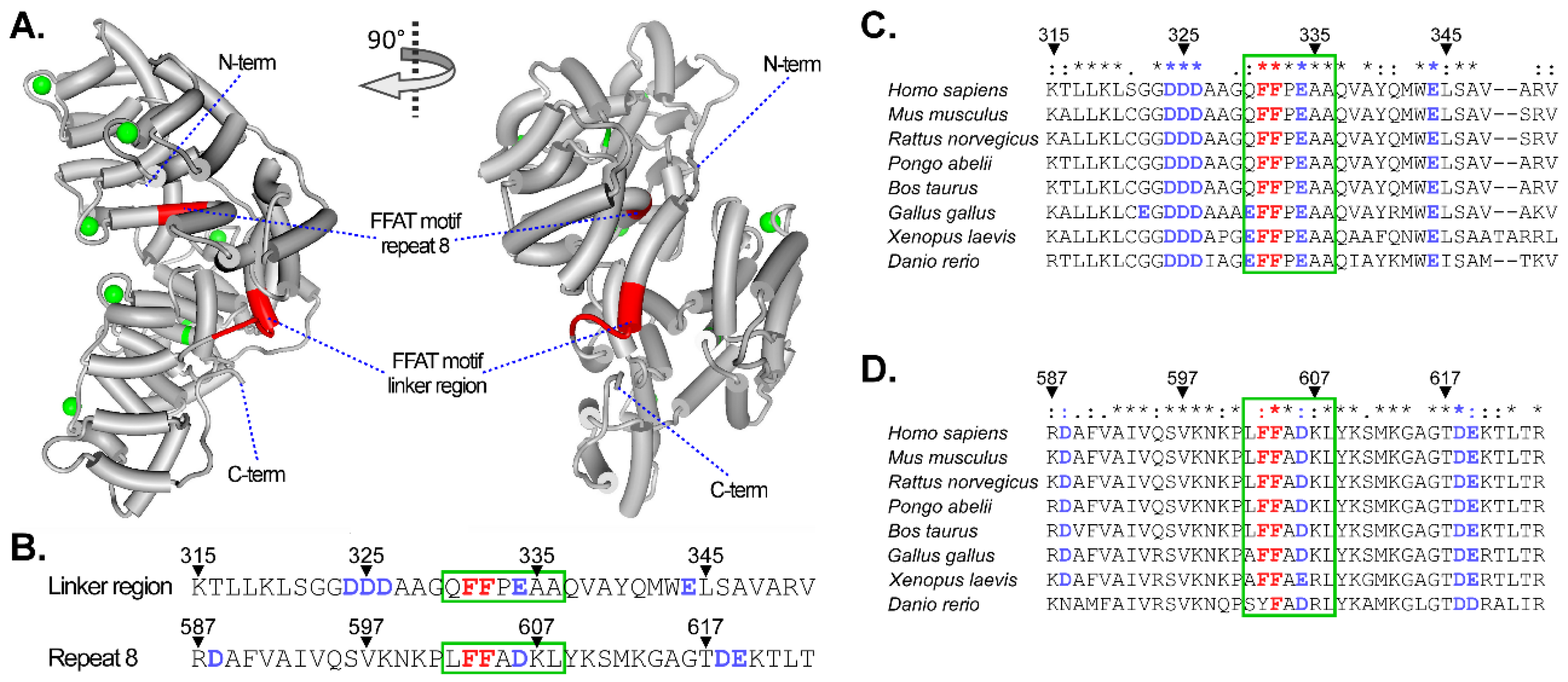
6. Annexins and Membrane Contact Sites: Close Encounters at the Interface of LE and the ER
7. Annexins and Biogenesis of Exosomes
8. Annexins and LE/Lys Positioning
9. Concluding Remarks and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Enrich, C.; Rentero, C.; de Muga, S.V.; Reverter, M.; Mulay, V.; Wood, P.; Koese, M.; Grewal, T. Annexin a6-linking Ca2+ signaling with cholesterol transport. Biochim. Biophys. Acta 2011, 1813, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Benz, J.; Bergner, A.; Hofmann, A.; Demange, P.; Gottig, P.; Liemann, S.; Huber, R.; Voges, D. The structure of recombinant human Annexin VI in crystals and membrane-bound. J. Mol. Biol. 1996, 260, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Avila-Sakar, A.J.; Creutz, C.E.; Kretsinger, R.H. Crystal structure of bovine Annexin VI in a calcium-bound state. Biochim. Biophys. Acta 1998, 1387, 103–116. [Google Scholar] [CrossRef]
- Avila-Sakar, A.J.; Kretsinger, R.H.; Creutz, C.E. Membrane-bound 3D structures reveal the intrinsic flexibility of Annexin VI. J. Struct. Biol. 2000, 130, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.J.; Crumpton, M.J. Isolation and characterization of a novel 68,000-Mr Ca2+-binding protein of lymphocyte plasma membrane. Biochem. J. 1984, 219, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Moss, S.E. Annexins and membrane dynamics. Biochim. Biophys. Acta 1997, 1357, 129–154. [Google Scholar] [CrossRef]
- Grewal, T.; Enrich, C. Molecular mechanisms involved in Ras inactivation: The Annexin A6-p120GAP complex. Bioessays 2006, 28, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Enrich, C. Annexins—Modulators of EGF receptor signalling and trafficking. Cell Signal 2009, 21, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Babiychuk, E.B.; Hostettler, A.; Rescher, U.; Draeger, A. Annexins as intracellular calcium sensors. Cell Calcium 2007, 41, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Babiychuk, E.B.; Draeger, A. The Annexins: Spatial and temporal coordination of signaling events during cellular stress. Cell Mol. Life Sci. 2009, 66, 2623–2642. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Rescher, U.; Gerke, V.; Moss, S.E. Annexin-actin interactions. Traffic 2004, 5, 571–576. [Google Scholar] [CrossRef] [PubMed]
- McNeil, A.K.; Rescher, U.; Gerke, V.; McNeil, P.L. Requirement for Annexin A1 in plasma membrane repair. J. Biol. Chem. 2006, 281, 35202–35207. [Google Scholar] [CrossRef] [PubMed]
- Raynal, P.; Pollard, H.B. Annexins: The problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim. Biophys. Acta 1994, 1197, 63–93. [Google Scholar] [CrossRef]
- Kourie, J.I.; Wood, H.B. Biophysical and molecular properties of Annexin-formed channels. Prog. Biophys. Mol. Biol. 2000, 73, 91–134. [Google Scholar] [CrossRef]
- Golczak, M.; Kicinska, A.; Bandorowicz-Pikula, J.; Buchet, R.; Szewczyk, A.; Pikula, S. Acidic ph-induced folding of Annexin VI is a prerequisite for its insertion into lipid bilayers and formation of ion channels by the protein molecules. FASEB J. 2001, 15, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Langen, R.; Isas, J.M.; Hubbell, W.L.; Haigler, H.T. A transmembrane form of Annexin XII detected by site-directed spin labeling. Proc. Natl. Acad. Sci. USA 1998, 95, 14060–14065. [Google Scholar] [CrossRef] [PubMed]
- Isas, J.M.; Cartailler, J.P.; Sokolov, Y.; Patel, D.R.; Langen, R.; Luecke, H.; Hall, J.E.; Haigler, H.T. Annexins V and XII insert into bilayers at mildly acidic pH and form ion channels. Biochemistry 2000, 39, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Hegde, B.G.; Isas, J.M.; Zampighi, G.; Haigler, H.T.; Langen, R. A novel calcium-independent peripheral membrane-bound form of Annexin B12. Biochemistry 2006, 45, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Kirilenko, A.; Pikula, S.; Bandorowicz-Pikula, J. Effects of mutagenesis of W343 in human Annexin A6 isoform 1 on its interaction with GTP: Nucleotide-induced oligomer formation and ion channel activity. Biochemistry 2006, 45, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Berendes, R.; Burger, A.; Schneider, M.; Karshikov, A.; Luecke, H.; Romisch, J.; Paques, E. Crystal and molecular structure of human Annexin V after refinement. Implications for structure, membrane binding and ion channel formation of the Annexin family of proteins. J. Mol. Biol. 1992, 223, 683–704. [Google Scholar] [CrossRef]
- Hawkins, T.E.; Merrifield, C.J.; Moss, S.E. Calcium signaling and Annexins. Cell Biochem. Biophys. 2000, 33, 275–296. [Google Scholar] [CrossRef]
- Enrich, C.; Rentero, C.; Meneses-Salas, E.; Tebar, F.; Grewal, T. Annexins: Ca2+ effectors determining membrane trafficking in the late endocytic compartment. Adv. Exp. Med. Biol. 2017, 981, 351–385. [Google Scholar] [PubMed]
- Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell. Biol. 2010, 11, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.P.; Patel, S. Signalling at membrane contact sites: Two membranes come together to handle second messengers. Curr. Opin. Cell Biol. 2016, 39, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Pryor, P.R.; Mullock, B.M.; Bright, N.A.; Gray, S.R.; Luzio, J.P. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000, 149, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Klumperman, J.; Raposo, G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb. Perspect Biol. 2014, 6, a016857. [Google Scholar] [CrossRef] [PubMed]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Spits, M.; Neefjes, J.; Berlin, I. The EGFR odyssey—From activation to destruction in space and time. J. Cell Sci. 2017, 130, 4087–4096. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J.; Griffiths, G.; Howell, K.E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol. 1989, 108, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Toret, C.P.; Drubin, D.G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006, 7, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Rab gtpases: Master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell 2017, 28, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Wandinger-Ness, A.; Zerial, M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb. Perspect. Biol. 2014, 6, a022616. [Google Scholar] [CrossRef] [PubMed]
- White, I.J.; Bailey, L.M.; Aghakhani, M.R.; Moss, S.E.; Futter, C.E. EGF stimulates Annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.R.; Sanchez-Heras, E.; Tsapara, A.; Sobota, A.; Levine, T.P.; Futter, C.E. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev. Cell 2016, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Emans, N.; Gorvel, J.P.; Walter, C.; Gerke, V.; Kellner, R.; Griffiths, G.; Gruenberg, J. Annexin ii is a major component of fusogenic endosomal vesicles. J. Cell Biol. 1993, 120, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Ghislat, G.; Knecht, E. New Ca2+-dependent regulators of autophagosome maturation. Commun. Integr. Biol. 2012, 5, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E.; White, I.J. Annexins and endocytosis. Traffic 2007, 8, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U.; Gerke, V. Annexins—Unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Boye, T.L.; Maeda, K.; Pezeshkian, W.; Sonder, S.L.; Haeger, S.C.; Gerke, V.; Simonsen, A.C.; Nylandsted, J. Annexin A4 and A6 induce membrane curvature and constriction during cell membrane repair. Nat. Commun. 2017, 8, 1623. [Google Scholar] [CrossRef] [PubMed]
- Drucker, P.; Pejic, M.; Galla, H.J.; Gerke, V. Lipid segregation and membrane budding induced by the peripheral membrane binding protein Annexin A2. J. Biol. Chem. 2013, 288, 24764–24776. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, D.; Gerke, V.; Heuer, A. Modeling of Annexin A2-membrane interactions by molecular dynamics simulations. PLoS ONE 2017, 12, e0185440. [Google Scholar] [CrossRef] [PubMed]
- Morozova, K.; Sridhar, S.; Zolla, V.; Clement, C.C.; Scharf, B.; Verzani, Z.; Diaz, A.; Larocca, J.N.; Hajjar, K.A.; Cuervo, A.M.; et al. Annexin A2 promotes phagophore assembly by enhancing Atg16L+ vesicle biogenesis and homotypic fusion. Nat. Commun. 2015, 6, 5856. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, V.; Poeter, M.; Zeuschner, D.; Gerke, V.; Rescher, U. Annexin A8 regulates late endosome organization and function. Mol. Biol. Cell 2008, 19, 5267–5278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goebeler, V.; Ruhe, D.; Gerke, V.; Rescher, U. Annexin A8 displays unique phospholipid and F-actin binding properties. FEBS Lett. 2006, 580, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U.; Ruhe, D.; Ludwig, C.; Zobiack, N.; Gerke, V. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J. Cell Sci. 2004, 117, 3473–3480. [Google Scholar] [CrossRef] [PubMed]
- Bayer, N.; Schober, D.; Prchla, E.; Murphy, R.F.; Blaas, D.; Fuchs, R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: Implications for viral uncoating and infection. J. Virol. 1998, 72, 9645–9655. [Google Scholar] [PubMed]
- Falguieres, T.; Luyet, P.P.; Bissig, C.; Scott, C.C.; Velluz, M.C.; Gruenberg, J. In vitro budding of intralumenal vesicles into late endosomes is regulated by Alix and Tsg101. Mol. Biol. Cell 2008, 19, 4942–4955. [Google Scholar] [CrossRef] [PubMed]
- Fairn, G.D.; Schieber, N.L.; Ariotti, N.; Murphy, S.; Kuerschner, L.; Webb, R.I.; Grinstein, S.; Parton, R.G. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J. Cell Biol. 2011, 194, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Gilbert, G.E.; Shi, J.; Silvius, J.; Kapus, A.; Grinstein, S. Membrane phosphatidylserine regulates surface charge and protein localization. Science 2008, 319, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E.; Felder, S.; Schlessinger, J.; Ullrich, A.; Hopkins, C.R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J. Cell Biol. 1993, 120, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Kellner, R.; Parton, R.G.; Gruenberg, J. Specific release of membrane-bound Annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol. Biol. Cell. 1997, 8, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tao, J.Q.; Zimmerman, U.J. Annexin II binds to the membrane of A549 cells in a calcium-dependent and calcium-independent manner. Cell Signal 1997, 9, 299–304. [Google Scholar] [CrossRef]
- Trotter, P.J.; Orchard, M.A.; Walker, J.H. EGTA-resistant binding of Annexin V to platelet membranes can be induced by physiological calcium concentrations. Biochem. Soc. Trans. 1995, 23, 37S. [Google Scholar] [CrossRef] [PubMed]
- Mayran, N.; Parton, R.G.; Gruenberg, J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 2003, 22, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Gruenberg, J. The p11/s100a10 light chain of Annexin A2 is dispensable for Annexin A2 association to endosomes and functions in endosomal transport. PLoS ONE 2007, 2, e1118. [Google Scholar] [CrossRef] [PubMed]
- Morel, E.; Parton, R.G.; Gruenberg, J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev. Cell 2009, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Poeter, M.; Radke, S.; Koese, M.; Hessner, F.; Hegemann, A.; Musiol, A.; Gerke, V.; Grewal, T.; Rescher, U. Disruption of the Annexin A1/S100A11 complex increases the migration and clonogenic growth by dysregulating epithelial growth factor (EGF) signaling. Biochim. Biophys. Acta 2013, 1833, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- De Diego, I.; Schwartz, F.; Siegfried, H.; Dauterstedt, P.; Heeren, J.; Beisiegel, U.; Enrich, C.; Grewal, T. Cholesterol modulates the membrane binding and intracellular distribution of Annexin 6. J. Biol. Chem. 2002, 277, 32187–32194. [Google Scholar] [CrossRef] [PubMed]
- Domon, M.M.; Besson, F.; Tylki-Szymanska, A.; Bandorowicz-Pikula, J.; Pikula, S. Interaction of AnxA6 with isolated and artificial lipid microdomains; importance of lipid composition and calcium content. Mol. Biosyst. 2013, 9, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Sanmartin, J. Cholesterol enhances phospholipid binding and aggregation of Annexins by their core domain. Biochem. Biophys. Res. Commun. 2001, 283, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Sanmartin, J.; Henry, J.P.; Pradel, L.A. Cholesterol regulates membrane binding and aggregation by Annexin 2 at submicromolar Ca2+ concentration. Biochim. Biophys. Acta 2001, 1510, 18–28. [Google Scholar] [CrossRef]
- Junker, M.; Creutz, C.E. Endonexin (Annexin IV)-mediated lateral segregation of phosphatidylglycerol in phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry 1993, 32, 9968–9974. [Google Scholar] [CrossRef] [PubMed]
- Menke, M.; Ross, M.; Gerke, V.; Steinem, C. The molecular arrangement of membrane-bound Annexin A2-S100A10 tetramer as revealed by scanning force microscopy. Chembiochem 2004, 5, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Lin-Moshier, Y.; Keebler, M.V.; Hooper, R.; Boulware, M.J.; Liu, X.; Churamani, D.; Abood, M.E.; Walseth, T.F.; Brailoiu, E.; Patel, S.; et al. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. USA 2014, 111, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Enrich, C.; Rentero, C.; Hierro, A.; Grewal, T. Role of cholesterol in SNARE-mediated trafficking on intracellular membranes. J. Cell Sci. 2015, 128, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Melero, A.; Reverter, M.; Hoque, M.; Meneses-Salas, E.; Koese, M.; Conway, J.R.; Johnsen, C.H.; Alvarez-Guaita, A.; Morales-Paytuvi, F.; Elmaghrabi, Y.A.; et al. Annexin A6 and late endosomal cholesterol modulate integrin recycling and cell migration. J. Biol. Chem. 2016, 291, 1320–1335. [Google Scholar] [CrossRef] [PubMed]
- Te Vruchte, D.; Lloyd-Evans, E.; Veldman, R.J.; Neville, D.C.; Dwek, R.A.; Platt, F.M.; van Blitterswijk, W.J.; Sillence, D.J. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J. Biol. Chem. 2004, 279, 26167–26175. [Google Scholar] [CrossRef] [PubMed]
- Drucker, P.; Pejic, M.; Grill, D.; Galla, H.J.; Gerke, V. Cooperative binding of Annexin A2 to cholesterol- and phosphatidylinositol-4,5-bisphosphate-containing bilayers. Biophys. J. 2014, 107, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Hulce, J.J.; Cognetta, A.B.; Niphakis, M.J.; Tully, S.E.; Cravatt, B.F. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods 2013, 10, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Cornely, R.; Rentero, C.; Enrich, C.; Grewal, T.; Gaus, K. Annexin A6 is an organizer of membrane microdomains to regulate receptor localization and signalling. IUBMB life 2011, 63, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, L.; Yang, H.; Song, B.L. Routes and mechanisms of post-endosomal cholesterol trafficking: A story that never ends. Traffic 2017, 18, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Chang, C.C.; Ohgami, N.; Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.J.; Voeltz, G.K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 2016, 17, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Wenzel, E.M.; Stenmark, H. ER-endosome contact sites: Molecular compositions and functions. EMBO J. 2015, 34, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Zobiack, N.; Rescher, U.; Ludwig, C.; Zeuschner, D.; Gerke, V. The Annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 2003, 14, 4896–4908. [Google Scholar] [CrossRef] [PubMed]
- Cubells, L.; Vila de Muga, S.; Tebar, F.; Wood, P.; Evans, R.; Ingelmo-Torres, M.; Calvo, M.; Gaus, K.; Pol, A.; Grewal, T.; et al. Annexin A6-induced alterations in cholesterol transport and caveolin export from the Golgi complex. Traffic 2007, 8, 1568–1589. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Rentero, C.; de Muga, S.V.; Alvarez-Guaita, A.; Mulay, V.; Cairns, R.; Wood, P.; Monastyrskaya, K.; Pol, A.; Tebar, F.; et al. Cholesterol transport from late endosomes to the Golgi regulates t-SNARE trafficking, assembly, and function. Mol. Biol. Cell 2011, 22, 4108–4123. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Rentero, C.; Garcia-Melero, A.; Hoque, M.; Vila de Muga, S.; Alvarez-Guaita, A.; Conway, J.R.; Wood, P.; Cairns, R.; Lykopoulou, L.; et al. Cholesterol regulates Syntaxin 6 trafficking at trans-Golgi network endosomal boundaries. Cell Rep. 2014, 7, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Kreutzberger, A.J.B.; Lee, J.; Kiessling, V.; Tamm, L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Urano, Y.; Watanabe, H.; Murphy, S.R.; Shibuya, Y.; Geng, Y.; Peden, A.A.; Chang, C.C.; Chang, T.Y. Transport of LDL-derived cholesterol from the NPC1 compartment to the ER involves the trans-Golgi network and the SNARE protein complex. Proc. Natl. Acad. Sci. USA 2008, 105, 16513–16518. [Google Scholar] [CrossRef] [PubMed]
- Umbrecht-Jenck, E.; Demais, V.; Calco, V.; Bailly, Y.; Bader, M.F.; Chasserot-Golaz, S. S100A10-mediated translocation of Annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic 2010, 11, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Guaita, A.; Vila de Muga, S.; Owen, D.M.; Williamson, D.; Magenau, A.; Garcia-Melero, A.; Reverter, M.; Hoque, M.; Cairns, R.; Cornely, R.; et al. Evidence for Annexin A6-dependent plasma membrane remodelling of lipid domains. Br. J. Pharmacol. 2015, 172, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Sztolsztener, M.E.; Dobrzyn, A.; Pikula, S.; Tylki-Szymanska, A.; Bandorowicz-Pikula, J. Impaired dynamics of the late endosome/lysosome compartment in human Niemann-Pick type C skin fibroblasts carrying mutation in NPC1 gene. Mol. Biosyst. 2012, 8, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Heitzig, N.; Kuhnl, A.; Grill, D.; Ludewig, K.; Schloer, S.; Galla, H.J.; Grewal, T.; Gerke, V.; Rescher, U. Cooperative binding promotes demand-driven recruitment of AnxA8 to cholesterol-containing membranes. Biochim. Biophys. Acta 2018, 1863, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Tebar, F.; Gelabert-Baldrich, M.; Hoque, M.; Cairns, R.; Rentero, C.; Pol, A.; Grewal, T.; Enrich, C. Annexins and endosomal signaling. Methods Enzymol. 2014, 535, 55–74. [Google Scholar]
- Flinn, R.J.; Backer, J.M. mTORC1 signals from late endosomes: Taking a TOR of the endocytic system. Cell Cycle 2010, 9, 1869–1870. [Google Scholar] [CrossRef] [PubMed]
- Amaya, C.; Colombo, M.I. Protein interactions in the last steps of the endosomal degradative pathway. J. Cell Commun. Signal. 2017, 2, 5. [Google Scholar]
- Mizuno-Yamasaki, E.; Rivera-Molina, F.; Novick, P. Gtpase networks in membrane traffic. Annu. Rev. Biochem. 2012, 81, 637–659. [Google Scholar] [CrossRef] [PubMed]
- Kloer, D.P.; Rojas, R.; Ivan, V.; Moriyama, K.; van Vlijmen, T.; Murthy, N.; Ghirlando, R.; van der Sluijs, P.; Hurley, J.H.; Bonifacino, J.S. Assembly of the biogenesis of lysosome-related organelles complex-3 (BLOC-3) and its interaction with Rab9. J. Biol. Chem. 2010, 285, 7794–7804. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.A.; Soldati, T.; Shapiro, A.D.; Lin, J.; Pfeffer, S.R. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J. Cell Biol. 1994, 125, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Ganley, I.G.; Pfeffer, S.R. Cholesterol accumulation sequesters Rab9 and disrupts late endosome function in NPC1-deficient cells. J. Biol. Chem. 2006, 281, 17890–17899. [Google Scholar] [CrossRef] [PubMed]
- Lebrand, C.; Corti, M.; Goodson, H.; Cosson, P.; Cavalli, V.; Mayran, N.; Faure, J.; Gruenberg, J. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 2002, 21, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Sudhof, T.C.; Anderson, R.G. Annexin VI is required for budding of clathrin-coated pits. Cell 1992, 70, 283–291. [Google Scholar] [CrossRef]
- Trischler, M.; Stoorvogel, W.; Ullrich, O. Biochemical analysis of distinct Rab5- and Rab11-positive endosomes along the transferrin pathway. J. Cell Sci. 1999, 112, 4773–4783. [Google Scholar] [PubMed]
- Creutz, C.E. The Annexins and exocytosis. Science 1992, 258, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M.R.; Moss, S.E.; Crumpton, M.J. Diversity in the lipocortin/calpactin family. Cell 1988, 55, 1–3. [Google Scholar] [CrossRef]
- Meers, P.; Hong, K.L.; Papaphadjopoulos, D. Role of specific lipids and Annexins in calcium-dependent membrane fusion. Ann. N. Y. Acad. Sci. 1991, 635, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.K.; Huang, F.; Kim, W.; Gygi, S.; Sorkin, A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J. Cell Biol. 2010, 189, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, A.; von Zastrow, M. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009, 10, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Clague, M.J.; Urbe, S. The interface of receptor trafficking and signalling. J. Cell Sci. 2001, 114, 3075–3081. [Google Scholar] [PubMed]
- Huang, F.; Jiang, X.; Sorkin, A. Tyrosine phosphorylation of the β2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J. Biol. Chem. 2003, 278, 43411–43417. [Google Scholar] [CrossRef] [PubMed]
- Rappoport, J.Z.; Simon, S.M. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J. Cell Sci. 2009, 122, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, S.; Salcini, A.E.; Puri, C.; Tacchetti, C.; Di Fiore, P.P. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J. Cell Biol. 2000, 150, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Koese, M.; Rentero, C.; Enrich, C. Annexin A6-regulator of the EGFR/Ras signalling pathway and cholesterol homeostasis. Int. J. Biochem. Cell Biol. 2010, 42, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Evans, R.; Rentero, C.; Tebar, F.; Cubells, L.; de Diego, I.; Kirchhoff, M.F.; Hughes, W.E.; Heeren, J.; Rye, K.A.; et al. Annexin A6 stimulates the membrane recruitment of p120GAP to modulate Ras and raf-1 activity. Oncogene 2005, 24, 5809–5820. [Google Scholar] [CrossRef] [PubMed]
- Koese, M.; Rentero, C.; Kota, B.P.; Hoque, M.; Cairns, R.; Wood, P.; Vila de Muga, S.; Reverter, M.; Alvarez-Guaita, A.; Monastyrskaya, K.; et al. Annexin A6 is a scaffold for pkcalpha to promote EGFR inactivation. Oncogene 2013, 32, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Vila de Muga, S.; Timpson, P.; Cubells, L.; Evans, R.; Hayes, T.E.; Rentero, C.; Hegemann, A.; Reverter, M.; Leschner, J.; Pol, A.; et al. Annexin A6 inhibits Ras signalling in breast cancer cells. Oncogene 2009, 28, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.R.; White, I.J.; Tsapara, A.; Futter, C.E. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat. Cell Biol. 2010, 12, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Nat. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mrna translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Thelen, A.M.; Zoncu, R. Emerging roles for the lysosome in lipid metabolism. Trends Cell Biol. 2017, 27, 833–850. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The Rag GTPase bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef] [PubMed]
- Duvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Goberdhan, D.C.; Wilson, C.; Harris, A.L. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metab. 2016, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Ogmundsdottir, M.H.; Heublein, S.; Kazi, S.; Reynolds, B.; Visvalingam, S.M.; Shaw, M.K.; Goberdhan, D.C. Proton-assisted amino acid transporter Pat1 complexes with Rag GTPase and activates TORC1 on late endosomal and lysosomal membranes. PLoS ONE 2012, 7, e36616. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dang, Y.; Ren, Y.R.; Liu, J.O. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4764–4769. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008, 14, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Castellano, B.M.; Thelen, A.M.; Moldavski, O.; Feltes, M.; van der Welle, R.E.; Mydock-McGrane, L.; Jiang, X.; van Eijkeren, R.J.; Davis, O.B.; Louie, S.M.; et al. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 2017, 355, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; de Araujo, M.E.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 2015, 519, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef] [PubMed]
- West, M.; Zurek, N.; Hoenger, A.; Voeltz, G.K. A 3d analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. J. Cell Biol. 2011, 193, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.R. The formation and function of ER-endosome membrane contact sites. Biochim. Biophys. Acta 2016, 1861, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Helle, S.C.; Kanfer, G.; Kolar, K.; Lang, A.; Michel, A.H.; Kornmann, B. Organization and function of membrane contact sites. Biochim. Biophys. Acta 2013, 1833, 2526–2541. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Roberts, P.; Chen, Y.; Kvam, E.; Shulga, N.; Huang, K.; Lemmon, S.; Goldfarb, D.S. Nucleus-vacuole junctions in saccharomyces cerevisiae are formed through the direct interaction of Vac8p with nvj1p. Mol. Biol. Cell 2000, 11, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 glued and late endosome positioning. J. Cell Biol. 2009, 185, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Rocha, N.; Zwart, W.; Jordens, I.; Janssen, L.; Kuijl, C.; Olkkonen, V.M.; Neefjes, J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RIPL-p150glued, ORP1L, and the receptor betalll spectrin. J. Cell Biol. 2007, 176, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Wenzel, E.M.; Pedersen, N.M.; Olsvik, H.; Schink, K.O.; Schultz, S.W.; Vietri, M.; Nisi, V.; Bucci, C.; Brech, A.; et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature 2015, 520, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Van der Kant, R.; Fish, A.; Janssen, L.; Janssen, H.; Krom, S.; Ho, N.; Brummelkamp, T.; Carette, J.; Rocha, N.; Neefjes, J. Late endosomal transport and tethering are coupled processes controlled by RIPL and the cholesterol sensor ORP1L. J. Cell Sci. 2013, 126, 3462–3474. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, T.; Uronen, R.L.; Wohlfahrt, G.; Bjorkhem, I.; Ikonen, E.; Olkkonen, V.M. Sterol binding by OSBP-related protein 1L regulates late endosome motility and function. Cell Mol. Life Sci. 2011, 68, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Rowland, A.A.; Chitwood, P.J.; Phillips, M.J.; Voeltz, G.K. ER contact sites define the position and timing of endosome fission. Cell 2014, 159, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Dibenedetto, J.R.; West, M.; Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 2013, 24, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Antonny, B.; Drin, G. Insights into the mechanisms of sterol transport between organelles. Cell Mol. Life Sci. 2013, 70, 3405–3421. [Google Scholar] [CrossRef] [PubMed]
- Van der Kant, R.; Neefjes, J. Small regulators, major consequences—Ca2+ and cholesterol at the endosome-ER interface. J. Cell Sci. 2014, 127, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Haj, F.G.; Sabet, O.; Kinkhabwala, A.; Wimmer-Kleikamp, S.; Roukos, V.; Han, H.M.; Grabenbauer, M.; Bierbaum, M.; Antony, C.; Neel, B.G.; et al. Regulation of signaling at regions of cell–cell contact by endoplasmic reticulum-bound protein-tyrosine phosphatase 1B. PLoS ONE 2012, 7, e36633. [Google Scholar] [CrossRef] [PubMed]
- Neefjes, J.; Jongsma, M.M.L.; Berlin, I. Stop or go? Endosome positioning in the establishment of compartment architecture, dynamics, and function. Trends Cell Biol. 2017, 27, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, S.G.; Peranen, J.; Ikonen, E. LDL-cholesterol transport to the endoplasmic reticulum: Current concepts. Curr. Opin. Lipidol. 2016, 27, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Brown, A.J.; Yang, H. Novel mechanisms of intracellular cholesterol transport: Oxysterol-binding proteins and membrane contact sites. Curr. Opin. Cell Biol. 2015, 35, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Wijdeven, R.H.; Jongsma, M.L.; Neefjes, J.; Berlin, I. ER contact sites direct late endosome transport. Bioessays 2015, 37, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Ridgway, N.D. Oxysterol-binding protein-related protein 1L regulates cholesterol egress from the endo-lysosomal system. Cell Rep. 2017, 19, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, L.P.; Tomasetto, C.; Alpy, F. Touche! STARD3 and STARD3NL tether the ER to endosomes. Biochem. Soc. Trans. 2016, 44, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Miwa, N.; Uebi, T.; Kawamura, S. S100-Annexin complexes—Biology of conditional association. FEBS J. 2008, 275, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- Rintala-Dempsey, A.C.; Rezvanpour, A.; Shaw, G.S. S100–Annexin complexes—Structural insights. FEBS J. 2008, 275, 4956–4966. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin A2 heterotetramer: Structure and function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.; Levine, T.P. A highly conserved binding site in vesicle-associated membrane protein-associated protein (VAP) for the FFAT motif of lipid-binding proteins. J. Biol. Chem. 2005, 280, 14097–14104. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.; Roy, A.; Levine, T.P. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 2003, 22, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Alpy, F.; Rousseau, A.; Schwab, Y.; Legueux, F.; Stoll, I.; Wendling, C.; Spiegelhalter, C.; Kessler, P.; Mathelin, C.; Rio, M.C.; et al. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J. Cell Sci. 2013, 126, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Levine, T.P. Vap, a versatile access point for the endoplasmic reticulum: Review and analysis of FFAT-like motifs in the vapome. Biochim. Biophys. Acta 2016, 1861, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Macias-Vidal, J.; Guerrero-Hernandez, M.; Estanyol, J.M.; Aguado, C.; Knecht, E.; Coll, M.J.; Bachs, O. Identification of lysosomal Npc1-binding proteins: Cathepsin D activity is regulated by NPC1. Proteomics 2016, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Torta, F.; Masai, K.; Lucast, L.; Czapla, H.; Tanner, L.B.; Narayanaswamy, P.; Wenk, M.R.; Nakatsu, F.; De Camilli, P. Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated er-plasma membrane contacts. Science 2015, 349, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kazim, A.S.; Brown, A.J.; Yang, H. An essential role of HRS/VPS27 in endosomal cholesterol trafficking. Cell Rep. 2012, 1, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Kumar, J.; Ferguson, C.; Schulz, T.A.; Ong, Y.S.; Hong, W.; Prinz, W.A.; Parton, R.G.; Brown, A.J.; Yang, H. A role for oxysterol-binding protein-related protein 5 in endosomal cholesterol trafficking. J. Cell Biol. 2011, 192, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Sanchez-Madrid, F. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, L.; Svetlova, M.; Fomina, A.F. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 2007, 212, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Falguieres, T.; Luyet, P.P.; Gruenberg, J. Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp. Cell. Res. 2009, 315, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Van der Goot, F.G.; Gruenberg, J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006, 16, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Sobo, K.; Le Blanc, I.; Luyet, P.P.; Fivaz, M.; Ferguson, C.; Parton, R.G.; Gruenberg, J.; van der Goot, F.G. Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS ONE 2007, 2, e851. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. ESCRTs are everywhere. EMBO J. 2015, 34, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009, 458, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Guo, Q.; Xia, B.; Zhang, Y.H.; Giesert, E.E.; Levy, S.; Zheng, J.J.; Zhang, X.A. Tetraspanins regulate the protrusive activities of cell membrane. Biochem. Biophys. Res. Commun. 2011, 415, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Chevallier, J.; Mayran, N.; Le Blanc, I.; Ferguson, C.; Faure, J.; Blanc, N.S.; Matile, S.; Dubochet, J.; Sadoul, R.; et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004, 303, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Desdin-Mico, G.; Mittelbrunn, M. Role of exosomes in the protection of cellular homeostasis. Cell Adhes. Migr. 2017, 11, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Carayon, K.; Chaoui, K.; Ronzier, E.; Lazar, I.; Bertrand-Michel, J.; Roques, V.; Balor, S.; Terce, F.; Lopez, A.; Salome, L.; et al. Proteolipidic composition of exosomes changes during reticulocyte maturation. J. Biol. Chem. 2011, 286, 34426–34439. [Google Scholar] [CrossRef] [PubMed]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.; Schwarzmann, G.; Mobius, W.; Hoernschemeyer, J.; Slot, J.W.; Geuze, H.J.; Stoorvogel, W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef] [PubMed]
- Boura, E.; Ivanov, V.; Carlson, L.A.; Mizuuchi, K.; Hurley, J.H. Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. J. Biol. Chem. 2012, 287, 28144–28151. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, J.; Chamoun, Z.; Jiang, G.; Prestwich, G.; Sakai, N.; Matile, S.; Parton, R.G.; Gruenberg, J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 2008, 283, 27871–27880. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.; Goebel, C.; Runz, H.; Mobius, W.; Weiss, S.; Feussner, I.; Simons, M.; Schneider, A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 2010, 285, 26279–26288. [Google Scholar] [CrossRef] [PubMed]
- Verweij, F.J.; Bebelman, M.P.; Jimenez, C.R.; Garcia-Vallejo, J.J.; Janssen, H.; Neefjes, J.; Knol, J.C.; de Goeij-de Haas, R.; Piersma, S.R.; Baglio, S.R.; et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell. Biol. 2018. [Google Scholar]
- Mathivanan, S. ExoCarta exosome database. Available online: http://exocarta.org (accessed on 1 January 2018).
- Wang, S.; Sun, H.; Tanowitz, M.; Liang, X.H.; Crooke, S.T. Annexin A2 facilitates endocytic trafficking of antisense oligonucleotides. Nucleic Acids Res. 2016, 44, 7314–7330. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.; Pol, A.; Biermer, M.; Jackle, S.; Enrich, C. Annexin VI defines an apical endocytic compartment in rat liver hepatocytes. J. Cell Sci. 1998, 111, 261–269. [Google Scholar] [PubMed]
- Kaushik, S.; Cuervo, A.M. Chaperone-mediated autophagy: A unique way to enter the lysosome world. Trends Cell Biol. 2012, 22, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Katsuda, T.; Gailhouste, L.; Kosaka, N.; Ochiya, T. Commitment of Annexin A2 in recruitment of micrornas into extracellular vesicles. FEBS Lett. 2015, 589, 4071–4078. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K. Functional association between regulatory RNAs and the Annexins. Int. J. Mol. Sci. 2018, 19, 591. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Guardia, C.M.; Keren-Kaplan, T.; Bonifacino, J.S. Mechanisms and functions of lysosome positioning. J. Cell. Sci. 2016, 129, 4329–4339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rydzewski, N.; Hider, A.; Zhang, X.; Yang, J.; Wang, W.; Gao, Q.; Cheng, X.; Xu, H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 2016, 18, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Noda, T.; Yoshimori, T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct. Funct. 2008, 33, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Dykes, S.S.; Gao, C.; Songock, W.K.; Bigelow, R.L.; Woude, G.V.; Bodily, J.M.; Cardelli, J.A. Zinc finger E-box binding homeobox-1 (Zeb1) drives anterograde lysosome trafficking and tumor cell invasion via upregulation of Na+/H+ Exchanger-1 (NHE1). Mol. Carcinog. 2017, 56, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, I.R.; Rainero, E.; Mitchell, L.E.; van den Berghe, P.V.; Speirs, C.; Dozynkiewicz, M.A.; Chaudhary, S.; Kalna, G.; Edwards, J.; Timpson, P.; et al. CLIC3 controls recycling of late endosomal MT1-MMP and dictates invasion and metastasis in breast cancer. J. Cell Sci. 2014, 127, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Marchesin, V.; Castro-Castro, A.; Lodillinsky, C.; Castagnino, A.; Cyrta, J.; Bonsang-Kitzis, H.; Fuhrmann, L.; Irondelle, M.; Infante, E.; Montagnac, G.; et al. ARF6-JIP3/4 regulate endosomal tubules for MT1-MMP exocytosis in cancer invasion. J Cell Biol. 2015, 211, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Guardia, C.M.; Pu, J.; Chen, Y.; Bonifacino, J.S. BORC coordinates encounter and fusion of lysosomes with autophagosomes. Autophagy 2017, 13, 1648–1663. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Ferreira, C.; Munro, S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev. Cell 2011, 21, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Wenzel, E.M.; Pedersen, N.M.; Stenmark, H. ER-endosome contact sites in endosome positioning and protrusion outgrowth. Biochem. Soc. Trans. 2016, 44, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, G.; Alifano, P.; Roberti, V.; Bruni, C.B.; Bucci, C. Rab-interacting lysosomal protein (RIPL): The Rab7 effector required for transport to lysosomes. EMBO J. 2001, 20, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Jordens, I.; Fernandez-Borja, M.; Marsman, M.; Dusseljee, S.; Janssen, L.; Calafat, J.; Janssen, H.; Wubbolts, R.; Neefjes, J. The Rab7 effector protein RIPL controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 2001, 11, 1680–1685. [Google Scholar] [CrossRef]
- Frasa, M.A.; Koessmeier, K.T.; Ahmadian, M.R.; Braga, V.M. Illuminating the functional and structural repertoire of human TBC/RABGAPs. Nat. Rev. Mol. Cell Biol. 2012, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Bucci, C. Multiple roles of the small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, T.; Patel, S.; Eden, E.R. Calcium signaling at ER membrane contact sites. Biochim. Biophys. Acta 2015, 1853, 2012–2017. [Google Scholar] [CrossRef] [PubMed]
- Derivery, E.; Sousa, C.; Gautier, J.J.; Lombard, B.; Loew, D.; Gautreau, A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell 2009, 17, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Duleh, S.N.; Welch, M.D. Wash and the Arp2/3 complex regulate endosome shape and trafficking. Cytoskeleton 2010, 67, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez, T.S.; Billadeau, D.D. A FAM21-containing Wash complex regulates retromer-dependent sorting. Dev. Cell 2009, 17, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Eskelinen, E.L.; Schmidt, C.K.; Neu, S.; Willenborg, M.; Fuertes, G.; Salvador, N.; Tanaka, Y.; Lullmann-Rauch, R.; Hartmann, D.; Heeren, J.; et al. Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol. Biol. Cell 2004, 15, 3132–3145. [Google Scholar] [CrossRef] [PubMed]
- Schneede, A.; Schmidt, C.K.; Holtta-Vuori, M.; Heeren, J.; Willenborg, M.; Blanz, J.; Domanskyy, M.; Breiden, B.; Brodesser, S.; Landgrebe, J.; et al. Role for LAMP-2 in endosomal cholesterol transport. J. Cell. Mol. Med. 2011, 15, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.K.; Eskelinen, E.L.; Scott, C.C.; Malevanets, A.; Saftig, P.; Grinstein, S. LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J. 2007, 26, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Krzewski, K.; Gil-Krzewska, A.; Nguyen, V.; Peruzzi, G.; Coligan, J.E. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood 2013, 121, 4672–4683. [Google Scholar] [CrossRef] [PubMed]
- Willett, R.; Martina, J.A.; Zewe, J.P.; Wills, R.; Hammond, G.R.V.; Puertollano, R. TFEB regulates lysosomal positioning by modulating TMEM55B expression and JIP4 recruitment to lysosomes. Nat. Commun. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Vergarajauregui, S.; Martina, J.A.; Puertollano, R. Identification of the penta-EF-hand protein ALG-2 as a Ca2+-dependent interactor of mucolipin-1. J. Biol. Chem. 2009, 284, 36357–36366. [Google Scholar] [CrossRef] [PubMed]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The bioplex network: A systematic exploration of the human interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Marchant, J.S.; Patel, S. Two-pore channels at the intersection of endolysosomal membrane traffic. Biochem. Soc. Trans. 2015, 43, 434–441. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentero, C.; Blanco-Muñoz, P.; Meneses-Salas, E.; Grewal, T.; Enrich, C. Annexins—Coordinators of Cholesterol Homeostasis in Endocytic Pathways. Int. J. Mol. Sci. 2018, 19, 1444. https://doi.org/10.3390/ijms19051444
Rentero C, Blanco-Muñoz P, Meneses-Salas E, Grewal T, Enrich C. Annexins—Coordinators of Cholesterol Homeostasis in Endocytic Pathways. International Journal of Molecular Sciences. 2018; 19(5):1444. https://doi.org/10.3390/ijms19051444
Chicago/Turabian StyleRentero, Carles, Patricia Blanco-Muñoz, Elsa Meneses-Salas, Thomas Grewal, and Carlos Enrich. 2018. "Annexins—Coordinators of Cholesterol Homeostasis in Endocytic Pathways" International Journal of Molecular Sciences 19, no. 5: 1444. https://doi.org/10.3390/ijms19051444
APA StyleRentero, C., Blanco-Muñoz, P., Meneses-Salas, E., Grewal, T., & Enrich, C. (2018). Annexins—Coordinators of Cholesterol Homeostasis in Endocytic Pathways. International Journal of Molecular Sciences, 19(5), 1444. https://doi.org/10.3390/ijms19051444