Meta-Analysis and Experimental Validation Identified FREM2 and SPRY1 as New Glioblastoma Marker Candidates
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Glioblastoma Datasets
2.2. Selection of New Glioblastoma Marker Candidates
2.3. Assessment of the Effectiveness of Selection Tests
2.4. Validation Tests with the Data from Other Datasets
2.5. Novelty Assessment of the Identified Marker Candidates
2.5.1. FREM2—Expression Patterns and Association with Disease
2.5.2. SPRY1—Expression Patterns and Association with Disease
2.5.3. Reported Relations between Glioblastoma Multiforme and FREM2/SPRY1
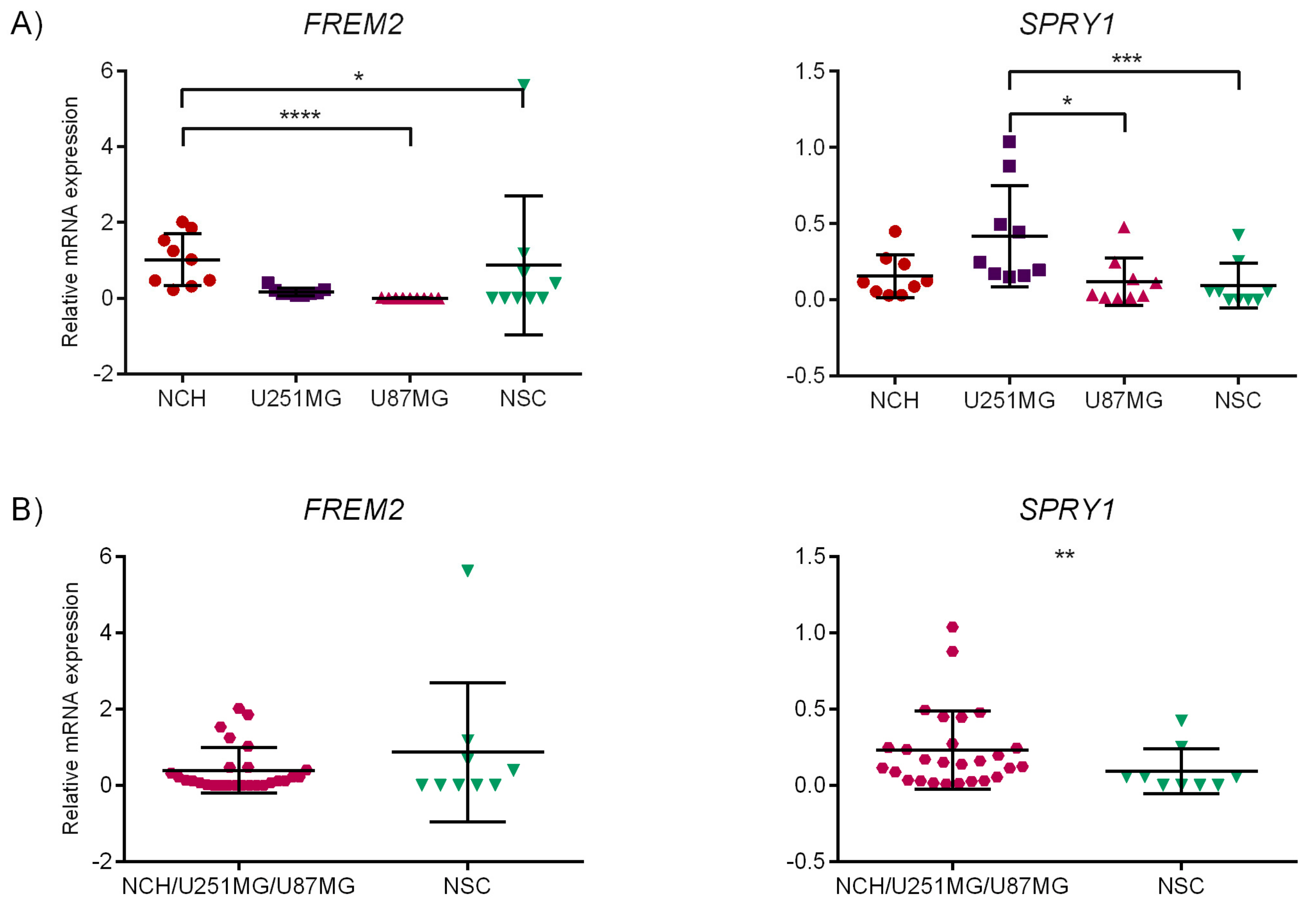
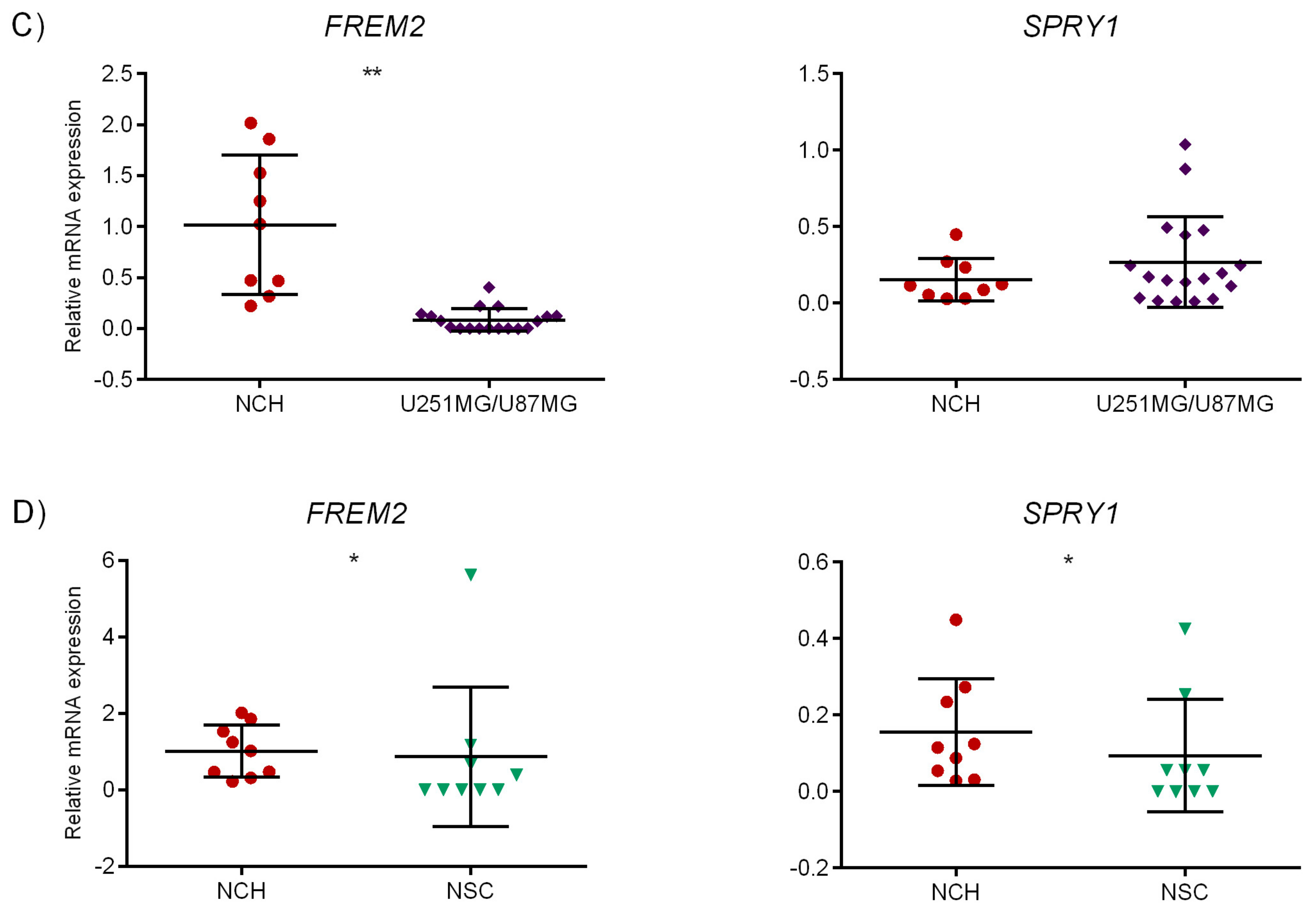
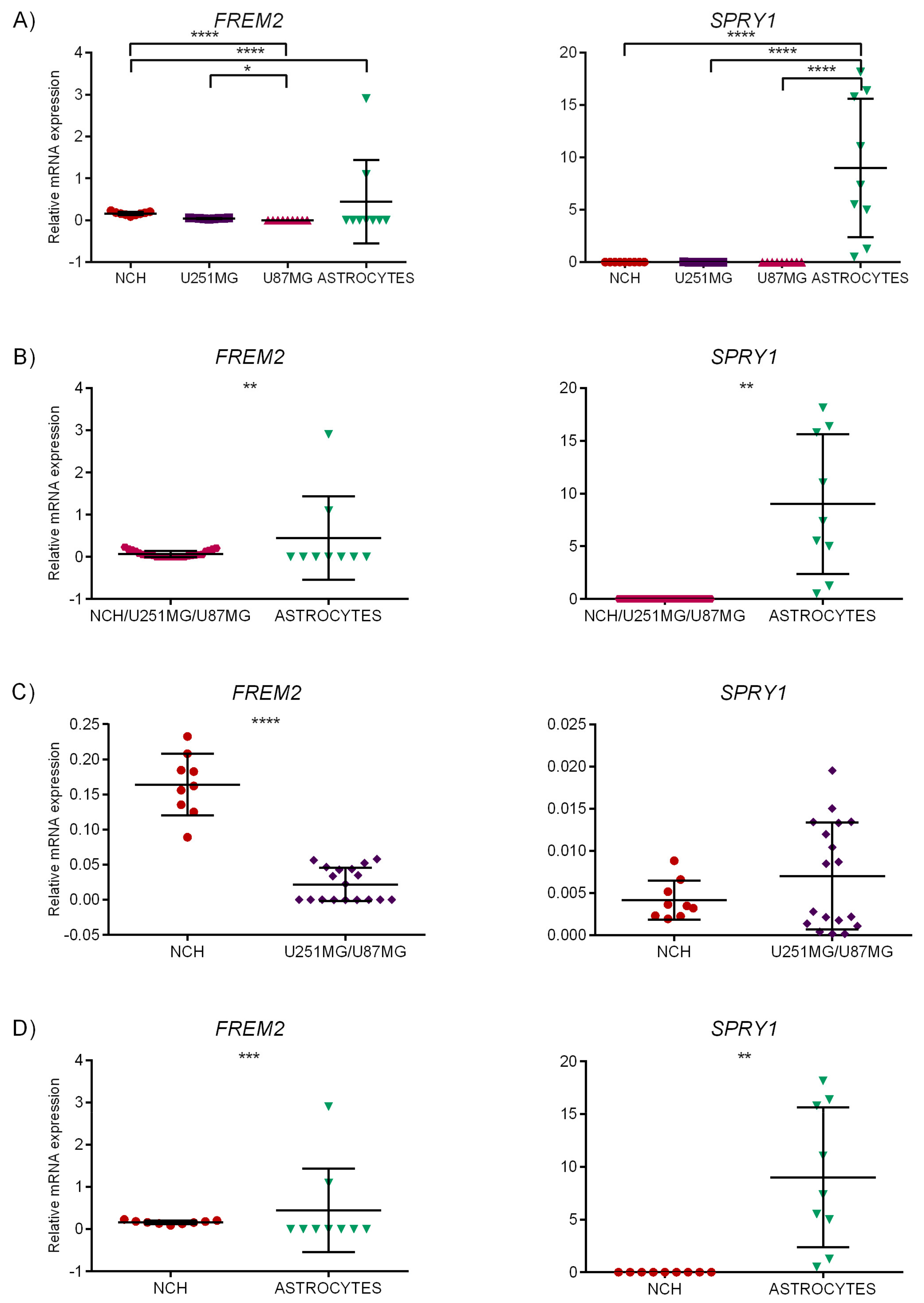
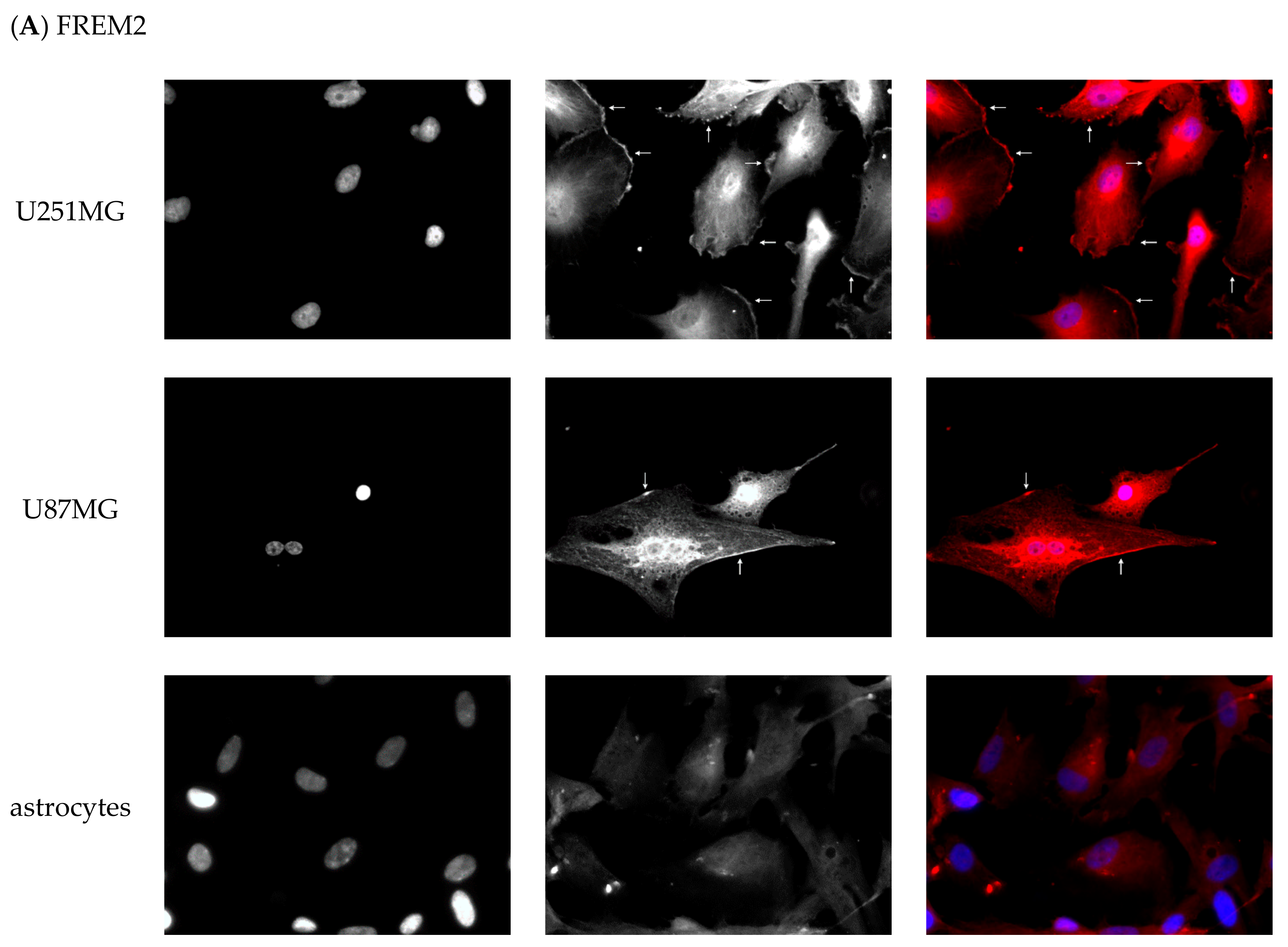
2.6. Experimental Validation of the Identified Marker Candidates
2.6.1. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
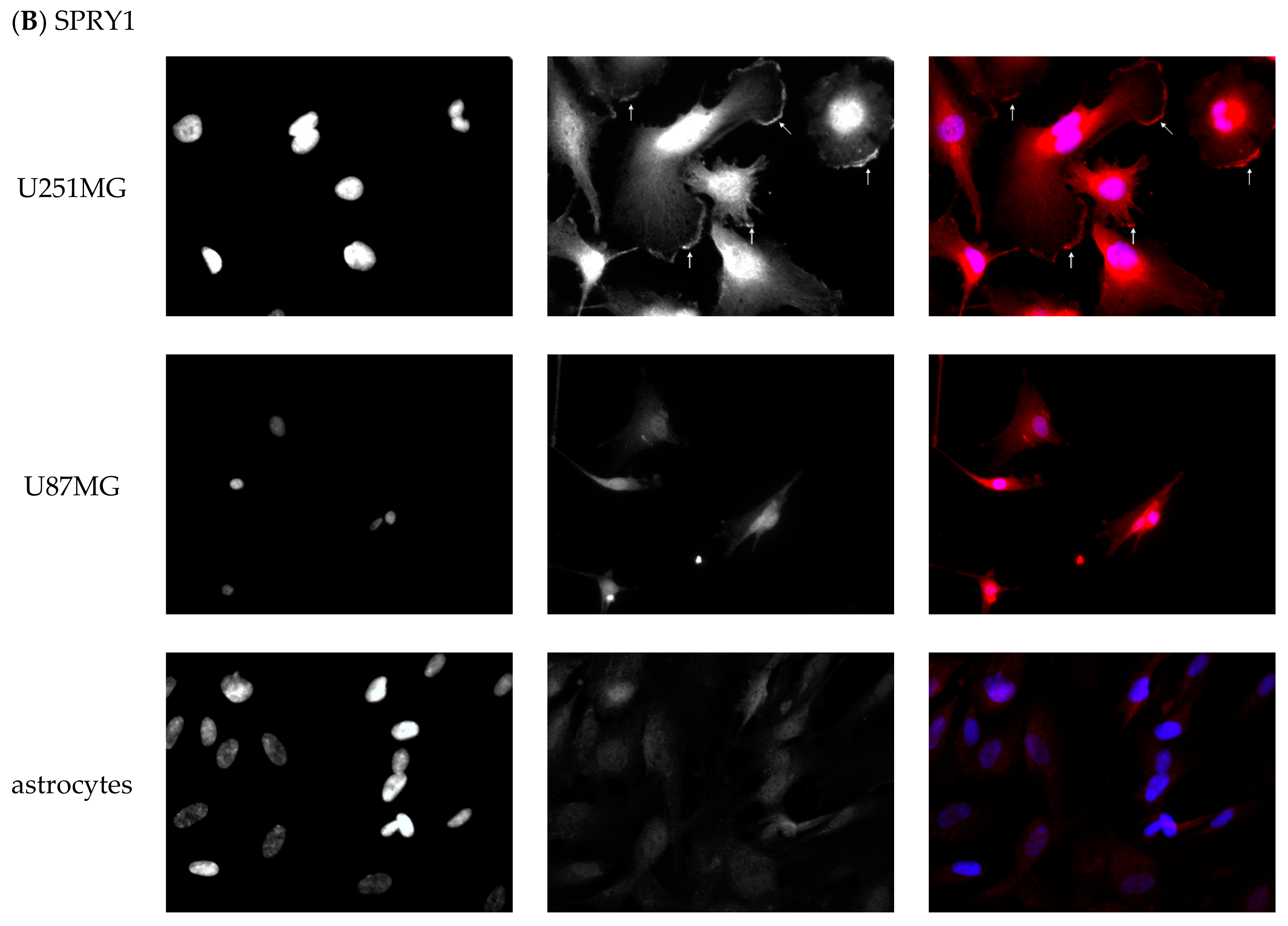
2.6.2. Immunofluorescent Staining
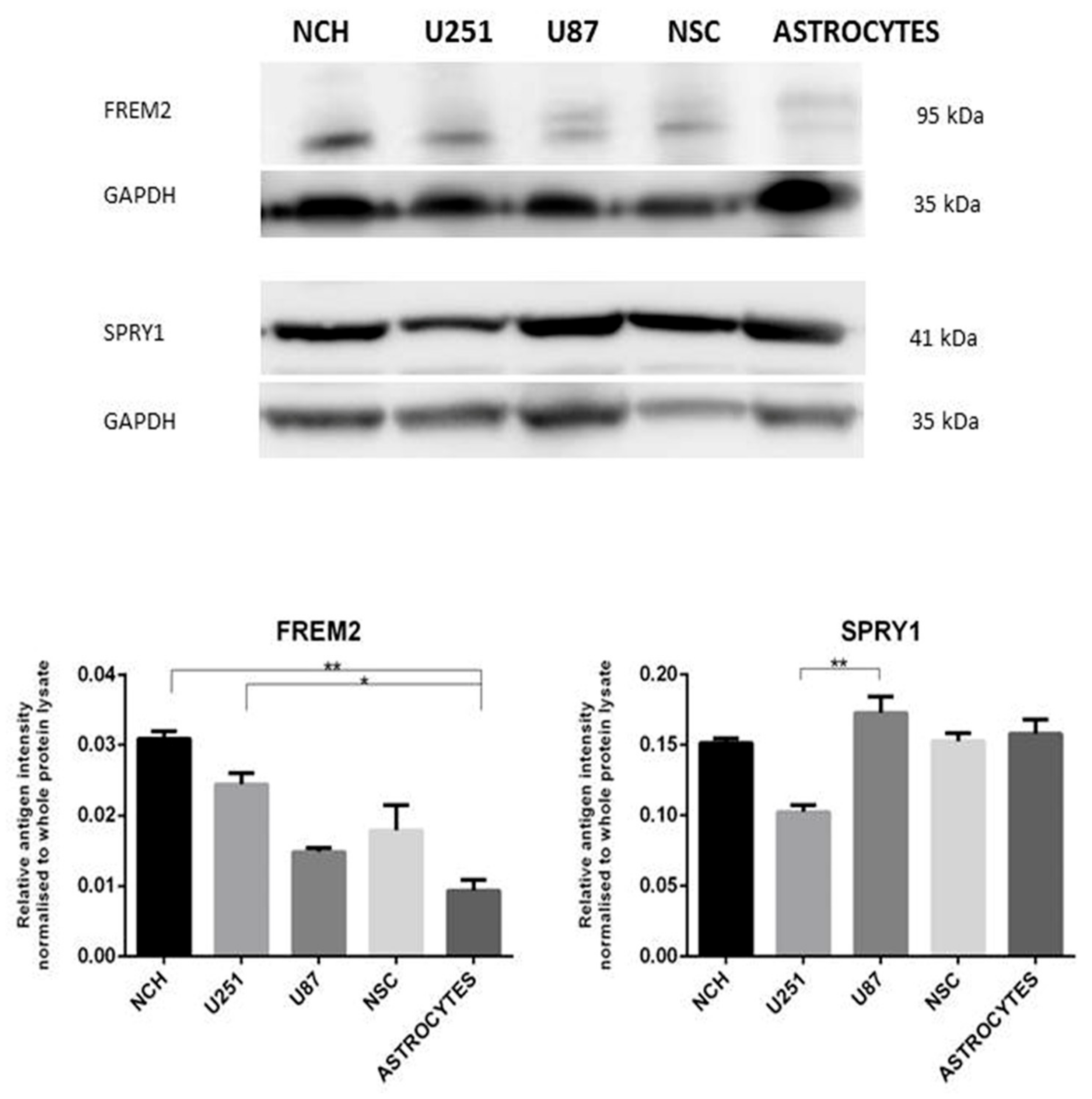
2.6.3. Western Blot Validation
3. Materials and Methods
3.1. Selection of Glioblastoma Datasets
3.2. Selection of New Marker Candidates
3.2.1. Differential Gene Expression Analysis with GEO2R
3.2.2. Analysis of Localization Data of Corresponding Proteins with COMPARTMENTS
3.3. Assessment of the Effectiveness of Selection Tests
3.4. Validation Tests with the Data from Other Datasets
3.5. Novelty Test of Genes Identified as Potential Markers
3.6. Experimental Validation
3.6.1. Cell Cultures
3.6.2. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
3.6.3. Immunofluorescent Staining
3.6.4. Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| APPT | average points per test |
| BSA | bovine serum albumin |
| CGCL | conventional glioblastoma cell lines |
| CSP | cell-surface protein |
| CSP gene | gene that encodes a cell-surface protein |
| CYC1 | cytochrome c1 |
| EGF(R) | epidermal growth factor (receptor) |
| EMBL-EBI | European Bioinformatics Institute |
| EMC7 | endoplasmatic reticulum membrane protein complex subunit 7 |
| FDR | false discovery rate |
| GB | glioblastoma multiforme |
| GEO | Gene Expression Omnibus |
| GPCR | G-protein coupled receptor |
| GSC | glioblastoma stem cells |
| GSCL | glioblastoma stem-like cell lines |
| H3F3A | H3 histone family 3A |
| HPRT1 | hypoxanthine phosphoribosyltransferase 1 |
| lfc | log2 of the ratio of average expression levels |
| limma | Linear Models for Microarray Analysis |
| MAPK | mitogen-activated protein kinase |
| miR | micro ribonucleic acid (micro RNA) |
| NCBI | National Center for Biotechnology Information |
| NSC | neural stem cells (non-malignant) |
| PBS | phosphate buffer saline |
| PI3K | phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PTEN | phosphatase and tensin homolog |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RMS | Rhabdomyosarcoma |
| RPLP0 | ribosomal protein lateral stalk subnit P0 |
| RTK | receptor tyrosine kinase |
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the united states before and during the temozolomide era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Lubman, D.M. Targeting glioblastoma stem cells: Cell surface markers. Curr. Med. Chem. 2012, 19, 6050–6055. [Google Scholar] [CrossRef] [PubMed]
- Cruceru, M.L.; Neagu, M.; Demoulin, J.B.; Constantinescu, S.N. Therapy targets in glioblastoma and cancer stem cells: Lessons from haematopoietic neoplasms. J. Cell. Mol. Med. 2013, 17, 1218–1235. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Han, I.; Wu, L.; Zeng, X. Targeted nanotechnology in glioblastoma multiforme. Front. Pharmacol. 2017, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Hau, P.; Proescholdt, M.; Lohmeier, A.; Wischhusen, J.; Oefner, P.J.; Aigner, L.; Brawanski, A.; Bogdahn, U.; Beier, C.P. Cd133(+) and cd133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007, 67, 4010–4015. [Google Scholar] [CrossRef] [PubMed]
- Ehtesham, M.; Mapara, K.Y.; Stevenson, C.B.; Thompson, R.C. Cxcr4 mediates the proliferation of glioblastoma progenitor cells. Cancer Lett. 2009, 274, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Ma, X.; Guan, Y.; Mizoguchi, M.; Nakamizo, A.; Amano, T.; Hata, N.; Kuga, D.; Sasaki, T. Expression of stem cell marker and receptor kinase genes in glioblastoma tissue quantified by real-time rt-pcr. Brain Tumor Pathol. 2011, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Clement, V.; Sanchez, P.; de Tribolet, N.; Radovanovic, I.; Ruiz i Altaba, A. Hedgehog-gli1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P.; Han, X.F. Cd9 modulates proliferation of human glioblastoma cells via epidermal growth factor receptor signaling. Mol. Med. Rep. 2015, 12, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Podergajs, N.; Motaln, H.; Rajčević, U.; Verbovšek, U.; Koršič, M.; Obad, N.; Espedal, H.; Vittori, M.; Herold-Mende, C.; Miletic, H.; et al. Transmembrane protein cd9 is glioblastoma biomarker, relevant for maintenance of glioblastoma stem cells. Oncotarget 2016, 7, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Kuppner, M.C.; Van Meir, E.; Gauthier, T.; Hamou, M.F.; de Tribolet, N. Differential expression of the cd44 molecule in human brain tumours. Int. J. Cancer 1992, 50, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. Ncbi geo: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, N.; Hastings, E.; Keays, M.; Melnichuk, O.; Tang, Y.A.; Williams, E.; Dylag, M.; Kurbatova, N.; Brandizi, M.; Burdett, T.; et al. Arrayexpress update--simplifying data submissions. Nucleic Acids Res. 2015, 43, D1113–D1116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Meng, F.; Wang, W.; Wang, Z.; Zhang, C.; Jiang, T. Comprehensive rna-seq transcriptomic profiling in the malignant progression of gliomas. Sci. Data 2017, 4, 170024. [Google Scholar] [CrossRef] [PubMed]
- Kraan, J.; van den Broek, P.; Verhoef, C.; Grunhagen, D.J.; Taal, W.; Gratama, J.W.; Sleijfer, S. Endothelial cd276 (b7-h3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br. J. Cancer 2014, 111, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Frati, L.; Leonetti, C.; Zuchegna, C.; Di Zazzo, E.; Calogero, A.; Porcellini, A. Dual-specificity phosphatase dusp6 has tumor-promoting properties in human glioblastomas. Oncogene 2011, 30, 3813–3820. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, F.E.; Molenaar, R.J.; Leenstra, S. Recent advances in the molecular understanding of glioblastoma. J. Neurooncol. 2012, 108, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Halatsch, M.E.; Löw, S.; Mursch, K.; Hielscher, T.; Schmidt, U.; Unterberg, A.; Vougioukas, V.I.; Feuerhake, F. Candidate genes for sensitivity and resistance of human glioblastoma multiforme cell lines to erlotinib. Laboratory investigation. J. Neurosurg. 2009, 111, 211–218. [Google Scholar] [CrossRef] [PubMed]
- De Kok, J.B.; Roelofs, R.W.; Giesendorf, B.A.; Pennings, J.L.; Waas, E.T.; Feuth, T.; Swinkels, D.W.; Span, P.N. Normalization of gene expression measurements in tumor tissues: Comparison of 13 endogenous control genes. Lab. Investig. 2005, 85, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Seaman, S.; Zhu, Z.; Saha, S.; Zhang, X.M.; Yang, M.Y.; Hilton, M.B.; Morris, K.; Szot, C.; Morris, H.; Swing, D.A.; et al. Eradication of tumors through simultaneous ablation of cd276/b7-h3-positive tumor cells and tumor vasculature. Cancer Cell 2017, 31, 501–515. [Google Scholar] [CrossRef] [PubMed]
- GeneCards. Frem2 Gene (Protein Coding)—Fras1 Related Extracellular Matrix Protein 2. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=FREM2&keywords=FREM2 (accessed on 4 May 2017).
- Jadeja, S.; Smyth, I.; Pitera, J.E.; Taylor, M.S.; van Haelst, M.; Bentley, E.; McGregor, L.; Hopkins, J.; Chalepakis, G.; Philip, N.; et al. Identification of a new gene mutated in fraser syndrome and mouse myelencephalic blebs. Nat. Genet. 2005, 37, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Saisawat, P.; Tasic, V.; Vega-Warner, V.; Kehinde, E.O.; Günther, B.; Airik, R.; Innis, J.W.; Hoskins, B.E.; Hoefele, J.; Otto, E.A.; et al. Identification of two novel cakut-causing genes by massively parallel exon resequencing of candidate genes in patients with unilateral renal agenesis. Kidney Int. 2012, 81, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Moon, S.H.; Kim, D.K.; Choi, C.; Song, J.; Park, K.S. Sprouty1 regulates neural and endothelial differentiation of mouse embryonic stem cells. Stem Cells Dev. 2012, 21, 554–561. [Google Scholar] [CrossRef] [PubMed]
- GeneCards. Spry1 Gene (Protein Coding)—Sprouty Rtk Signaling Antagonist 1. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=SPRY1&keywords=SPRY1 (accessed on 4 May 2017).
- Schaaf, G.; Hamdi, M.; Zwijnenburg, D.; Lakeman, A.; Geerts, D.; Versteeg, R.; Kool, M. Silencing of spry1 triggers complete regression of rhabdomyosarcoma tumors carrying a mutated ras gene. Cancer Res. 2010, 70, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Ivliev, A.E.; AC’t Hoen, P.; Sergeeva, M.G. Coexpression network analysis identifies transcriptional modules related to proastrocytic differentiation and sprouty signaling in glioma. Cancer Res. 2010, 70, 10060–10070. [Google Scholar] [CrossRef] [PubMed]
- Nagaishi, M.; Kim, Y.H.; Mittelbronn, M.; Giangaspero, F.; Paulus, W.; Brokinkel, B.; Vital, A.; Tanaka, Y.; Nakazato, Y.; Legras-Lachuer, C.; et al. Amplification of the stoml3, frem2, and lhfp genes is associated with mesenchymal differentiation in gliosarcoma. Am. J. Pathol. 2012, 180, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Forte, S.; Pagliuca, A.; Maniscalchi, E.T.; Gulino, R.; Calabrese, G.; Ricci-Vitiani, L.; Pallini, R.; Signore, M.; Parenti, R.; De Maria, R.; et al. Gene expression analysis of pten positive glioblastoma stem cells identifies dub3 and wee1 modulation in a cell differentiation model. PLoS ONE 2013, 8, e81432. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, B.; Sagardoy, A.; Nogueira, L.; Guruceaga, E.; Grande, L.; Huse, J.T.; Aznar, M.A.; Díez-Valle, R.; Tejada-Solís, S.; Alonso, M.M.; et al. Involvement of mirnas in the differentiation of human glioblastoma multiforme stem-like cells. PLoS ONE 2013, 8, e77098. [Google Scholar] [CrossRef] [PubMed]
- Berezovsky, A.D.; Poisson, L.M.; Cherba, D.; Webb, C.P.; Transou, A.D.; Lemke, N.W.; Hong, X.; Hasselbach, L.A.; Irtenkauf, S.M.; Mikkelsen, T.; et al. Sox2 promotes malignancy in glioblastoma by regulating plasticity and astrocytic differentiation. Neoplasia 2014, 16, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Okawa, S.; Gagrica, S.; Blin, C.; Ender, C.; Pollard, S.M.; Krijgsveld, J. Proteome and secretome characterization of glioblastoma-derived neural stem cells. Stem Cells 2017, 35, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Kapoor, G.S.; Buonato, J.M.; Mathew, L.K.; Bi, Y.; Davuluri, R.V.; Martinez-Lage, M.; Simon, M.C.; O’Rourke, D.M.; Lazzara, M.J. Sprouty2 drives drug resistance and proliferation in glioblastoma. Mol. Cancer Res. 2015, 13, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Grassian, A.R.; Schafer, Z.T.; Brugge, J.S. Erbb2 stabilizes epidermal growth factor receptor (EGFR) expression via erk and sprouty2 in extracellular matrix-detached cells. J. Biol. Chem. 2011, 286, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bachoo, R.M.; Maher, E.A.; Ligon, K.L.; Sharpless, N.E.; Chan, S.S.; You, M.J.; Tang, Y.; DeFrances, J.; Stover, E.; Weissleder, R.; et al. Epidermal growth factor receptor and ink4a/arf: Convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 2002, 1, 269–277. [Google Scholar] [CrossRef]
- Radaelli, E.; Ceruti, R.; Patton, V.; Russo, M.; Degrassi, A.; Croci, V.; Caprera, F.; Stortini, G.; Scanziani, E.; Pesenti, E.; et al. Immunohistopathological and neuroimaging characterization of murine orthotopic xenograft models of glioblastoma multiforme recapitulating the most salient features of human disease. Histol. Histopathol. 2009, 24, 879–891. [Google Scholar] [PubMed]
- The-Human-Protein-Atlas. Frem2. Available online: https://www.proteinatlas.org/ENSG00000150893-FREM2/tissue (accessed on 26 April 2018).
- UniProt. Uniprotkb—q5szk8 (Frem2_Human). Available online: http://www.uniprot.org/uniprot/Q5SZK8 (accessed on 26 April 2018).
- Stretch, C.; Khan, S.; Asgarian, N.; Eisner, R.; Vaisipour, S.; Damaraju, S.; Graham, K.; Bathe, O.F.; Steed, H.; Greiner, R.; et al. Effects of sample size on differential gene expression, rank order and prediction accuracy of a gene signature. PLoS ONE 2013, 8, e65380. [Google Scholar] [CrossRef] [PubMed]
- Gene-Expression-Omnibus. About Geo Datasets. Available online: https://www.ncbi.nlm.nih.gov/geo/info/datasets.html#record (accessed on 4 May 2017).
- Gene-Expression-Omnibus. About geo2r. Available online: https://www.ncbi.nlm.nih.gov/geo/info/geo2r.html (accessed on 4 May 2017).
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar]
- Bioinformatics-&-Evolutionary-Genomics. Venn Diagram: Calculate and Draw Custom Venn Diagrams. Available online: http://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on 18 October 2017).
- Binder, J.X.; Pletscher-Frankild, S.; Tsafou, K.; Stolte, C.; O’Donoghue, S.I.; Schneider, R.; Jensen, L.J. Compartments: Unification and visualization of protein subcellular localization evidence. Database (Oxford) 2014, 2014, bau012. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding rnas in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Grube, S.; Göttig, T.; Freitag, D.; Ewald, C.; Kalff, R.; Walter, J. Selection of suitable reference genes for expression analysis in human glioma using rt-qpcr. J. Neurooncol. 2015, 123, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kośla, K.; Nowakowska, M.; Pospiech, K.; Bednarek, A.K. Wwox modulates the gene expression profile in the t98g glioblastoma cell line rendering its phenotype less malignant. Oncol. Rep. 2014, 32, 1362–1368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demuth, T.; Rennert, J.L.; Hoelzinger, D.B.; Reavie, L.B.; Nakada, M.; Beaudry, C.; Nakada, S.; Anderson, E.M.; Henrichs, A.N.; McDonough, W.S.; et al. Glioma cells on the run—The migratory transcriptome of 10 human glioma cell lines. BMC Genomics 2008, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Ixcells-Biotech. Product Information: Human Schwann Cells (HSWC). Available online: http://www.ixcellsbiotech.com/image/data/Human%20Schwann%20Cells%20(HSwC)/Human%20Schwann%20Cells%20(HSwC).pdf (accessed on 3 May 2018).
- Invitrogen. Gibco Human Neural Stem Cells (h9 HESC-Derived). Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/GIBCO_hNSC_man.pdf (accessed on 3 May 2018).
- CLS. Cls Product Information: Nch644. Available online: https://clsgmbh.de/pdf/nch644.pdf (accessed on 3 May 2018).
- CLS. Cls Product Information: Nch421k. Available online: https://www.clsgmbh.de/pdf/nch421k.pdf (accessed on 3 May 2018).
- CLS. Cls Product Information: U-251 mg. Available online: https://www.clsgmbh.de/pdf/u-251-mg.pdf (accessed on 3 May 2018).
- ATCC. Product Sheet: U-87 mg (ATCC htb-14). Available online: https://www.lgcstandards-atcc.org/en/Products/All/HTB-14.aspx#documentation (accessed on 3 May 2018).
- PrimerBank. Pcr Primers for Gene Expression Detection and Quantification. Available online: https://pga.mgh.harvard.edu/primerbank/ (accessed on 14 November 2017).
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative rt-pcr data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
| Database | GEO | ArrayExpress | GLIOMASdb | ||
|---|---|---|---|---|---|
| Query | »glioblastoma« | »glioblastoma, grade« | »glioblastoma, stem cells« | »glioblastoma, neurospheres« | / |
| Filters | »homo sapiens«, »DataSets« | »homo sapiens«, »RNA assay«, »array assay« | / | ||
| All datasets | 36 | 76 | 69 | 14 | 1 |
| Datasets > 20 samples | 11 | 58 | 17 | 3 | 1 |
| Rejected—Reason 1 | 5 | 35 | 11 | 1 | 0 |
| Rejected—Reason 2 | 2 | 16 | 3 | 2 | 0 |
| Rejected—Reason 3 | 0 | 4 | 1 | 0 | 0 |
| Appropriate datasets | 4 | 3 | 1 + 1 conditionally * | 0 | 1 |
| Appropriate datasets (list) | GSE4412/GDS1975 GSE4412/GDS1976 GSE4290/GDS1962 GSE23806/GDS3885 | E-GEOD-16011 E-GEOD-52009 E-GEOD-68848 | E-GEOD-4536 E-GEOD-74571 * | / | GLIOMASdb |
| Dataset | Category | NPC | Rank | Curated | Use of Dataset |
|---|---|---|---|---|---|
| GSE4412/GDS1975 | Grade | 1 | 5 | yes | validation |
| GSE4412/GDS1976 | Grade | 1 | 5 | yes | validation |
| GSE4290/GDS1962 | Grade | 3 | 1 *** | yes | selection (winner) |
| E-GEOD-16011 | Grade | 2 | 3 | no | validation |
| E-GEOD-52009 | Grade | 2 | 3 | no | validation |
| E-GEOD-68848 | Grade | 3 | 1 *** | no | validation |
| GSE23806/GDS3885 | Neuro/GSCL | 4 | 1 | yes | selection (winner) |
| E-GEOD-4536 | Neuro/GSCL | 2 | 2 | no | validation |
| E-GEOD-74571 | Neuro/GSCL | 2 | 2 | no | validation |
| GLIOMASdb | Grade | 1 | / | yes | selection (directly qualified) |
| Stage | Test | First Group (Overexpression of GSC Marker Candidates Expected) | Second Group | DATA ORIGIN | |||
|---|---|---|---|---|---|---|---|
| Tissue/Cells | N | Tissue/Cells | N | Database | Dataset | ||
| Selection | 1 | GB | 77 | Non-malignant brain tissue | 23 | GEO | GSE4290/GDS1962 |
| 2 | GB | 77 | Grade II glioma | 45 | GEO | GSE4290/GDS1962 | |
| 3 | GB | 77 | oligodendroglioma | 50 | GEO | GSE4290/GDS1962 | |
| 4 | GSCL | 27 | GB tumor tissue | 12 | GEO | GSE23806/GDS3885 | |
| 5 | GSCL | 27 | Conventional GB cell lines | 32 | GEO | GSE23806/GDS3885 | |
| 6 | neurospheres | 17 | GB tumor tissue | 12 | GEO | GSE23806/GDS3885 | |
| 7 | neurospheres | 17 | Conventional GB cell lines | 32 | GEO | GSE23806/GDS3885 | |
| 8 | GB | 143 | Grade II glioma | 110 | GLIOMASdb | / | |
| Validation | v1-1 | GB | 228 | Non-malignant brain tissue | 28 | ArrayExpress | E-GEOD-68848 |
| v2-1 | GB | 24 | Grade II glioma | 63 | ArrayExpress | E-GEOD-52009 | |
| v2-2 | GB | 134 | Grade II glioma | 99 | ArrayExpress | E-GEOD-68848 | |
| v2-3 | GB | 159 | Grade II glioma | 24 | ArrayExpress | E-GEOD-16011 | |
| v3-1 | GB | 59 | oligodendroglioma | 11 | GEO | GSE4412/GDS1975 | |
| v3-2 | GB | 59 | oligodendroglioma | 11 | GEO | GSE4412/GDS1976 | |
| v3-3 | GB | 24 | oligodendroglioma | 13 | ArrayExpress | E-GEOD-52009 | |
| v3-4 | GB | 228 | oligodendroglioma | 67 | ArrayExpress | E-GEOD-68848 | |
| v3-5 | GB | 159 | oligodendroglioma | 52 | ArrayExpress | E-GEOD-16011 | |
| v4-1 | GSCL | 28 | GB tumor tissue | 17 | ArrayExpress | E-GEOD-4536 | |
| v5-1 | GSCL | 28 | Conventional GB cell lines | 26 | ArrayExpress | E-GEOD-4536 | |
| v6-1 | neurospheres | 9 | GB tumor tissue | 9 | ArrayExpress | E-GEOD-74571 | |
| v7-1 | neurospheres | 9 | Conventional GB cell lines | 10 | ArrayExpress | E-GEOD-74571 | |
| Gene | Success Rate | CSP Status | Already Linked to Glioblastoma Carcinogenesis | Single Failed Test (p > 0.001 or lfc < 1) | Results of the Failed Test (Only for CSP Genes with 7/8 Success Rate) | |
|---|---|---|---|---|---|---|
| p < 0.05 | lfc > 0 | |||||
| CD276 | 8/8 | yes | yes [19] | none | / | / |
| HIST1H2BH | 8/8 | no | no | none | / | / |
| DUSP6 | 8/8 | no | yes [20] | none | / | / |
| YME1L1 | 7/8 | no | yes [21] | Test 8 | / | / |
| DHRSX | 7/8 | no | no | Test 8 | / | / |
| B3GNT5 | 7/8 | no | no | Test 7 | / | / |
| FREM2 | 7/8 | yes | no | Test 6 | yes | yes |
| DUSP4 | 7/8 | no | yes [22] | Test 3 | / | / |
| SPRY1 | 7/8 | yes | no | Test 3 | yes | yes |
| SLC47A1 | 7/8 | yes | no | Test 1 | yes | yes |
| Test | Points | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New Candidates | Established Candidates | Control | |||||||||||||
| CD 276 | FREM2 | SPRY1 | SLC47A1 | CD133 | CXCR4 | MELK | PTCH1 | CD9 | CD44 | CYC1 | HPRT1 | H3F3A | RPLP0 | EMC7 | |
| 1 | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 3 | 3 | 4 | 0 | 0 | 4 | 4 | 0 |
| 2 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 0 | 3 | 4 | 0 | 0 | 0 | 0 | 0 |
| 3 | 4 | 4 | 3 | 4 | 3 | 4 | 4 | 0 | 3 | 4 | 0 | 0 | 0 | 0 | 0 |
| 4 | 4 | 4 | 4 | 4 | 0 | 0 | 4 | 3 | 0 | 3 | 0 | 4 | 0 | 0 | 0 |
| 5 | 4 | 4 | 4 | 4 | 3 | 4 | 0 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 0 |
| 6 | 4 | 3 | 4 | 4 | 0 | 0 | 3 | 3 | 0 | 1 | 0 | 3 | 0 | 0 | 3 |
| 7 | 4 | 4 | 4 | 4 | 3 | 4 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 3 | 0 |
| 8 | 4 | 4 | 4 | 4 | / | 4 | 4 | 0 | / | 4 | / | / | 0 | 0 | 0 |
| APPT | 4.000 | 3.875 | 3.875 | 3.875 | 2.143 | 3.000 | 2.875 | 1.875 | 2.143 | 2.500 | 0.000 | 1.000 | 0.875 | 0.875 | 0.375 |
| Test | Points | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New Candidates | Established Candidates | Control | |||||||||||||
| CD 276 | FREM2 | SPRY1 | SLC47A1 | CD133 | CXCR4 | MELK | PTCH1 | CD9 | CD44 | CYC1 | HPRT1 | H3F3A | RPLP0 | EMC7 | |
| v1-1 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 0 | 3 | 4 | 0 | 0 | 3 | 4 | 0 |
| v2-1 | 3 | / | 3 | 3 | 0 | 0 | 4 | 0 | 0 | 3 | 0 | 0 | 3 | 0 | 0 |
| v2-2 | 3 | 4 | 4 | 4 | 3 | 3 | 4 | 0 | 3 | 4 | 0 | 3 | 0 | 0 | 0 |
| v2-3 | 3 | / | 4 | 4 | 3 | 4 | 4 | 0 | 3 | 4 | 0 | 3 | / | / | / |
| v3-1 | / | / | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| v3-2 | 3 | 0 | 3 | / | / | / | / | 0 | 0 | 0 | / | / | / | / | 0 |
| v3-3 | 3 | / | 0 | 3 | 0 | 0 | 3 | 1 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| v3-4 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 0 | 3 | 4 | 0 | 3 | 0 | 0 | 0 |
| v3-5 | 3 | / | 3 | 0 | 4 | 4 | 3 | 0 | 3 | 4 | 0 | 0 | / | / | / |
| v4-1 | 4 | 4 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 | 0 | 0 | 3 | 3 |
| v5-1 | 3 | 4 | 4 | 4 | 4 | 4 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| v6-1 | 3 | 3 | 1 | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 3 | 3 |
| v7-1 | 3 | 4 | 4 | 4 | 3 | 4 | 0 | 3 | 1 | 0 | 3 | 0 | 3 | 1 | 0 |
| APPT | 3.167 | 3.250 | 2.846 | 2.667 | 1.917 | 2.250 | 2.583 | 0.615 | 1.692 | 2.077 | 0.750 | 0.750 | 1.000 | 1.100 | 0.545 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidak, M.; Jovcevska, I.; Samec, N.; Zottel, A.; Liovic, M.; Rozman, D.; Dzeroski, S.; Juvan, P.; Komel, R. Meta-Analysis and Experimental Validation Identified FREM2 and SPRY1 as New Glioblastoma Marker Candidates. Int. J. Mol. Sci. 2018, 19, 1369. https://doi.org/10.3390/ijms19051369
Vidak M, Jovcevska I, Samec N, Zottel A, Liovic M, Rozman D, Dzeroski S, Juvan P, Komel R. Meta-Analysis and Experimental Validation Identified FREM2 and SPRY1 as New Glioblastoma Marker Candidates. International Journal of Molecular Sciences. 2018; 19(5):1369. https://doi.org/10.3390/ijms19051369
Chicago/Turabian StyleVidak, Marko, Ivana Jovcevska, Neja Samec, Alja Zottel, Mirjana Liovic, Damjana Rozman, Saso Dzeroski, Peter Juvan, and Radovan Komel. 2018. "Meta-Analysis and Experimental Validation Identified FREM2 and SPRY1 as New Glioblastoma Marker Candidates" International Journal of Molecular Sciences 19, no. 5: 1369. https://doi.org/10.3390/ijms19051369
APA StyleVidak, M., Jovcevska, I., Samec, N., Zottel, A., Liovic, M., Rozman, D., Dzeroski, S., Juvan, P., & Komel, R. (2018). Meta-Analysis and Experimental Validation Identified FREM2 and SPRY1 as New Glioblastoma Marker Candidates. International Journal of Molecular Sciences, 19(5), 1369. https://doi.org/10.3390/ijms19051369






