Ethylene Responsive Factor MeERF72 Negatively Regulates Sucrose synthase 1 Gene in Cassava
Abstract
1. Introduction
2. Results
2.1. Full-Length cDNA Library for Y1H Screening
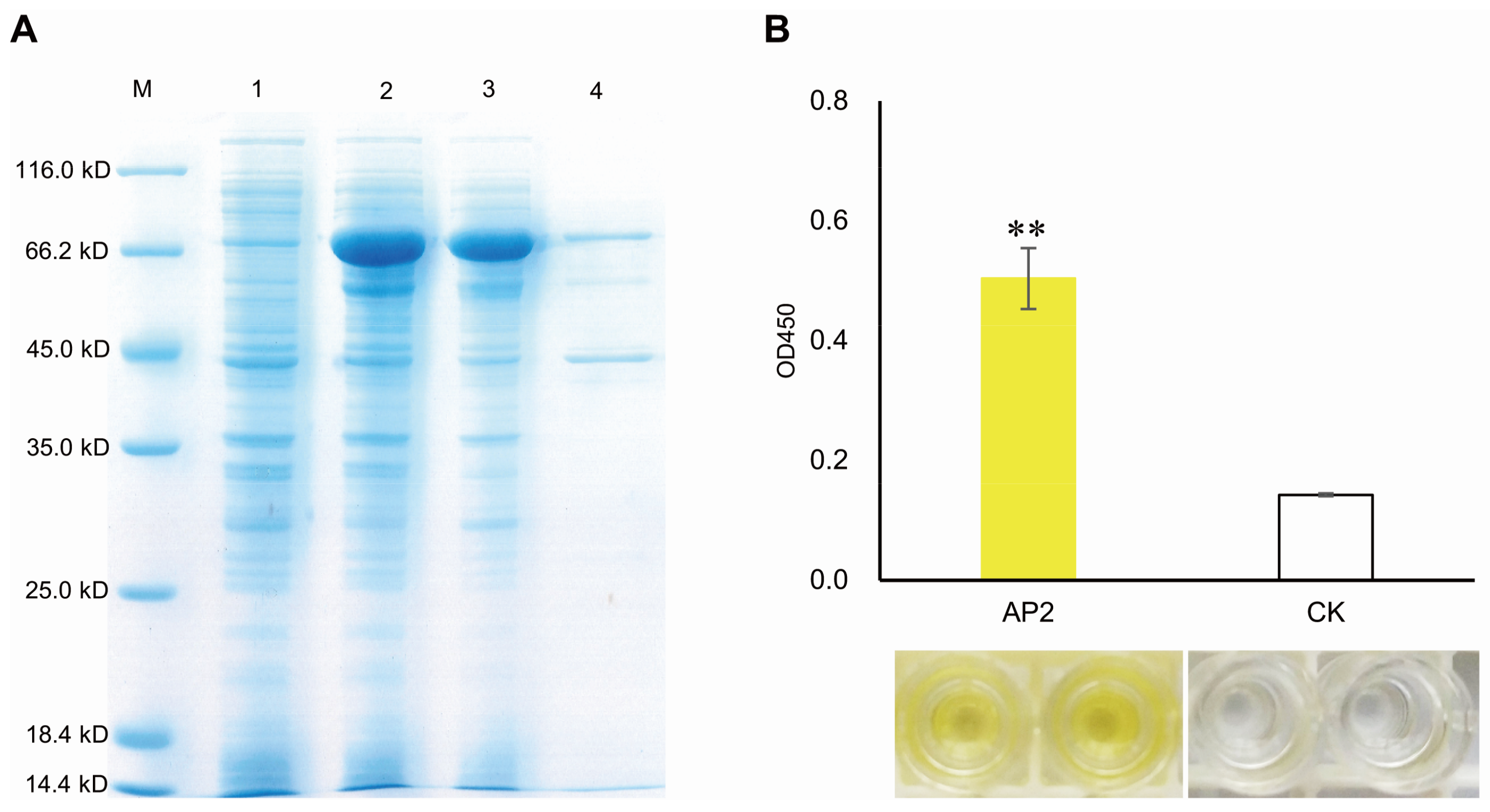
2.2. Cloning and Characterization of MeERF72
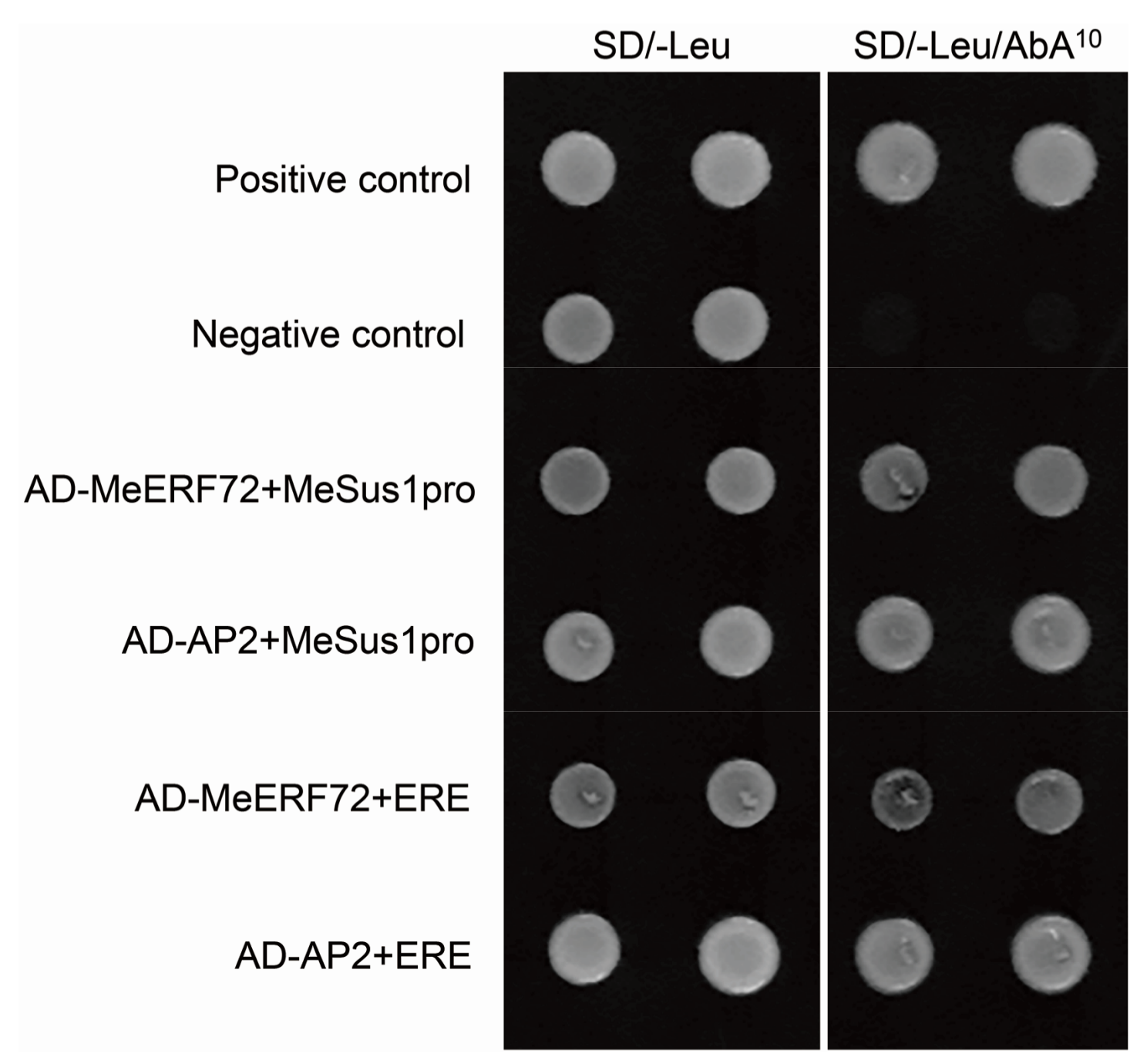
2.3. MeERF72 Binds to MeSus1 Promoter through AP2 Domain
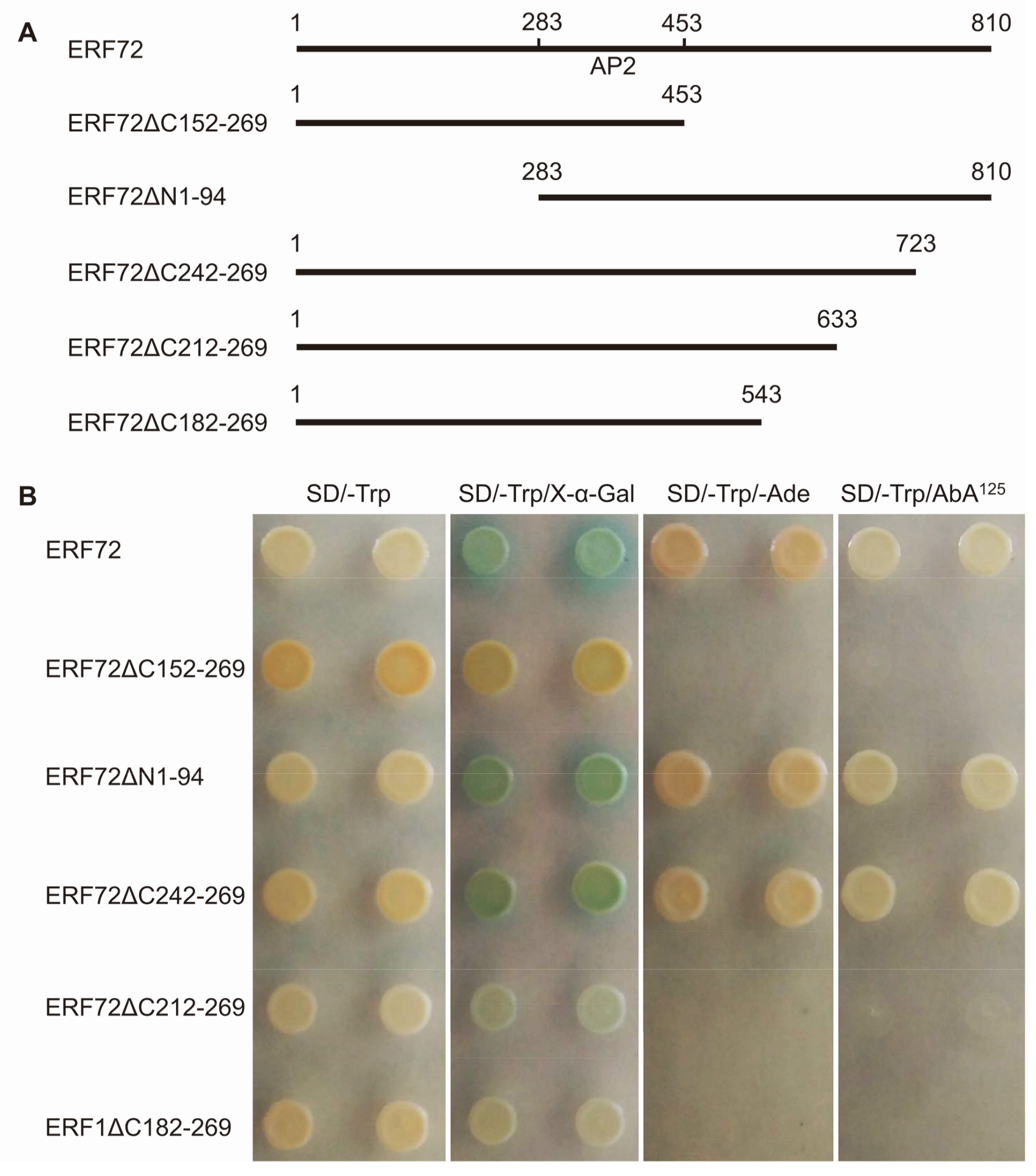
2.4. The Activated Domain Is Located in the aa212-aa241 Region of MeERF72 Protein
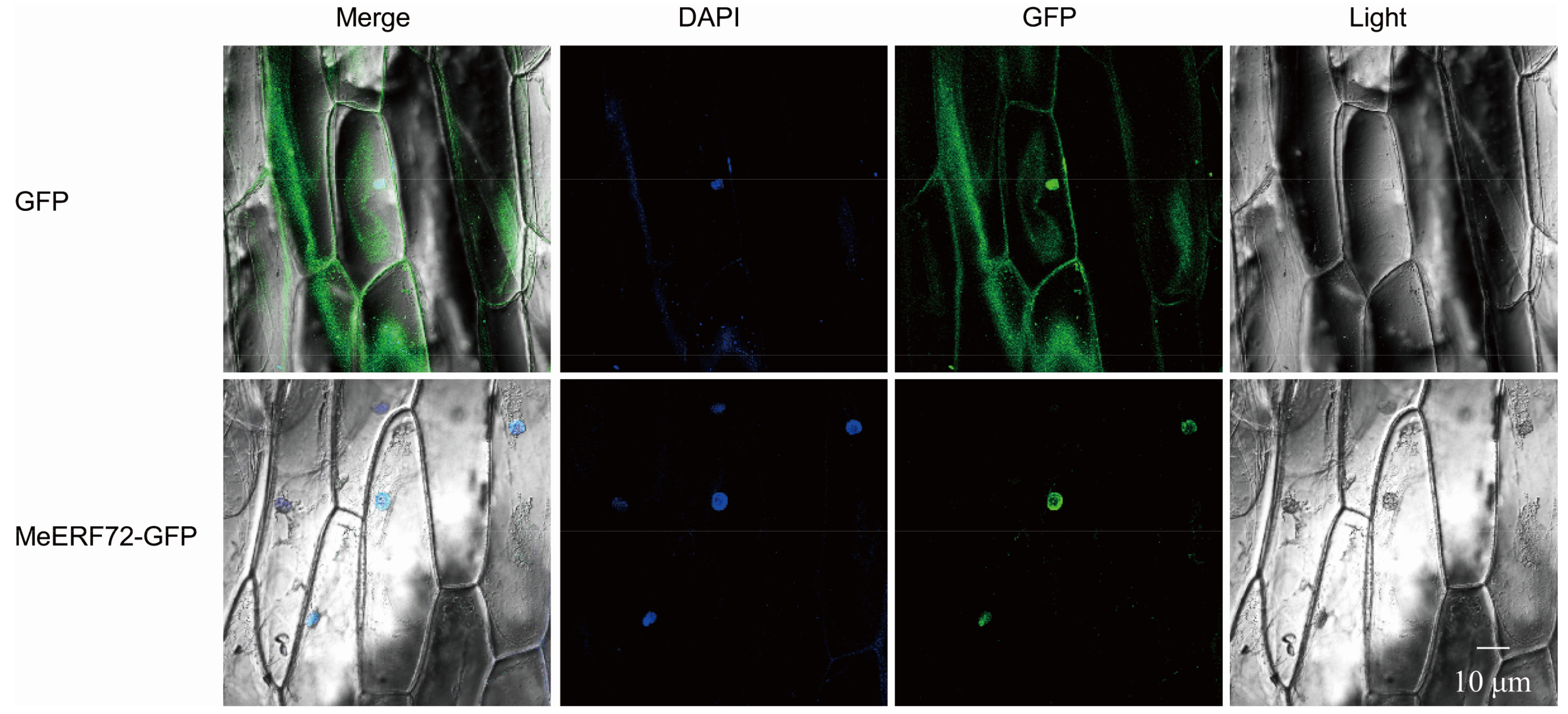
2.5. MeERF72 Is a Nuclear-Localized Protein
2.6. MeERF72 Can Bind to the MeSus1 Promoter In Vitro
2.7. Repression of MeSus1 Promoter Activity by MeERF72
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. SMART III cDNA Library Construction
4.3. Yeast One-Hybrid Library Screening
4.4. Subcellular Location of MeERF72 Protein
4.5. DNA and Protein Interaction Confirmation in Yeast Cell
4.6. DNA-Protein-Interaction Enzyme-Linked Immunosorbent Assay (DPI-ELISA)
4.7. Transcription Activation Assay
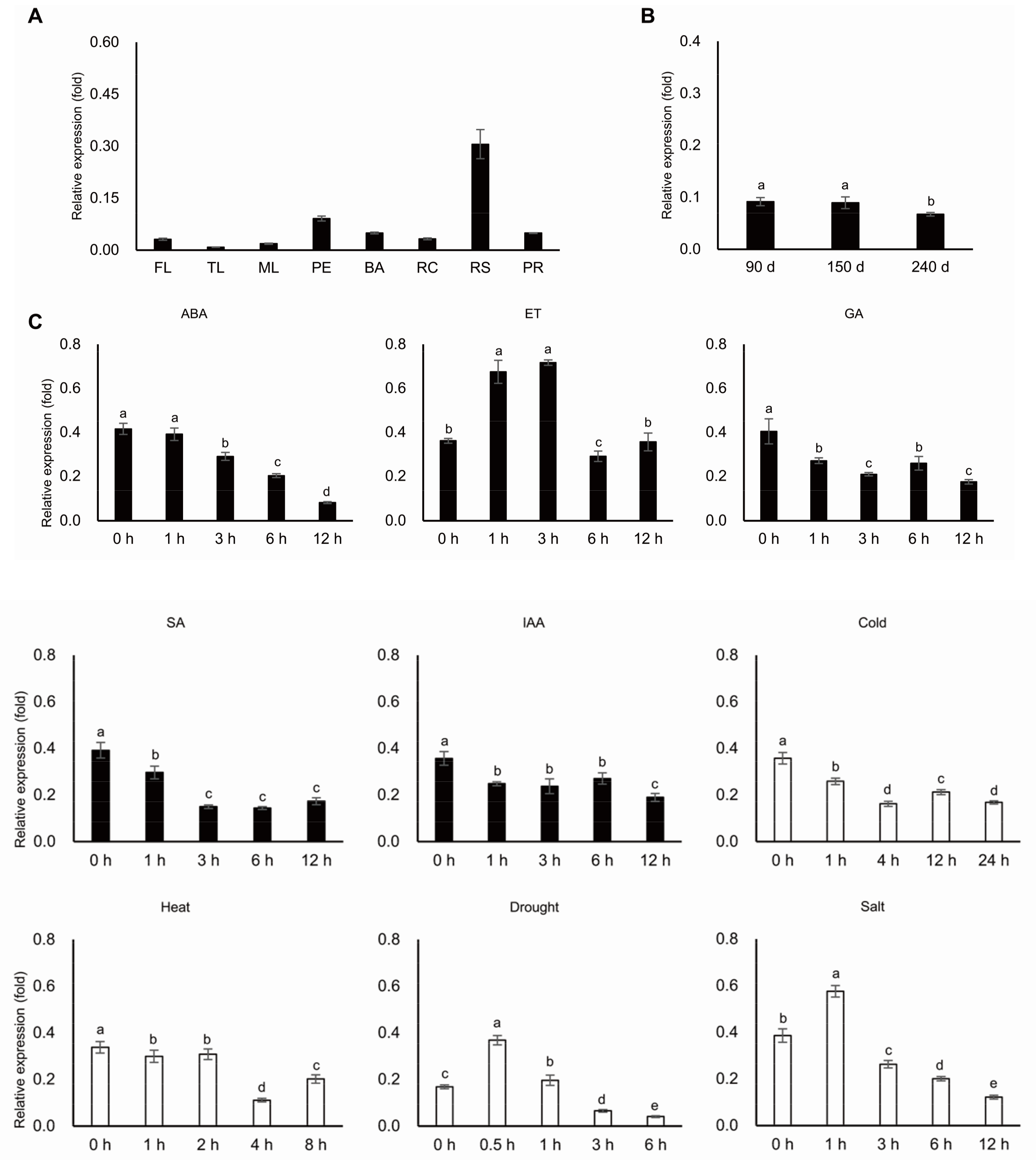
4.8. Expression Profile of MeERF72 By Quantitative PCR
4.9. Plant One-Hybrid
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zeng, Y.; Wu, Y.; Avigne, W.T.; Koch, K.E. Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Physiol. 1998, 116, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Fernandez, E.; Munoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baroja-Fernandez, E.; Bahaji, A.; Munoz, F.J.; Ovecka, M.; Montero, M.; Sesma, M.T.; Alonso-Casajus, N.; Almagro, G.; Sanchez-Lopez, A.M.; et al. Enhancing sucrose synthase activity results in increased levels of starch and ADP-glucose in maize (Zea mays L.) seed endosperms. Plant Cell Physiol. 2013, 54, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Qu, Z.; Zhang, L.; Zhao, S.; Bi, Z.; Ji, X.; Wang, X.; Wei, H. Overexpression of poplar xylem sucrose synthase in tobacco leads to a thickened cell wall and increased height. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.A.; Luan, S.; Wi, S.G.; Bae, H.; Lee, D.S.; Bae, H.J. Pronounced phenotypic changes in transgenic tobacco plants overexpressing sucrose synthase may reveal a novel sugar signaling pathway. Front. Plant Sci. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E., Jr. Ethylene in Plant Biology, 2nd ed.; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Naik, P.K.; Mohapatra, P.K. Ethylene inhibitors enhanced sucrose synthase activity and promoted grain filling of basal rice kernels. Aust. J. Plant Physiol. 2000, 27, 997–1008. [Google Scholar] [CrossRef]
- Chen, Y.F.; Etheridge, N.; Schaller, G.E. Ethylene signal transduction. Ann. Bot. 2005, 95, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, M.D.; Chang, C. Ethylene signaling: New levels of complexity and regulation. Curr. Opin. Plant Biol. 2008, 11, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.F.; Xue, H.W. Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010, 154, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Schippers, J.H.; Mieulet, D.; Watanabe, M.; Hoefgen, R.; Guiderdoni, E.; Mueller-Roeber, B. SALT-RESPONSIVE ERF1 is a negative regulator of grain filling and gibberellin-mediated seedling establishment in rice. Mol. Plant 2014, 7, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Xu, H.; Zhu, Y.; Liu, Q.Q.; Cai, X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Yi, Q.; Hu, Y.; Liu, H.; Liu, Y.; Huang, Y. Novel role of ZmaNAC36 in co-expression of starch synthetic genes in maize endosperm. Plant Mol. Biol. 2014, 84, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yi, Q.; Cao, Y.; Wei, B.; Zheng, L.; Xiao, Q.; Xie, Y.; Gu, Y.; Li, Y.; Huang, H.; et al. ZmbZIP91 regulates expression of starch synthesis-related genes by binding to ACTCAT elements in their promoters. J. Exp. Bot. 2016, 67, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cai, X.L.; Wang, Z.Y.; Hong, M.M. An interaction between a MYC protein and an EREBP protein is involved in transcriptional regulation of the rice Wx gene. J. Biol. Chem. 2003, 278, 47803–47811. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Munne-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Scarpeci, T.E.; Frea, V.S.; Zanor, M.I.; Valle, E.M. Overexpression of AtERF019 delays plant growth and senescence, and improves drought tolerance in Arabidopsis. J. Exp. Bot. 2017, 68, 673–685. [Google Scholar] [PubMed]
- Yao, W.; Wang, L.; Zhou, B.; Wang, S.; Li, R.; Jiang, T. Over-expression of poplar transcription factor ERF76 gene confers salt tolerance in transgenic tobacco. J. Plant Physiol. 2016, 198, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhang, X.; Zhou, B.; Zhao, K.; Li, R.; Jiang, T. Expression pattern of erf gene family under multiple abiotic stresses in Populus simonii x P. nigra. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Lestari, R.; Rio, M.; Martin, F.; Leclercq, J.; Woraathasin, N.; Roques, S.; Dessailly, F.; Clement-Vidal, A.; Sanier, C.; Fabre, D.; et al. Overexpression of Hevea brasiliensis ethylene response factor HbERF-IXc5 enhances growth and tolerance to abiotic stress and affects laticifer differentiation. Plant Biotechnol. J. 2018, 16, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, X.; Yang, J.; Messing, J.; Wu, Y. Maize endosperm-specific transcription factors O2 and PBF network the regulation of protein and starch synthesis. Proc. Natl. Acad. Sci. USA 2016, 113, 10842–10847. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Tian, H.; Wang, S.; Chen, J.G. The small ethylene response factor ERF96 is involved in the regulation of the abscisic acid response in Arabidopsis. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Hai, M.; Guo, Y.; Ding, Z.; Tie, W.; Ding, X.; Yan, Y.; Wei, Y.; Liu, Y.; Wu, C.; et al. The ERF transcription factor family in cassava: Genome-wide characterization and expression analyses against drought stress. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Li, Y.; Yang, Y.; Wang, G.; Peng, M. Exposure to various abscission-promoting treatments suggests substantial ERF subfamily transcription factors involvement in the regulation of cassava leaf abscission. BMC Genom. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Qi, J.; Li, H.; Zhang, C.; Wang, Y. A convenient and efficient protocol for isolating high-quality RNA from latex of Hevea brasiliensis (para rubber tree). J. Biochem. Biophys. Methods 2007, 70, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H. Large-scale high-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Rawat, R.; Xu, Z.F.; Yao, K.M.; Chye, M.L. Identification of cis-elements for ethylene and circadian regulation of the Solanum melongena gene encoding cysteine proteinase. Plant Mol. Biol. 2005, 57, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Chen, X.; Liu, C.; Meng, Y.; Xia, Z.; Zeng, C.; Lu, C.; Wang, W. MeSAUR1, Encoded by a small auxin-up RNA gene, acts as a transcription regulator to positively regulate adp-glucose pyrophosphorylase small subunit1a gene in cassava. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.H.; Kirchler, T.; Hummel, S.; Chaban, C.; Wanke, D. DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods 2010, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Chen, X.; Ma, P.; Zhang, S.; Zeng, C.; Jiang, X.; Wang, W. Ethylene Responsive Factor MeERF72 Negatively Regulates Sucrose synthase 1 Gene in Cassava. Int. J. Mol. Sci. 2018, 19, 1281. https://doi.org/10.3390/ijms19051281
Liu C, Chen X, Ma P, Zhang S, Zeng C, Jiang X, Wang W. Ethylene Responsive Factor MeERF72 Negatively Regulates Sucrose synthase 1 Gene in Cassava. International Journal of Molecular Sciences. 2018; 19(5):1281. https://doi.org/10.3390/ijms19051281
Chicago/Turabian StyleLiu, Chen, Xin Chen, Ping’an Ma, Shengkui Zhang, Changying Zeng, Xingyu Jiang, and Wenquan Wang. 2018. "Ethylene Responsive Factor MeERF72 Negatively Regulates Sucrose synthase 1 Gene in Cassava" International Journal of Molecular Sciences 19, no. 5: 1281. https://doi.org/10.3390/ijms19051281
APA StyleLiu, C., Chen, X., Ma, P., Zhang, S., Zeng, C., Jiang, X., & Wang, W. (2018). Ethylene Responsive Factor MeERF72 Negatively Regulates Sucrose synthase 1 Gene in Cassava. International Journal of Molecular Sciences, 19(5), 1281. https://doi.org/10.3390/ijms19051281



