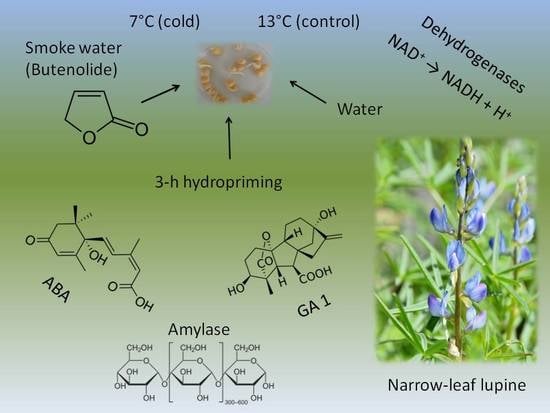
Seed Hydropriming and Smoke Water Significantly Improve Low-Temperature Germination of Lupinus angustifolius L.
Abstract
:1. Introduction
2. Results
2.1. Seed Germination Vigour
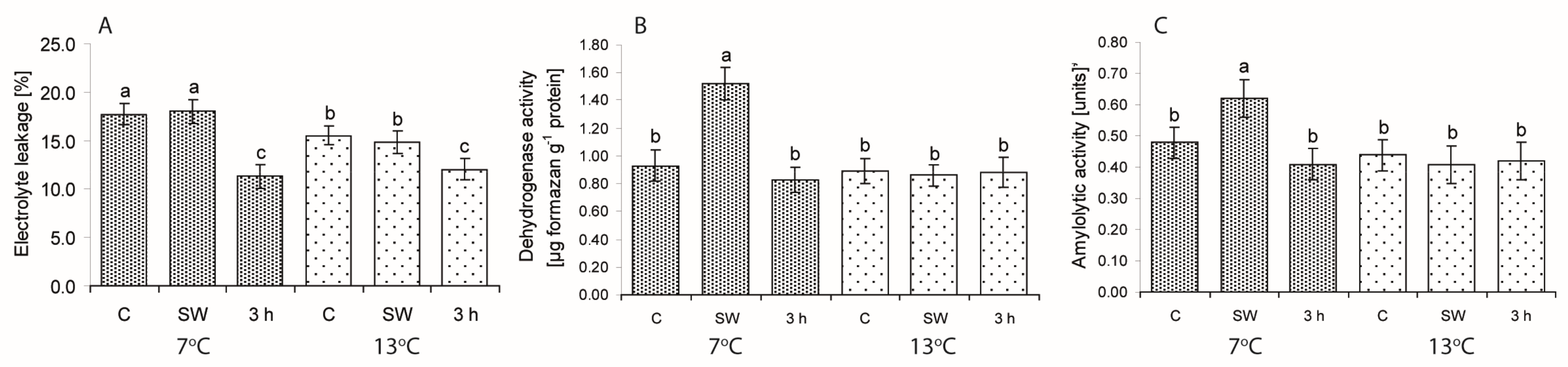
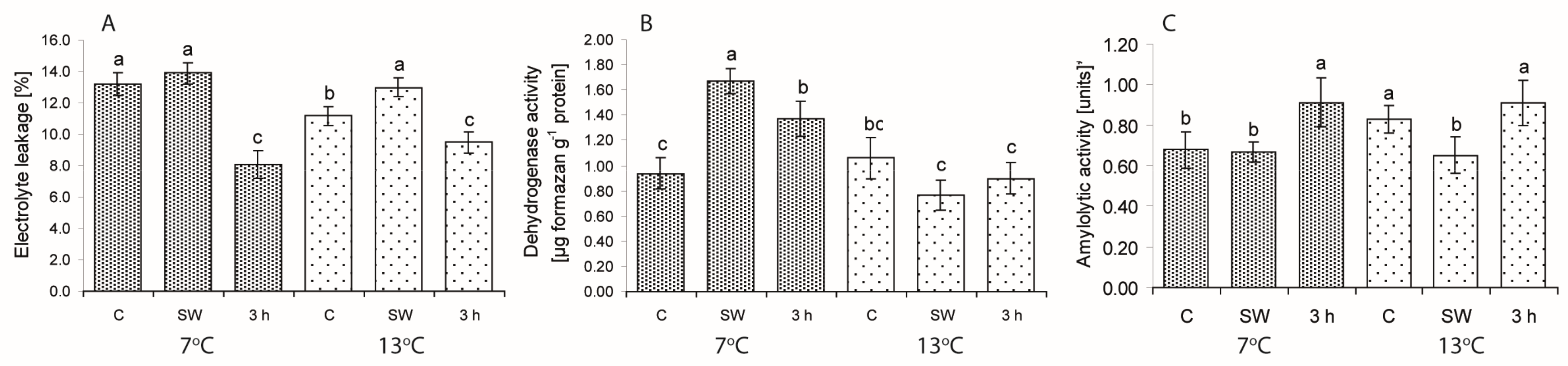
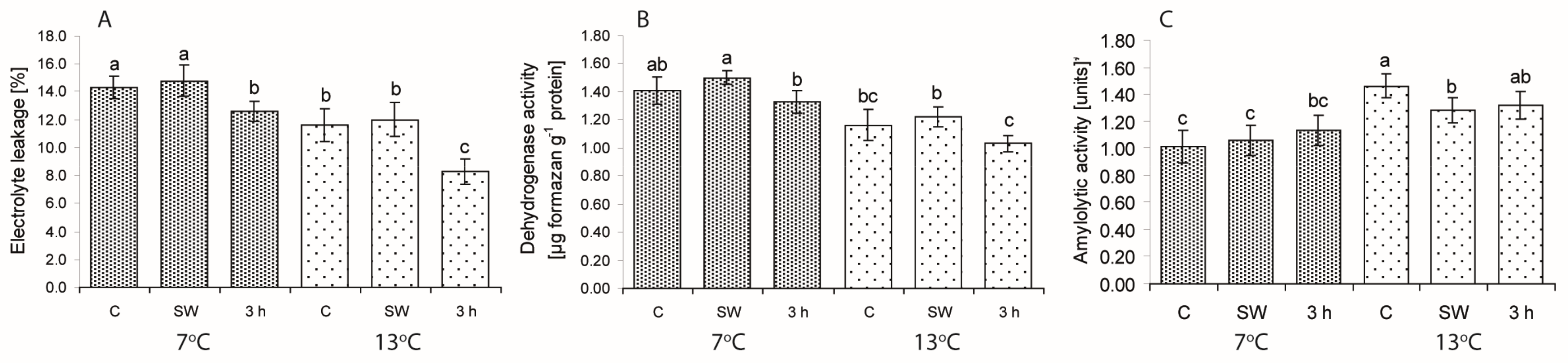
2.2. Ion Leakage (EL) and Dehydrogenase and Amylolytic Activity
2.3. Hormone Content
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Seed Germination Vigour (V)
4.3. Electrolyte Leakage (EL)
4.4. Dehydrogenase Activity Assay
4.5. Amylolytic Activity (A) Assay
4.6. Analyses of GA and ABA Content
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3 h | seed hydropriming for 3 h at 20 °C |
| ABA | abscisic acid |
| A | amylolytic activity |
| DA | activity of dehydrogenase pool measured by 2,3,5-triphenyltetrazolium chloride reduction |
| GAs | gibberellins |
| EL | electrolyte leakage |
| SW | smoke water |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| V | seed germination vigour |
References
- Wolko, B.; Clements, J.C.; Naganowska, B.; Nelson, M.N.; Yang, H. Lupinus. In Wild Crop Relatives: Genomic and Breeding Resources: Legume Crops and Forages; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 56–61. ISBN 978-3-642-14386-1. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.M.; Ross, E.E.; Manalo, R. Vigour of garden bean seeds and seedlings influenced by initial seed moisture, substrate, oxygen and imbibition temperature. J. Am. Soc. Hortic. Sci. 1969, 94, 577–584. [Google Scholar]
- Hobbs, P.R.; Obendorf, R.L. Interaction of initial seed moisture and imbibitional temperature on germination and productivity of soybean. Crop Sci. 1972, 12, 664–667. [Google Scholar] [CrossRef]
- Dubert, F.; Filek, W. The low temperature drying of soybean (Glycine max) seeds as a factor reducing injuries caused by exposure to chill during subsequent germination. Bull. Pol. Acad. Sci. Biol. Sci. 1995, 43, 51–55. [Google Scholar]
- Steponkus, P.L. Role of the plasma membrane in freezing injury and cold acclimation. Ann. Rev. Plant Physiol. 1984, 35, 534–584. [Google Scholar] [CrossRef]
- Lee, S.; Cheng, H.; King, K.E.; Wang, W.; He, Y.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Gene Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Graeber, K.; Linkies, A.; Muller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-species approaches to seed dormancy and germination: Conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol. Biol. 2010, 73, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.S.; Smith, S.M. KAI2- and MAX2-mediated responses to karrikins and strigolactones are largely indepent of HY5 in Arabidopsis seedlings. Mol. Plant 2013, 6, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Teixeira da Silva, J.A.; Ma, G. Effects of smoke water and karrikin on seed germination of 13 species growing in China. Cent. Eur. J. Biol. 2014, 9, 1108–1116. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J. Smoke-induced seed germination in California chaparral. Ecology 1998, 79, 2320–2336. [Google Scholar] [CrossRef]
- Rokich, D.P.; Dixon, K.W.; Sivasithamparam, K.; Meney, K.A. Smoke, mulch, and seed broadcasting effects on woodland restoration in Western Australia. Restor. Ecol. 2002, 10, 185–194. [Google Scholar] [CrossRef]
- Van Staden, J.; Jäger, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Dixon, K.W.; Merrit, D.J.; Flematti, G.R.; Ghisalberti, E.L. Karrinkinolide—A phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Soós, V.; Sebestyén, E.; Juhász, A.; Light, M.E.; Kohout, L.; Szalai, G.; Tandori, J.; Van Staden, J.; Balázs, E. Transcriptome analysis of germinating maize kernels exposed to smoke-water and the active compound KAR1. BMC Plant Biol. 2010, 10, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Van Staden, J. A smoke-derived butenolide improve early growth of tomato seedlings. Plant Growth Regul. 2006, 50, 139–148. [Google Scholar] [CrossRef]
- Dodd, G.L.; Donovan, L.A. Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am. J. Bot. 1999, 86, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, F.; Paciolla, C.; De Pinto, M.; De Gara, L. A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J. Exp. Bot. 2001, 52, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Hourmant, A.; Pradet, A. Oxidative phosphorylation in germinating lettuce seeds (Lactuca sativa) during the first hours of imbibition. Am. Soc. Plant Biol. 1981, 68, 631–635. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Bailly, C.; Szafrańska, K.; Janas, K.M.; Corbineau,, F. Antioxidant enzymes and isoflavonoids in chilled soybean (Glycine max (L.) Merr.) seedlings. J. Plant Physiol. 2005, 162, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Deneaujon, I.; Koornnef, M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef]
- Penfield, S.; Josse, E.M.; Kannagara, R.; Gilday, A.D.; Halliday, K.J.; Graham, I.A. Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr. Biol. 2005, 15, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kwak, K.J.; Oh, T.R.; Kim, Y.O.; Kang, H. Cold shock domain proteins affect seed germination and growth of Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol. 2009, 50, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Nordborg, M.; Bergelson, J. The effect of seed and rosette cold treatment on germination and flowering time in some Arabidopsis thaliana (Brassicaceae) ecotypes. Am. J. Bot. 1999, 86, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kumar, S.; Nayyar, H.; Upadhaya, H.D. Cold stress injury during the pod-filling phase in chickpea (Cicer arietinum L.): Effects on quantitative and qualitative components of seeds. J. Agron. Crop Sci. 2008, 194, 457–464. [Google Scholar]
- Leopold, A.C.; Musgrave, M.E. Respiratory changes with chilling injury of soybeans. Plant Physiol. 1979, 64, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Riseborough, J.A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 2009, 149, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Kępczyński, J.; Chembrowska, D.; Van Staden, J. Releasing primary dormancy in Avena fatua L. caryopses by smoke-derived butenolide. Plant Growth Regul. 2010, 62, 85–91. [Google Scholar] [CrossRef]
- Hilhorst, H.W.M.; Karssen, C.M. Seed dormancy and germination: The role of abscisic acid and gibberellins and the importance of hormone mutants. Plant Growth Regul. 1992, 11, 225–238. [Google Scholar] [CrossRef]
- Halińska, A.; Lewak, S. Free and conjugated gibberellins in dormancy and germination of apple seeds. Physiol Plant. 1987, 69, 523–530. [Google Scholar] [CrossRef]
- Chien, C.T.; Kuo-Huang, L.; Lin, T.P. Changes in ultrastructure and abscisic acid level, and response to applied gibberellins in Taxus mairei seeds treated with warm and cold stratification. Ann. Bot. 1998, 81, 41–47. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.J.; Reid, J.B.; Swain, S.M.; Hasan, O.; Poole, A.T.; Hedden, P.; Willis, C.L. Genetic regulation of gibberellin deactivation in Pisum. Plant J. 1995, 7, 513–523. [Google Scholar] [CrossRef]
- Urbanová, T.; Tarkowská, D.; Novák, O.; Hedden, P.; Strnad, M. Analysis of gibberellins as free acids by ultra-performance liquid chromatography-tandem mass spectrometry. Talanta 2013, 112, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Nagatani, A.; Zhao, Y.J.; Kang, B.J.; Kendrick, R.E.; Kamiya, Y. Effects of gibberellins on seed germination of phytochrome-deficient mutants of Arabidopsis thaliana. Plant Cell Physiol. 1995, 36, 1205–1211. [Google Scholar] [PubMed]
- Cowling, R.J.; Kamiya, Y.; Seto, H.; Harberd, N.P. Gibberellin dose-response regulation of GA4 gene transcript levels in Arabidopsis. Plant Physiol. 1998, 117, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Chiwocha, S.D.S.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.-A.M.; Smith, S.M.; Stevens, J.C. Karrikins a new family of plant growth regulators in smoke. Plant Sci. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Braun, J.W.; Khan, A.A. Endogenous abscisic acid levels in germinating and nongerminating lettuce seeds. Plant Physiol. 1975, 56, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Walton, D.C. Does ABA play a role in seed germination? Isr. J. Bot. 1980, 29, 168–180. [Google Scholar]
- Humplík, J.F.; Bergougnoux, V.; Jandová, M.; Šimura, J.; Pěnčík, A.; Tomanec, O.; Rolčík, J.; Novák, O.; Fellner, M. Endogenous abscisic acid promotes hypocotyl growth and affects endoreduplication during dark-induced growth in tomato (Solanum lycopersicum L.). PLoS ONE 2015, 10, e0117793. [Google Scholar] [CrossRef] [PubMed]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heinkel, H. Comparison of various methods of determining blood diastases. Klin. Wochschr. 1956, 34, 155–157. [Google Scholar] [CrossRef]
- Hura, T.; Dziurka, M.; Hura, K.; Ostrowska, A.; Dziurka, K.; Gadzinowska, J. Wheat and rye genome confer specific phytohormone profile features and interplay under water stress in two phenotypes of triticale. Plant Physiol. Biochem. 2017, 118, 494–509. [Google Scholar] [CrossRef] [PubMed]
- Dziurka, M.; Janeczko, A.; Juhász, C.; Gullner, G.; Oklestková, J.; Novák, O.; Saja, D.; Skoczowski, A.; Tóbiász, J.; Barna, B. Local and systemic hormonal responses in pepper leaves during compatible and incompatible pepper-tobamovirus interactions. Plant Physiol. Biochem. 2016, 109, 355–364. [Google Scholar] [CrossRef] [PubMed]
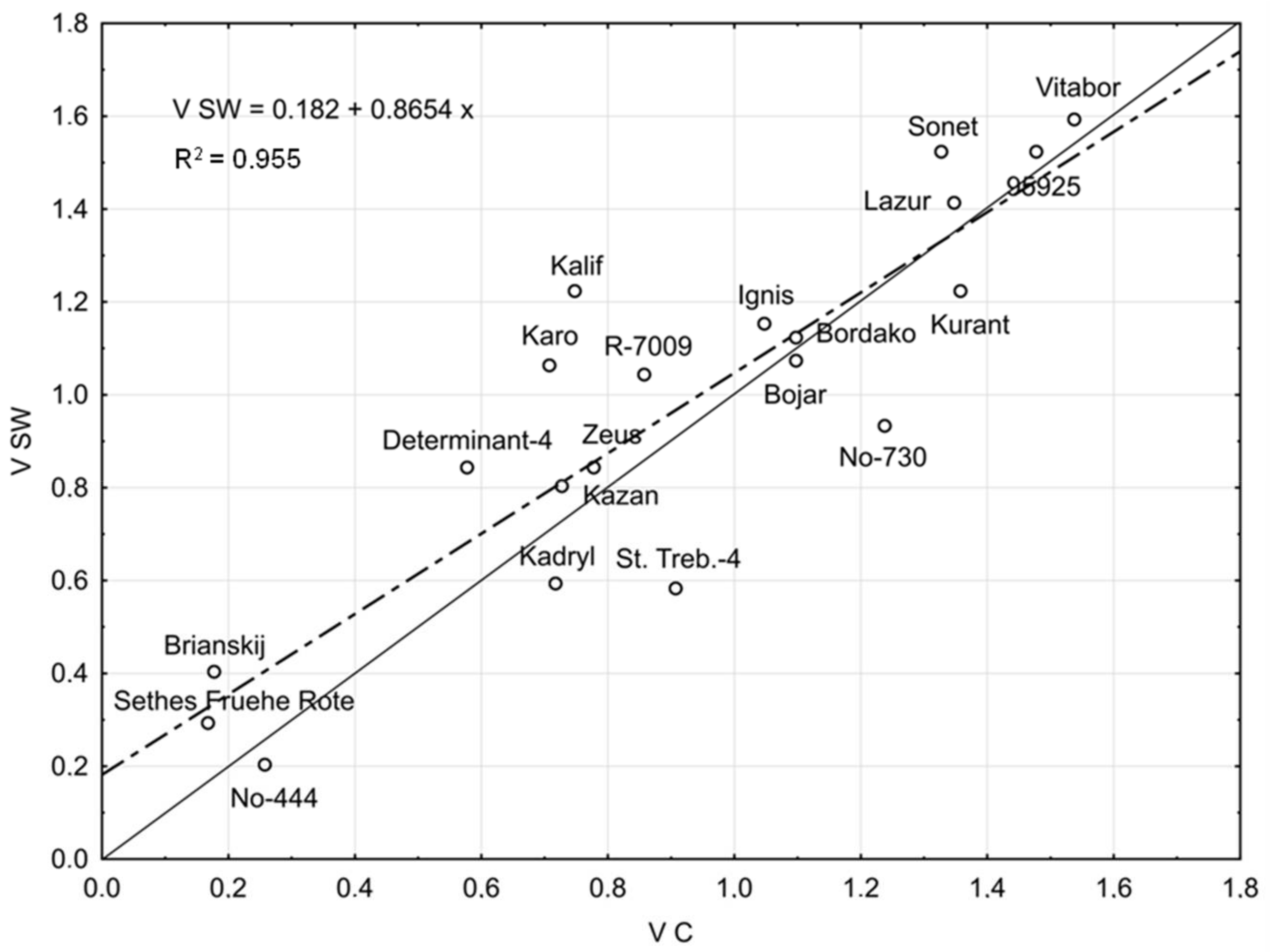
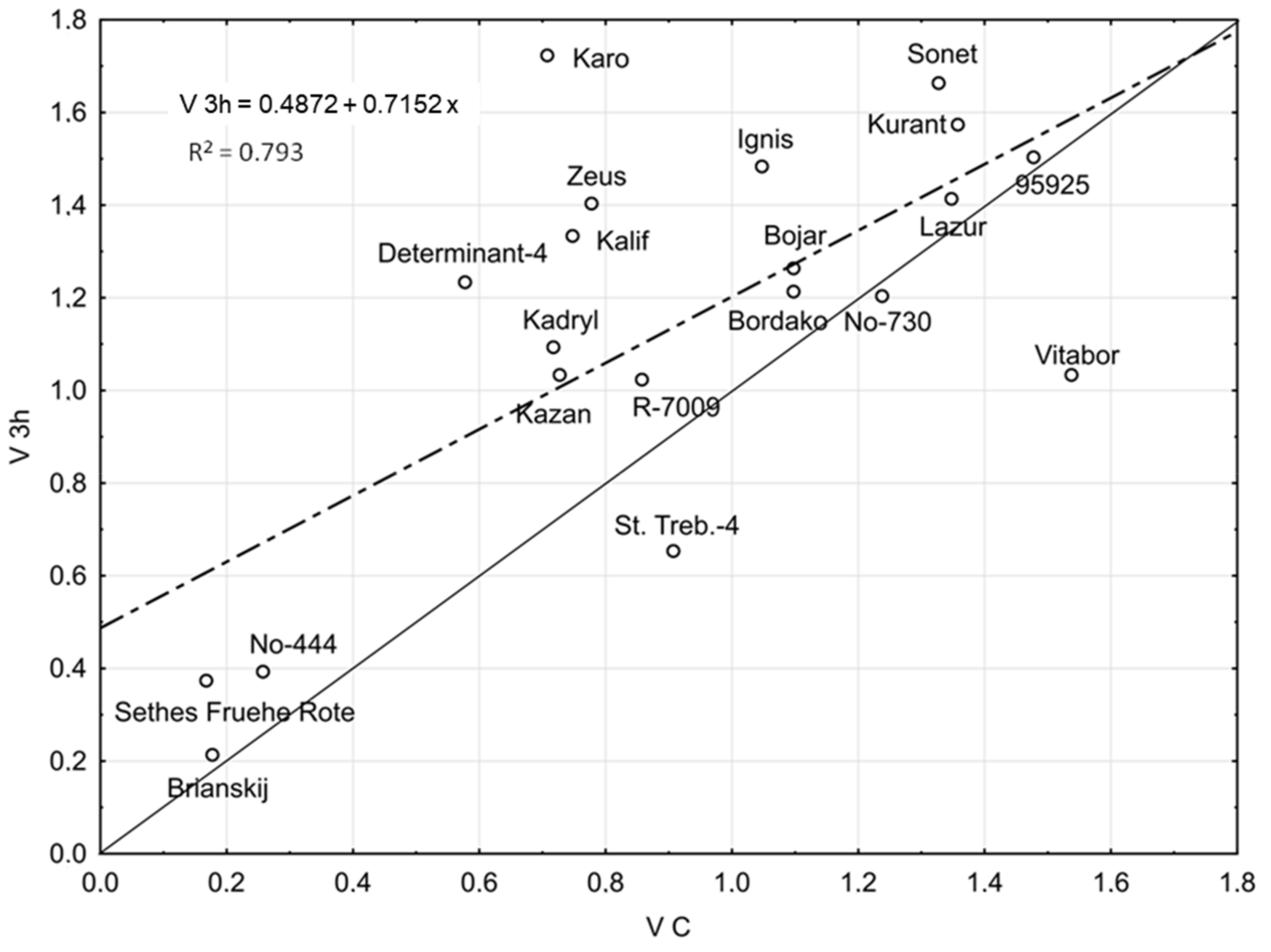
| Line/Cultivar and Origin | Temperature [°C] | V | ||
|---|---|---|---|---|
| Control | SW | 3 h | ||
| Brianskij (Russia) | 7 | 0.18 ± 0.03 c | 0.40 ± 0.06 a | 0.22 ± 0.03 b |
| 13 | 0.13 ± 0.02 c | 0.63 ± 0.09 a | 0.21 ± 0.03 b | |
| Sethes Fruehe Rote (Germany) | 7 | 0.17 ± 0.03 c | 0.29 ± 0.04 b | 0.37 ± 0.04 a |
| 13 | 1.01 ± 0.15 b | 1.44 ± 0.22 a | 1.06 ± 0.16 b | |
| No-444 (Russia) | 7 | 0.26 ± 0.04 b | 0.20 ± 0.03 b | 0.39 ± 0.06 a |
| 13 | 0.40 ± 0.05 a | 0.44 ± 0.06 a | 0.26 ± 0.04 b | |
| Determinant-4 (Belarus) | 7 | 0.58 ± 0.07 c | 0.84 ± 0.09 b | 1.23 ± 0.12 a |
| 13 | 0.60 ± 0.07 b | 0.64 ± 0.07 b | 1.06 ± 0.13 a | |
| Kalif (Poland) | 7 | 0.75 ± 0.11 b | 1.22 ± 0.18 a | 1.33 ± 0.16 a |
| 13 | 0.72 ± 0.11 c | 0.94 ± 0.14 b | 1.92 ± 0.29 a | |
| Kadryl (Poland) | 7 | 0.72 ± 0.11 b | 0.59 ± 0.09 c | 1.09 ± 0.12 a |
| 13 | 0.78 ± 0.12 c | 1.04 ± 0.15 b | 1.51 ± 0.23 a | |
| Kazan (Poland) | 7 | 0.73 ± 0.09 b | 0.85 ± 0.12 b | 1.03 ± 0.15 a |
| 13 | 0.88 ± 0.13 b | 0.89 ± 0.13 b | 1.52 ± 0.19 a | |
| Zeus (Poland) | 7 | 0.78 ± 0.12 b | 0.84 ± 0.11 b | 1.40 ± 0.22 a |
| 13 | 0.96 ± 0.13 b | 0.99 ± 0.13 b | 1.78 ± 0.24 a | |
| R-7009 × Chittick (Poland) | 7 | 0.86 ± 0.14 b | 1.04 ± 0.16 a | 1.02 ± 0.14 a |
| 13 | 1.21 ± 0.18 a | 1.03 ± 0.15 a | 0.85 ± 0.12 b | |
| Karo (Poland) | 7 | 0.71 ± 0.09 c | 1.06 ± 0.16 b | 1.72 ± 0.24 a |
| 13 | 2.45 ± 0.37 b | 3.09 ± 0.40 a | 2.34 ± 0.35 b | |
| Lazur (Poland) | 7 | 1.35 ± 0.21 a | 1.41 ± 0.19 a | 1.42 ± 0.21 a |
| 13 | 1.47 ± 0.22 b | 1.43 ± 0.20 b | 1.95 ± 027 a | |
| Ignis (Poland) | 7 | 1.05 ± 0.16 b | 1.15 ± 0.17 b | 1.48 ± 0.22 a |
| 13 | 3.06 ± 0.46 a | 3.12 ± 0.47 a | 2.87 ± 0.40 a | |
| Vitabor (Germany) | 7 | 1.53 ± 0.23 a | 1.59 ± 0.22 a | 1.06 ± 0.15 b |
| 13 | 2.67 ± 0.37 ab | 2.96 ± 0.44 a | 2.33 ± 0.33 b | |
| No-730 (Russia) | 7 | 1.24 ± 0.19 a | 0.93 ± 0.13 a | 1.20 ± 0.18 b |
| 13 | 3.34 ± 0.51 a | 3.35 ± 0.50 a | 3.32 ± 0.49 a | |
| Sonet (Poland) | 7 | 1.33 ± 0.20 a | 1.52 ± 0.23 a | 1.66 ± 0.23 a |
| 13 | 2.53 ± 0.38 a | 2.85 ± 0.43 a | 3.06 ± 0.45 a | |
| St. Treb.-4 (Germany) | 7 | 0.91 ± 0.14 a | 0.58 ± 0.07 b | 0.65 ± 0.11 b |
| 13 | 2.86 ± 0.43 a | 2.61 ± 0.39 a | 2.85 ± 0.43 a | |
| 95925 (Russia) | 7 | 1.48 ± 0.22 a | 1.52 ± 0.21 a | 1.50 ± 0.21 a |
| 13 | 3.00 ± 0.42 a | 2.39 ± 0.26 b | 2.56 ± 0.31 ab | |
| Bojar (Poland) | 7 | 1.10 ± 0.15 a | 1.07 ± 0.16 a | 1.26 ± 0.18 a |
| 13 | 0.79 ± 0.12 b | 0.80 ± 0.11 b | 1.30 ± 0.19 a | |
| Bordako (Germany) | 7 | 1.10 ± 0.17 a | 1.12 ± 0.17 a | 1.21 ± 0.18 a |
| 13 | 3.27 ± 0.50 a | 2.80 ± 0.42 a | 3.26 ± 0.48 a | |
| Kurant (Poland) | 7 | 1.36 ± 0.16 a | 1.22 ± 0.18 a | 1.57 ± 0.19 a |
| 13 | 1.17 ± 0.18 c | 1.62 ± 0.23 b | 2.53 ± 0.33 a | |
| Line/Cultivar | Temperature [°C] | V | ||
|---|---|---|---|---|
| Control | SW | 3 h | ||
| Brianskij | 7 | 41.2 ± 3.7 a | 37.1 ± 3.0 b | 28.1 ± 2.4 c |
| 13 | 30.8 ± 2.7 a | 7.5 ± 0.6 b | 2.6 ± 0.2 c | |
| Sethes Fruehe Rote | 7 | 40.6 ± 3.6 b | 49.2 ± 4.1 a | 30.8 ± 3.2 c |
| 13 | 22.9 ± 1.8 a | 13.6 ± 1.4 b | 3.1 ± 0.3 c | |
| No-444 | 7 | 2.9 ± 0.3 b | 2.9 ± 0.2 b | 8.8 ± 0.9 a |
| 13 | 0 b | 2.6 ± 0.2 a | 2.6 ± 0.2 a | |
| Determinant-4 | 7 | 3.0 ± 0.3 a | 0 b | 0 b |
| 13 | 0 a | 0 a | 0 a | |
| Kalif | 7 | 4.4 ± 0.4 a | 1.6 ± 0.2 b | 1.4 ± 0.1 b |
| 13 | 2.9 ± 0.2 b | 1.5 ± 0.1 c | 4.3 ± 0.4 a | |
| Kadryl | 7 | 13.4 ± 1.2 a | 1.8 ± 1.1 c | 4.5 ± 0.3 b |
| 13 | 6.6 ± 0.7 a | 2.7 ± 0.1 c | 4.0 ± 0.4 b | |
| Kazan | 7 | 11.1 ± 1.1 b | 19.4 ± 1.7 a | 12.5 ± 1.3 b |
| 13 | 0 a | 0 a | 0 a | |
| Zeus | 7 | 8.2 ± 0.8 a | 6.2 ± 0.7 b | 3.1 ± 0.2 c |
| 13 | 1.5 ± 0.1 b | 4.3 ± 0.4 a | 0 c | |
| R-7009 × Chittick | 7 | 2.0 ± 0.1 a | 2.0 ± 0.1 a | 2.0 ± 0.1 a |
| 13 | 3.8 ± 0.3 a | 2.9 ± 0.2 b | 3.8 ± 0.3 a | |
| Karo | 7 | 1.5 ± 0.1 b | 0 c | 3.0 ± 0.3 a |
| 13 | 7.9 ± 0.8 a | 1.6 ± 0.1 b | 0 c | |
| Lazur | 7 | 7.7 ± 0.5 a | 2.8 ± 0.3 b | 0 c |
| 13 | 1.5 ± 0.2 a | 0 b | 0 b | |
| Ignis | 7 | 6.4 ± 0.4 a | 4.5 ± 0.3 b | 6.2 ± 0.5 a |
| 13 | 5.8 ± 0.6 a | 4.3 ± 0.5 b | 4.2 ± 0.3 b | |
| Vitabor | 7 | 7.9 ± 0.5 a | 1.6 ± 0.1 b | 0 c |
| 13 | 1.6 ± 0.2 a | 0 b | 0 b | |
| No-730 | 7 | 3.2 ± 0.3 b | 0 c | 4.5 ± 0.4 a |
| 13 | 3.2 ± 0.3 a | 1.4 ± 0.1 b | 1.4 ± 0.1 b | |
| Sonet | 7 | 5.9 ± 0.5 a | 4.5 ± 0.5 b | 0 c |
| 13 | 4.2 ± 0.4 a | 4.2 ± 0.3 a | 1.4 ± 0.1 b | |
| St. Treb.-4 | 7 | 6.0 ± 0.5 c | 11.9 ± 1.2 a | 7.8 ± 0.8 b |
| 13 | 4.3 ± 0.5 b | 5.3 ± 0.6 a,b | 5.8 ± 0.6 a | |
| 95925 | 7 | 3.2 ± 0.3 a | 1.5 ± 0.1 b | 0 c |
| 13 | 0 a | 0 a | 0 a | |
| Bojar | 7 | 10.9 ± 0.8 a | 8.6 ± 0.7 b | 8.6 ± 0.7 b |
| 13 | 5.8 ± 0.6 a | 5.9 ± 0.6 a | 2.9 ± 0.2 b | |
| Bordako | 7 | 7.7 ± 0.7 a | 7.8 ± 0.8 a | 4.6 ± 0.3 b |
| 13 | 5.8 ± 0.6 a | 1.4 ± 0.2 b | 0 c | |
| Kurant | 7 | 1.5 ± 0.2 b | 1.5 ± 0.1 b | 3.1 ± 0.2 a |
| 13 | 0 b | 1.4 ± 0.1 a | 0 b | |
| Temperature [°C] | Treatment | Group I | Group II | Group III | |||
|---|---|---|---|---|---|---|---|
| GA8 | GAs | GA8 | GAs | GA8 | GAs | ||
| 7 | Control | 128 ± 15 b | 988 ± 129 b | 458 ± 52 b | 598 ± 77 b | 594 ± 71 a | 904 ± 91 a |
| SW | 74 ± 8 c | 666 ± 80 d | 601 ± 72 a | 810 ± 97 a | 392 ± 43 c | 675 ± 61 c | |
| 3 h | 149 ± 17 a | 1183 ± 153 a | 433 ± 47 b | 449 ± 53 c | 427 ± 46 b | 783 ± 74 b | |
| 13 | Control | 64 ± 7 d | 478 ± 57 e | 420 ± 50 b | 704 ± 77 b | 304 ± 33 c | 630 ± 72 c |
| SW | 115 ± 13 b | 750 ± 97 c | 384 ± 46 c | 644 ± 84 b | 326 ± 35 c | 642 ± 73 c | |
| 3 h | 40 ± 5 e | 338 ± 44 f | 442 ± 48 b | 466 ± 55 c | 308 ± 36 c | 839 ± 89 a | |
| Gibberellin | Control | Smoke Water | 3-h Hydropriming | |||
|---|---|---|---|---|---|---|
| 7 °C | 13 °C | 7 °C | 13 °C | 7 °C | 13 °C | |
| Group I | ||||||
| GA1 | 450 ± 49 a | 167 ± 16 d | 210 ± 18 c | 205 ± 18 c | 367 ± 33 b | 108 ± 9 e |
| GA3 | 229 ± 25 b | 138 ± 12 d | 165 ± 14 c | 239 ± 22 b | 413 ± 37 a | 59 ± 6 e |
| GA4 | 183 ± 20 c | 120 ± 10 e | 184 ± 17 c | 214 ± 19 b | 262 ± 24 a | 140 ± 15 d |
| GA5 | 33 ± 3 a | 18 ± 2 b | 6 ± 3 b | 17 ± 2 b | 13 ± 2 c | 13 ± 1 c |
| GA6 | 93 ± 11 b | 5 ± 3 d | 92 ± 8 b | 52 ± 4 c | 128 ± 12 a | 8 ± 2 e |
| Group II | ||||||
| GA1 | 401 ± 40 a | 411 ± 36 a | 436 ± 39 a | 392 ± 43 a | 277 ± 25 b | 273 ± 22 b |
| GA3 | 89 ± 9 a | 43 ± 9 b | 54 ± 5 b | 87 ± 9 a | 85 ± 8 a | 2 ± 8 a |
| GA4 | 41 ± 3 c | 42 ± 9 c | 9 ± 8 a | 74 ± 6 b | 8 ± 3 c | 41 ± 4 c |
| GA5 | 26 ± 2 a | 22 ± 2 b | 13 ± 2 d | 7 ± 3 a | 13 ± 2 d | 7 ± 2 c |
| GA6 | 41 ± 5 d | 187 ± 17 b | 208 ± 19 a | 2 ± 9 c | 6 ± 3 e | 44 ± 4 d |
| Group III | ||||||
| GA1 | 321 ± 28 a,b | 267 ± 24 b | 295 ± 21 b | 294 ± 26 b | 366 ± 32 a | 281 ± 25 b |
| GA3 | 236 ± 21 a | 94 ± 7 d | 186 ± 17 b | 135 ± 12 c | 198 ± 22 b | 158 ± 14 c |
| GA4 | 63 ± 6 c | 7 ± 7 b | 4 ± 4 c | 60 ± 7 c | 98 ± 9 b | 220 ± 20 a |
| GA5 | 87 ± 8 a | 61 ± 5 b | 45 ± 3 c | 3 ± 4 c | 32 ± 3 d | 21 ± 2 e |
| GA6 | 197 ± 17 a | 111 ± 9 c | 96 ± 9 c,d | 108 ± 18 c | 89 ± 9 d | 159 ± 14 b |
| Temperature [°C] | Treatment | Group I | Group II | Group III | |||
|---|---|---|---|---|---|---|---|
| ABA-glc | ABA | ABA-glc | ABA | ABA-glc | ABA | ||
| 7 | Control | 573 ± 63 c | 595 ± 71 a | 1684 ± 202 a | 425 ± 51 a | 1764 ± 194 a | 204 ± 26 b |
| SW | 632 ± 69 b | 184 ± 23 d | 1324 ± 145 b | 469 ± 62 a | 908 ± 100 c | 279 ± 39 a | |
| 3 h | 842 ± 92 a | 306 ± 42 b | 1140 ± 125 c | 249 ± 29 b | 1196 ± 143 b | 143 ± 17 c | |
| 13 | Control | 642 ± 70 b | 578 ± 10 a | 1319 ± 145 b | 419 ± 62 a | 931 ± 102 c | 216 ± 27 b |
| SW | 652 ± 71 b | 242 ± 31 c | 1046 ± 104 c | 284 ± 36 b | 752 ± 90 d | 105 ± 12 e | |
| 3 h | 628 ± 75 b | 178 ± 21 d | 708 ± 77 d | 276 ± 35 b | 754 ± 90 d | 126 ± 16 d | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Wolko, B. Seed Hydropriming and Smoke Water Significantly Improve Low-Temperature Germination of Lupinus angustifolius L. Int. J. Mol. Sci. 2018, 19, 992. https://doi.org/10.3390/ijms19040992
Płażek A, Dubert F, Kopeć P, Dziurka M, Kalandyk A, Pastuszak J, Wolko B. Seed Hydropriming and Smoke Water Significantly Improve Low-Temperature Germination of Lupinus angustifolius L. International Journal of Molecular Sciences. 2018; 19(4):992. https://doi.org/10.3390/ijms19040992
Chicago/Turabian StylePłażek, Agnieszka, Franciszek Dubert, Przemysław Kopeć, Michał Dziurka, Agnieszka Kalandyk, Jakub Pastuszak, and Bogdan Wolko. 2018. "Seed Hydropriming and Smoke Water Significantly Improve Low-Temperature Germination of Lupinus angustifolius L." International Journal of Molecular Sciences 19, no. 4: 992. https://doi.org/10.3390/ijms19040992
APA StylePłażek, A., Dubert, F., Kopeć, P., Dziurka, M., Kalandyk, A., Pastuszak, J., & Wolko, B. (2018). Seed Hydropriming and Smoke Water Significantly Improve Low-Temperature Germination of Lupinus angustifolius L. International Journal of Molecular Sciences, 19(4), 992. https://doi.org/10.3390/ijms19040992