Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report
Abstract
:1. Introduction
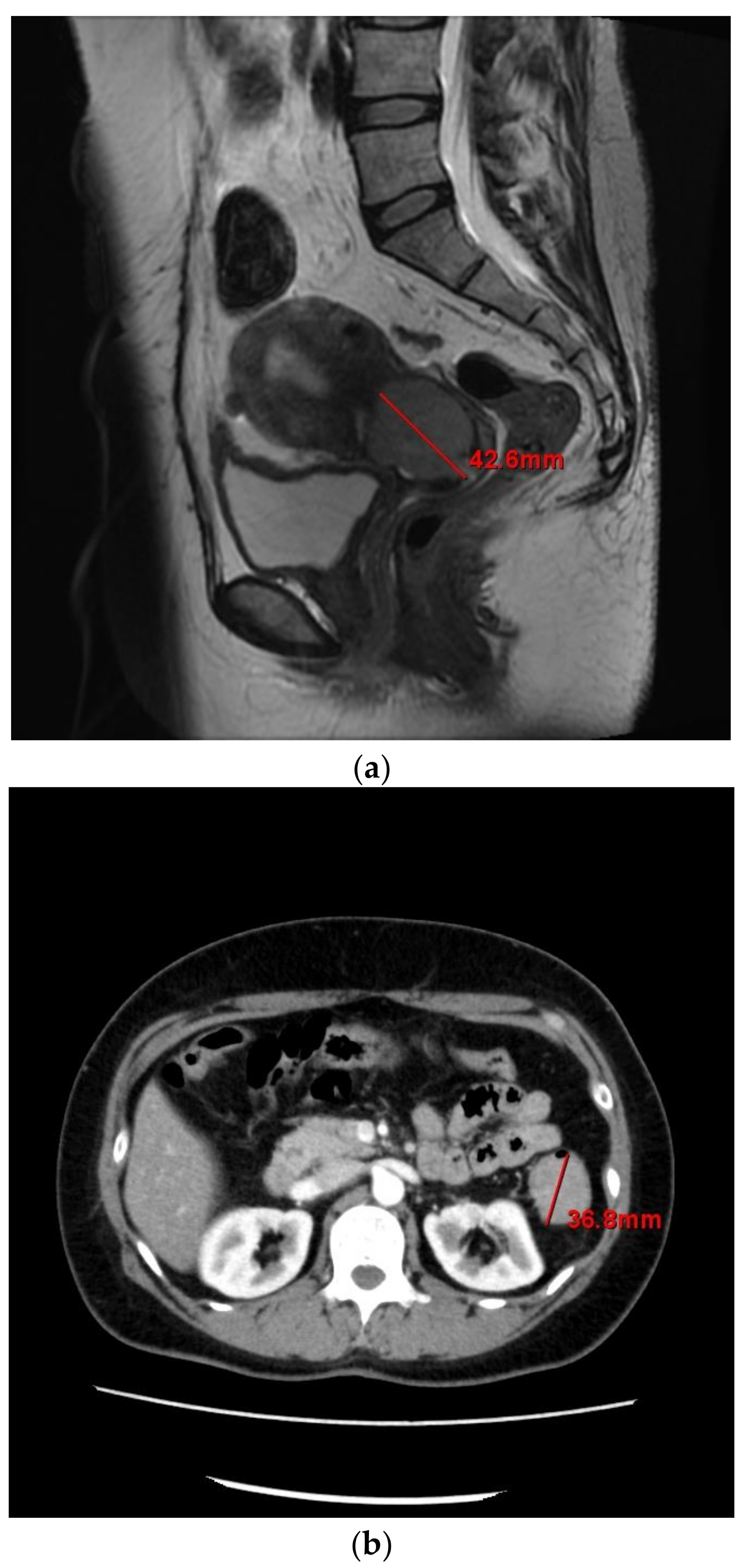
2. Case
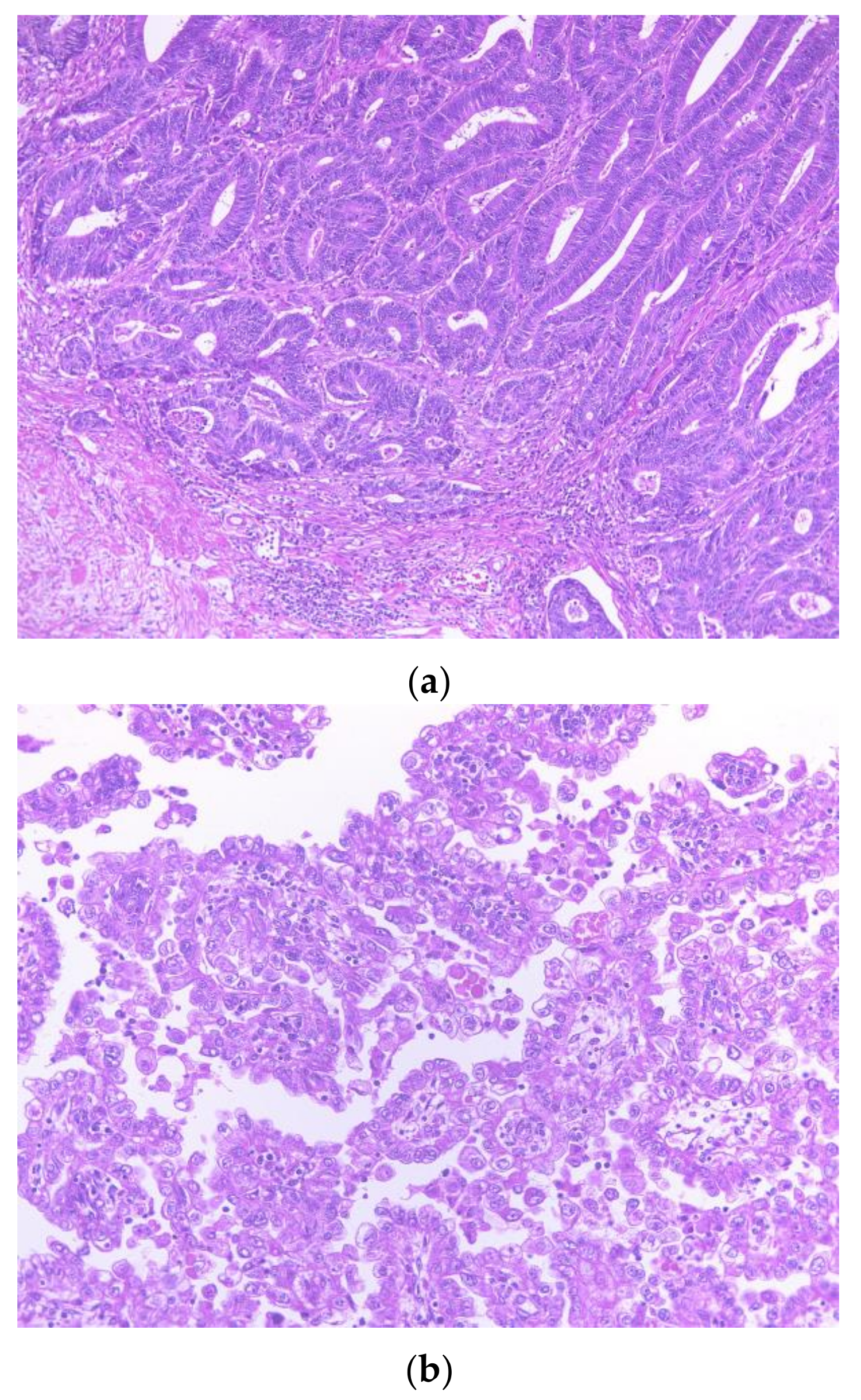
2.1. Pathological Findings
2.2. Immunohistochemical and MSI Analysis Findings
3. Discussion
Author Contributions
Conflicts of Interest
Abbreviations
| MMR | mismatch repair |
| MLH1 | MutL Homolog 1 |
| MSH2 | MutS Homolog 2 |
| MSH6 | MutS Homolog 6 |
| PMS2 | PMS1 Homolog 2 |
| MSI | microsatellite instability |
| CA | carbohydrate antigen |
| CT | computed tomography |
| DES | diethylstilbesterol |
References
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Baglietto, L.; Lindor, N.M.; Dowty, J.G.; White, D.M.; Wagner, A.; Gomez Garcia, E.B.; Vriends, A.H.; Cartwright, N.R.; Barnetson, R.A.; Farrington, S.M.; et al. Risks of Lynch syndrome cancers for MSH6 mutation carriers. J. Natl. Cancer Inst. 2010, 102, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Senter, L.; Clendenning, M.; Sotamaa, K.; Hampel, H.; Green, J.; Potter, J.D.; Lindblom, A.; Lagerstedt, K.; Thibodeau, S.N.; Lindor, N.M.; et al. The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology 2008, 135, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Bonadona, V.; Bonaiti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.J.; Caron, O.; et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011, 305, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Dowty, J.G.; Win, A.K.; Buchanan, D.D.; Lindor, N.M.; Macrae, F.A.; Clendenning, M.; Antill, Y.C.; Thibodeau, S.N.; Casey, G.; Gallinger, S.; et al. Cancer risks for MLH1 and MSH2 mutation carriers. Hum. Mutat. 2013, 34, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Mongiat-Artus, P.; Miquel, C.; Flejou, J.F.; Coulet, F.; Verine, J.; Buhard, O.; Soliman, H.; Teillac, P.; Praz, F. Spectrum of molecular alterations in colorectal, upper urinary tract, endocervical, and renal carcinomas arising in a patient with hereditary non-polyposis colorectal cancer. Virchows Arch. 2006, 449, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Curtin, J.P.; Mittal, K.; Hiotis, K.L. Cervical adenocarcinoma in a patient with Lynch syndrome, Muir-Torre variant. J. Clin. Oncol. 2012, 30, E5–E6. [Google Scholar] [CrossRef] [PubMed]
- Moat, M.; O’Donnell, R.L.; McCluggage, W.G.; Ralte, A.; Edmondson, R.J. Gastric-type adenocarcinoma of the cervix in a patient with Lynch syndrome: A case report. Gynecol. Oncol. Rep. 2014, 13, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L. Behavior of estrogen-associated female genital tract cancer and its relation to neoplasia following intrauterine exposure to diethylstilbestrol (DES). Gynecol. Oncol. 2000, 76, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, P.F.; Maier, R.C. Clear cell adenocarcinoma of the cervix unrelated to diethylstilbestrol exposure. Obstet. Gynecol. 1983, 62, 720–727. [Google Scholar] [PubMed]
- Ryan, S.; Jenkins, M.A.; Win, A.K. Risk of prostate cancer in Lynch syndrome: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Win, A.K.; Lindor, N.; Jenkins, M. Risk of breast cancer in Lynch syndrome: A systematic review. Breast Cancer Res. 2013, 15, R27. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Nakayama, K.; Minamoto, T.; Ishibashi, T.; Ohnishi, K.; Yamashita, H.; Ono, R.; Sasamori, H.; Razia, S.; Hossain, M.M.; et al. Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report. Int. J. Mol. Sci. 2018, 19, 979. https://doi.org/10.3390/ijms19040979
Nakamura K, Nakayama K, Minamoto T, Ishibashi T, Ohnishi K, Yamashita H, Ono R, Sasamori H, Razia S, Hossain MM, et al. Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report. International Journal of Molecular Sciences. 2018; 19(4):979. https://doi.org/10.3390/ijms19040979
Chicago/Turabian StyleNakamura, Kohei, Kentaro Nakayama, Toshiko Minamoto, Tomoka Ishibashi, Kaori Ohnishi, Hitomi Yamashita, Ruriko Ono, Hiroki Sasamori, Sultana Razia, Mohammad Mahmud Hossain, and et al. 2018. "Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report" International Journal of Molecular Sciences 19, no. 4: 979. https://doi.org/10.3390/ijms19040979
APA StyleNakamura, K., Nakayama, K., Minamoto, T., Ishibashi, T., Ohnishi, K., Yamashita, H., Ono, R., Sasamori, H., Razia, S., Hossain, M. M., Kamrunnahar, S., Ishikawa, M., Ishikawa, N., & Kyo, S. (2018). Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report. International Journal of Molecular Sciences, 19(4), 979. https://doi.org/10.3390/ijms19040979



