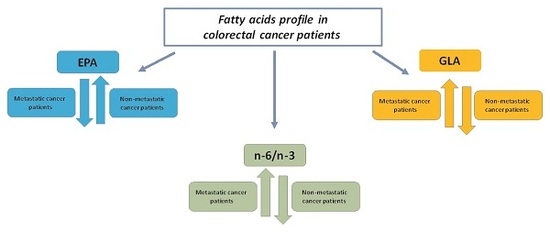
Differential Tissue Fatty Acids Profiling between Colorectal Cancer Patients with and without Synchronous Metastasis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. In Vitro Isolation of Erythrocytes
4.3. Fatty Acids Extraction and Preparation ofFatty Acid Methyl Esters from Erythrocytes and Tissue Samples
4.4. Gas Chromatography and Fatty Acids Quantification
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liao, F.; He, W.; Jiang, C.; Yin, C.; Guo, G.; Chen, X.; Qiu, H.; Rong, Y.; Zhang, B.; Xu, D.; et al. A high LDL-C to HDL-C ratio predicts poor prognosis for initially metastatic colorectal cancer patients with elevations in LDL-C. Onco Targets Ther. 2015, 5, 3135–3142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, T.T.; Shen, D.; Chen, X.P.; Wu, X.H.; Hua, D. Preoperative serum lipid profile and outcome in nonmetastatic colorectal cancer. Chronic Dis. Transl. Med. 2016, 2, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, P.; Xuan, J.; Zhu, C.; Liu, J.; Shan, L.; Du, Q.; Ren, Y.; Ye, J. Cholesterol enhances colorectal cancer progression via ROS elevation and MAPK signaling pathway activation. Cell. Physiol. Biochem. 2017, 42, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Radisauskas, R.; Kuznickiene, I.; Milinaviciene, E.; Everatt, R. Hypertension, serum lipids and cancer risk: A review of epidemiological evidence. Medicina (Kaunas) 2016, 52, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tian, Z. Dyslipidemia and colorectal cancer risk: A meta-analysis of prospective studies. Cancer Causes Control 2015, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Altomare, D.F.; Correale, M.; Ruggeri, E.; D’Attoma, B.; Mastrosimini, A.; Guerra, V.; Caruso, M.G. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology 2005, 68, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Qian, H.X.; Qin, L.; Zhou, X.J.; Zhang, B. Serum LDL-C and LDL-C/HDL-C ratio are positively correlated to lymph node stages in males with colorectal cancer. Hepato-Gastroenterology 2011, 58, 383–387. [Google Scholar] [PubMed]
- Muntoni, S.; Atzori, L.; Mereu, R.; Satta, G.; Macis, M.D.; Congia, M.; Tedde, A.; Desogus, A.; Muntoni, S. Serumlipoproteinsand cancer. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, H.; Nair, J.; Owen, R.W. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: Emerging evidence for their role as risk modifiers. Carcinogenesis 1999, 20, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front. Oncol. 2013, 3, 224. [Google Scholar] [CrossRef] [PubMed]
- May-Wilson, S.; Sud, A.; Law, P.J.; Palin, K.; Tuupanen, S.; Gylfe, A.; Hänninen, U.A.; Cajuso, T.; Tanskanen, T.; Kondelin, J.; et al. Pro-inflammatory fatty acid profile and colorectal cancer risk: A Mendelian randomisation analysis. Eur. J. Cancer 2017, 84, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Altomare, D.F.; Calvani, M.; Orlando, A.; Bifulco, M.; D’Attoma, B.; Caruso, M.G. Fatty acid synthase hyperactivation in human colorectal cancer. Oncology 2007, 71, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Notarnicola, M.; Caruso, M.G.; Scavo, M.P.; Viggiani, M.T.; Tutino, V.; Polimeno, L.; Pesetti, B.; Di Leo, A.; Francavilla, A. Olive oil and omega-3 polyunsaturated fatty acids suppress intestinal polyp growth by modulating the apoptotic process in ApcMin/+ mice. Carcinogenesis 2014, 35, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Cottet, V.; Vaysse, C.; Scherrer, M.L.; Ortega-Deballon, P.; Lakkis, Z.; Delhorme, J.B.; Deguelte-Lardière, S.; Combe, N.; Bonithon-Kopp, C. Fatty acid composition of adipose tissue and colorectal cancer: A case-control study. Am. J. Clin. Nutr. 2015, 101, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Coviello, G.; Tutino, V.; Notarnicola, M.; Caruso, M.G. Erythrocyte membrane fatty acids profile in colorectal cancer patients: A preliminary study. Anticancer Res. 2014, 34, 4775–4779. [Google Scholar] [PubMed]
- Tutino, V.; Caruso, M.G.; De Leonardis, G.; De Nunzio, V.; Notarnicola, M. Tissue Fatty Acid Profile Is Differently Modulated From Olive Oil and Omega-3 Polyunsaturated fatty acids in ApcMin/+ Mice. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Asiago, V.; Alvarado, L.Z.; Shanaiah, N.; Gowda, G.A.; Owusu-Sarfo, K.; Ballas, R.A.; Raftery, D. Early detection of recurrent breast cancer using metabolic profiling. Cancer Res. 2010, 70, 8309–8318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Han, Y.; Wang, X. Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Lett. 2014, 345, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, H.; Kim, S.H.; Jin, H.; Bae, J.; Choi, H.K. Discovery of potential biomarkers in human melanoma cells with different metastatic potential by metabolic and lipidomic profiling. Sci. Rep. 2017, 7, 8864. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, K.M.; Kim, S.H.; Kwon, Y.J.; Chun, Y.J.; Choi, H.K. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget 2016, 41, 6711–67128. [Google Scholar] [CrossRef] [PubMed]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Roselli, M.; Palmirotta, R.; Della-Morte, D.; Ferroni, P.; Guadagni, F. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J. Gastroenterol. 2014, 20, 5177–5190. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.; Yehuda-Shnaidman, E. Putative role of adipose tissue in growth and metabolism of colon cancer cells. Front. Oncol. 2014, 4, 164. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Miccolis, A.; Tutino, V.; Lorusso, D.; Caruso, M.G. Low levels of lipogenic enzymes in peritumoral adipose tissue of colorectal cancer patients. Lipids 2012, 47, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Tutino, V.; De Nunzio, V.; Dituri, F.; Caruso, M.G.; Giannelli, G. Dietary omega-3 polyunsaturated fatty acids inhibit tumorgrowth in transgenic ApcMin/+ mice, correlating with CB1 receptor upregulation. Int. J. Mol. Sci. 2017, 18, 485. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Messa, C.; Refolo, M.G.; Tutino, V.; Miccolis, A.; Caruso, M.G. Synergic effect of eicosapentaenoic acid and lovastatin on gene expression of HMGCoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Messa, C.; Refolo, M.G.; Tutino, V.; Miccolis, A.; Caruso, M.G. Polyunsaturated fatty acids reduce fatty acid synthase and hydroxy-methyl-glutaryl CoA-reductase gene expression and promote apoptosis in HepG2 cell line. Lipids Health Dis. 2011, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Adair, J.E.; Lih, F.B.; His, L.C.; Rubino, M.; Eling, T.E.; Tomer, T.E.; Yashiro, M.; Hirakawa, K.; Olden, K.; et al. Elevated dietary linoleic acid increases gastric carcinoma cell invasion and metastasis in mice. Br. J. Cancer 2010, 103, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Trans. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, T.; Nikkari, T. The effect of storage on the fatty acid composition of human serum. Clin. Chim. Acta 1981, 114, 111–116. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. J. Vis. Exp. 2014, 85, e51445. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; Bonfiglio, C.; Cozzolongo, R.; Giannuzzi, V.; De Nunzio, V.; De Leonardis, G.; Abbrescia, D.I.; Franco, I.; et al. Significant decrease of saturation index in erythrocytes membrane from subjects with non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 2017, 16, 160. [Google Scholar] [CrossRef] [PubMed]
| Patients (n = 51) | |
|---|---|
| Age (mean ± SD) | 68.3 ± 13.3 |
| Sex | |
| Male | 29 |
| Female | 22 |
| BMI (kg/m2) a | 22.2 ± 4.03 |
| Tumor Side b | |
| Right | 16 |
| Left | 35 |
| Tumor Stage c | |
| Stage I | 7 |
| Stage II | 17 |
| Stage III | 16 |
| Stage IV | 11 |
| Histological Grading | |
| Well-differentiated (G1) | 2 |
| Moderately-differentiated (G2) | 27 |
| Poorly-differentiated (G3) | 22 |
| Metastases | 25 |
| No metastases | 26 |
| CRC Patients | |||
|---|---|---|---|
| Fatty acids | No Metastases | Metastases | p-Value ° |
| SFA | 55.02 ± 6.2 | 53.7 ± 8.3 | 0.92 |
| MUFA | 22.02 ± 3.8 | 21.11 ± 4.1 | 0.81 |
| PUFAs | 23.94 ± 5.6 | 25.1 ± 3.9 | 0.75 |
| n6/n3 | 4.5 ± 1.6 | 4.6 ± 1.2 | 0.52 |
| Stearic acid | 21.5 ± 4.6 | 22.3 ± 6.71 | 0.85 |
| Oleic acid | 12.7 ± 2.3 | 12.9 ± 3.2 | 0.95 |
| SI * | 1.9 ± 0.5 | 2.2 ± 1.4 | 0.88 |
| GLA | 0.03 ± 0.1 | 0.09 ± 0.2 | 0.25 |
| EPA | 0.44 ± 0.58 | 0.53 ± 0.3 | 0.70 |
| DHA | 2.76 ± 1.5 | 2.8 ± 1.4 | 0.96 |
| Normal Mucosa | Tumor Tissue | |||||
|---|---|---|---|---|---|---|
| Fatty acids | No Metastases | Metastases | p-Value ° | No Metastases | Metastases | p-Value ° |
| SFAs | 42.09 ± 5.1 | 47.54 ± 7.8 | 0.62 | 49.97 ± 4.2 | 48.53 ± 3.9 | 0.84 |
| MUFAs | 40.54 ± 3.5 | 38.22 ± 4.0 | 0.73 | 32.20 ± 2.7 | 33.71 ± 4.3 | 0.90 |
| PUFAs | 17.37 ± 2.0 | 15.99 ± 1.9 | 0.60 | 17.83 ± 3.1 | 16.7 ± 0.8 | 0.77 |
| n-6/n-3 | 8.85 ± 5.82 | 15.10 ± 16.2 | 0.40 | 6.2 ± 4.5 | 15.2 ± 9.2 | 0.04 |
| Stearic acid | 15.2 ± 6.8 | 18.8 ± 7.8 | 0.22 | 18.56 ± 7.5 | 19.6 ± 6.7 | 0.71 |
| Oleic acid | 28.2 ± 8.4 | 35.5 ± 6.6 | 0.30 | 18.6 ± 4.5 | 21.3 ± 3.9 | 0.62 |
| SI * | 0.95 ± 1.5 | 0.72 ± 1.42 | 0.51 | 0.98 ± 0.49 | 1.5 ± 0.7 | 0.86 |
| GLA | 0.16 ± 0.6 | 0.26 ± 0.8 | 0.83 | 0.09 ± 0.11 | 0.34 ± 0.2 | 0.05 |
| EPA | 0.47 ± 0.6 | 0.36 ± 0.7 | 0.32 | 0.99 ± 0.54 | 0.46 ± 0.54 | 0.002 |
| DHA | 0.4 ± 0.35 | 0.2 ± 0.18 | 0.30 | 0.69 ± 0.48 | 0.54 ± 0.6 | 0.24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notarnicola, M.; Lorusso, D.; Tutino, V.; De Nunzio, V.; De Leonardis, G.; Marangelli, G.; Guerra, V.; Veronese, N.; Caruso, M.G.; Giannelli, G. Differential Tissue Fatty Acids Profiling between Colorectal Cancer Patients with and without Synchronous Metastasis. Int. J. Mol. Sci. 2018, 19, 962. https://doi.org/10.3390/ijms19040962
Notarnicola M, Lorusso D, Tutino V, De Nunzio V, De Leonardis G, Marangelli G, Guerra V, Veronese N, Caruso MG, Giannelli G. Differential Tissue Fatty Acids Profiling between Colorectal Cancer Patients with and without Synchronous Metastasis. International Journal of Molecular Sciences. 2018; 19(4):962. https://doi.org/10.3390/ijms19040962
Chicago/Turabian StyleNotarnicola, Maria, Dionigi Lorusso, Valeria Tutino, Valentina De Nunzio, Giampiero De Leonardis, Gisella Marangelli, Vito Guerra, Nicola Veronese, Maria Gabriella Caruso, and Gianluigi Giannelli. 2018. "Differential Tissue Fatty Acids Profiling between Colorectal Cancer Patients with and without Synchronous Metastasis" International Journal of Molecular Sciences 19, no. 4: 962. https://doi.org/10.3390/ijms19040962
APA StyleNotarnicola, M., Lorusso, D., Tutino, V., De Nunzio, V., De Leonardis, G., Marangelli, G., Guerra, V., Veronese, N., Caruso, M. G., & Giannelli, G. (2018). Differential Tissue Fatty Acids Profiling between Colorectal Cancer Patients with and without Synchronous Metastasis. International Journal of Molecular Sciences, 19(4), 962. https://doi.org/10.3390/ijms19040962