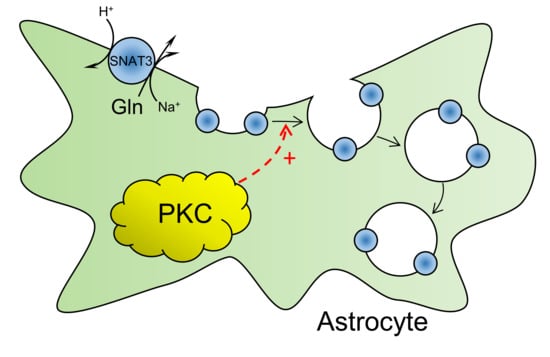
PKC-Mediated Modulation of Astrocyte SNAT3 Glutamine Transporter Function at Synapses in Situ
Abstract
:1. Introduction
2. Results
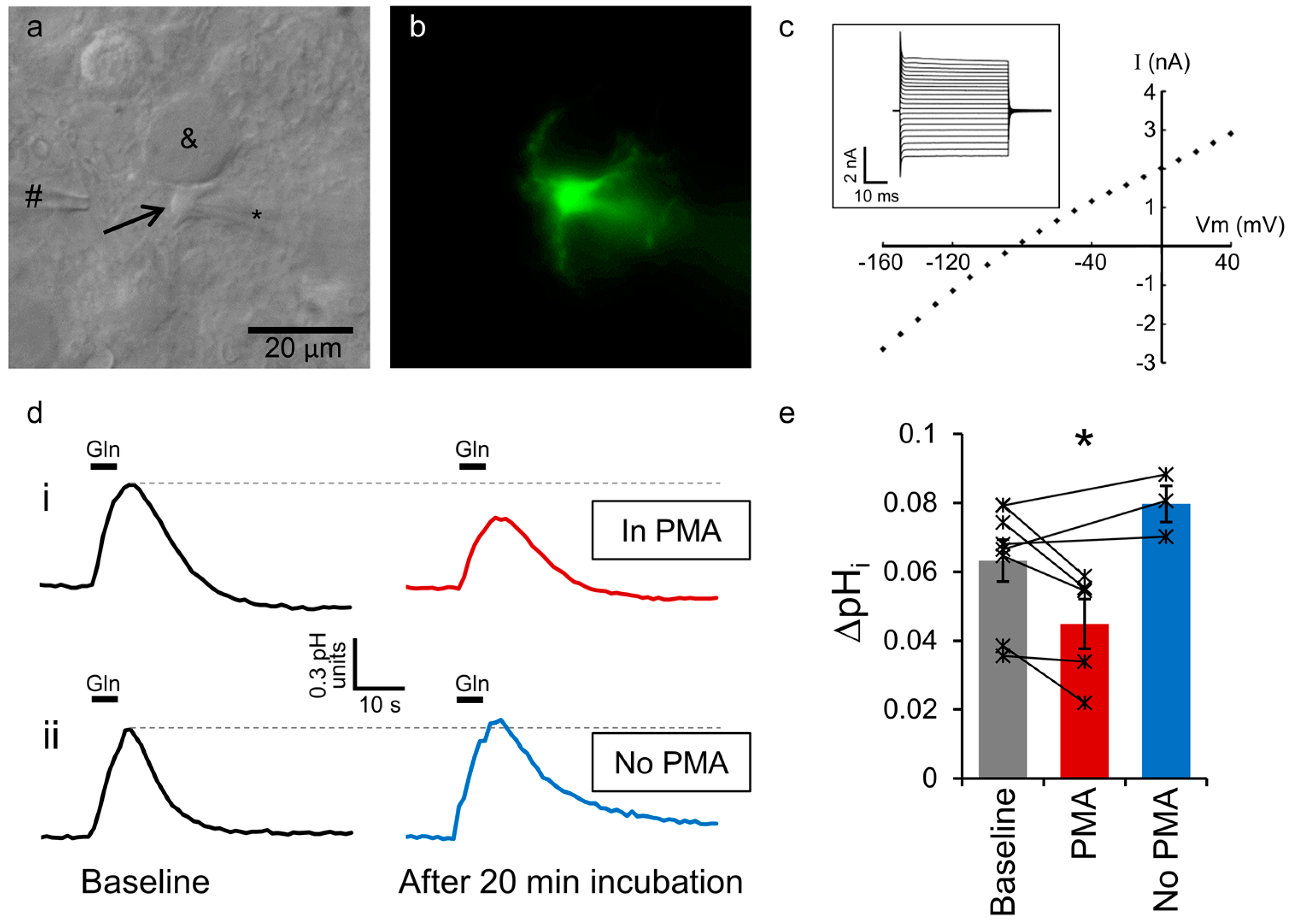
2.1. Astrocytic SNAT3 Glutamine Transport in Acutely Isolated Brain Slices
2.2. SNAT3 Function is Reduced by PKC Activation
2.3. Conventional PKC Isoforms Inhibit SNAT3 Function
2.4. PKC Inhibition of Mouse SNAT3 Function
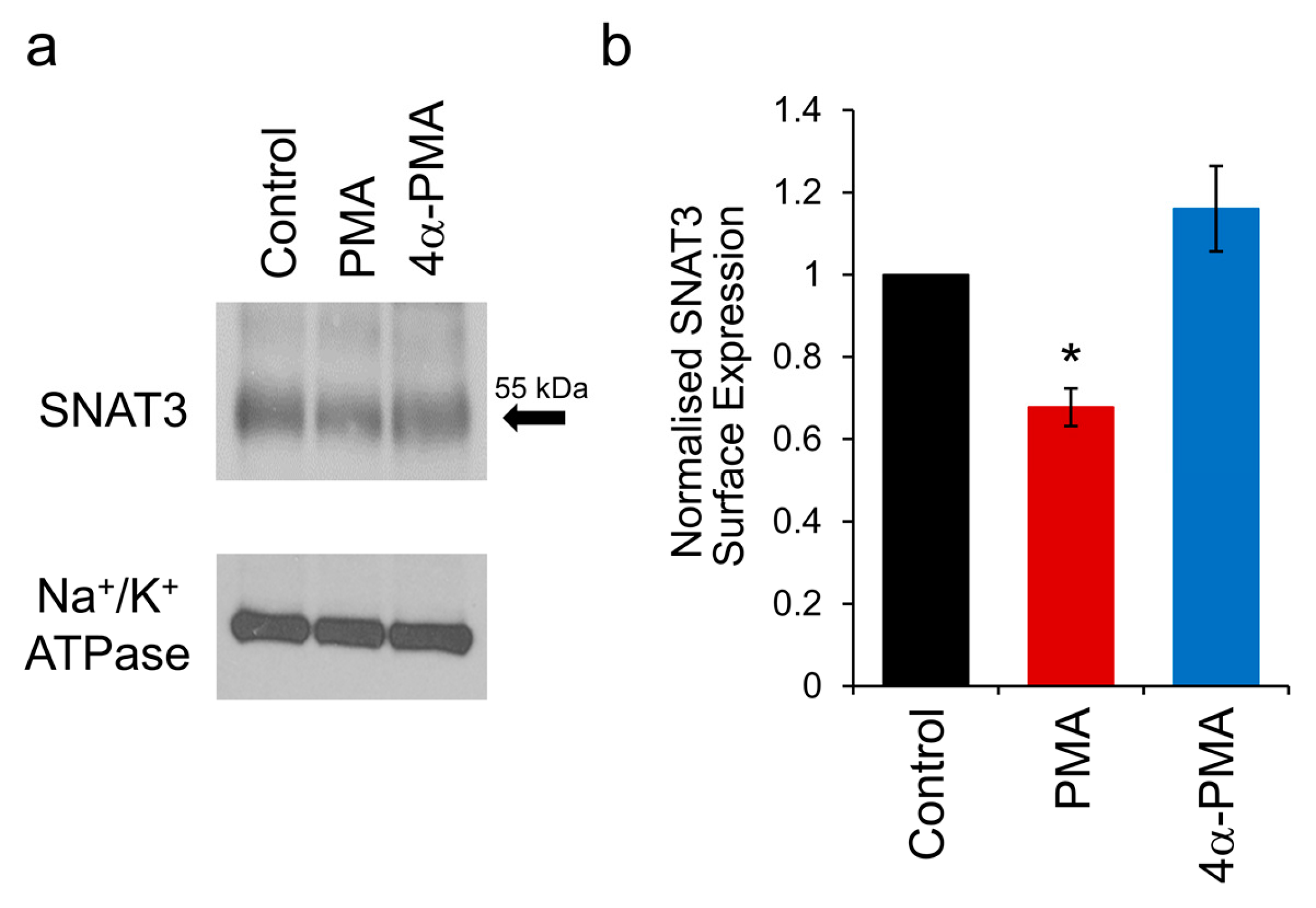
2.5. PKC Activation Induces Internalisation of SNAT3
3. Discussion
4. Materials and Methods
4.1. Brain Slice Preparation
4.2. Electrophysiological Recording
4.3. Fluorescent pH Imaging
4.4. Surface Biotinylation
4.5. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newsholme, P.; Procopio, J.; Lima, M.M.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Daikhin, Y.; Yudkoff, M. Compartmentation of brain glutamate metabolism in neurons and glia. J. Nutr. 2000, 130, 1026S–1031S. [Google Scholar] [CrossRef] [PubMed]
- Hertz, L.; Dringen, R.; Schousboe, A.; Robinson, S.R. Astrocytes: Glutamate producers for neurons. J. Neurosci. Res. 1999, 57, 417–428. [Google Scholar] [CrossRef]
- Rothman, D.L.; Behar, K.L.; Hyder, F.; Shulman, R.G. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: Implications for brain function. Annu. Rev. Physiol. 2003, 65, 401–427. [Google Scholar] [CrossRef] [PubMed]
- Billups, D.; Marx, M.C.; Mela, I.; Billups, B. Inducible presynaptic glutamine transport supports glutamatergic transmission at the calyx of Held synapse. J. Neurosci. 2013, 33, 17429–17434. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Dulla, C.G.; Farzampour, Z.; Taylor-Weiner, A.; Huguenard, J.R.; Reimer, R.J. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron 2014, 81, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.C.; Marx, M.C.; Hulme, S.R.; Broer, S.; Billups, B. SNAT3-mediated glutamine transport in perisynaptic astrocytes in situ is regulated by intracellular sodium. Glia 2017, 65, 900–916. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.C.; Billups, D.; Billups, B. Maintaining the presynaptic glutamate supply for excitatory neurotransmission. J. Neurosci. Res. 2015, 93, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Hare, N.; Bubb, W.A.; McEwan, S.R.; Broer, A.; McQuillan, J.A.; Balcar, V.J.; Conigrave, A.D.; Broer, S. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: Further evidence for metabolic compartmentation. J. Neurochem. 2003, 85, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Fricke, M.N.; Jones-Davis, D.M.; Mathews, G.C. Glutamine uptake by System A transporters maintains neurotransmitter GABA synthesis and inhibitory synaptic transmission. J. Neurochem. 2007, 102, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Yan, X.; Weng, H.R. Glial glutamate transporter and glutamine synthetase regulate GABAergic synaptic strength in the spinal dorsal horn. J. Neurochem. 2012, 121, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.L.; Carlson, G.C.; Coulter, D.A. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J. Neurosci. 2006, 26, 8537–8548. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cox, C.L. Attenuation of inhibitory synaptic transmission by glial dysfunction in rat thalamus. Synapse 2011, 65, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. The SLC38 family of sodium-amino acid co-transporters. Pflugers Arch. 2014, 466, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Broer, A.; Albers, A.; Setiawan, I.; Edwards, R.H.; Chaudhry, F.A.; Lang, F.; Wagner, C.A.; Broer, S. Regulation of the glutamine transporter SN1 by extracellular pH and intracellular sodium ions. J. Physiol. 2002, 539, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Krizaj, D.; Larsson, P.; Reimer, R.J.; Wreden, C.; Storm-Mathisen, J.; Copenhagen, D.; Kavanaugh, M.; Edwards, R.H. Coupled and uncoupled proton movement by amino acid transport system N. EMBO J. 2001, 20, 7041–7051. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Reimer, R.J.; Krizaj, D.; Barber, D.; Storm-Mathisen, J.; Copenhagen, D.R.; Edwards, R.H. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell 1999, 99, 769–780. [Google Scholar] [CrossRef]
- Jacobson, I.; Sandberg, M.; Hamberger, A. Mass transfer in brain dialysis devices—A new method for the estimation of extracellular amino acids concentration. J. Neurosci. Methods 1985, 15, 263–268. [Google Scholar] [CrossRef]
- Kanamori, K.; Ross, B.D. Quantitative determination of extracellular glutamine concentration in rat brain, and its elevation in vivo by system A transport inhibitor, alpha-(methylamino)isobutyrate. J. Neurochem. 2004, 90, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Dolgodilina, E.; Imobersteg, S.; Laczko, E.; Welt, T.; Verrey, F.; Makrides, V. Brain interstitial fluid glutamine homeostasis is controlled by blood-brain barrier SLC7A5/LAT1 amino acid transporter. J. Cereb. Blood Flow Metab. 2016, 36, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Roderick, H.L.; Camacho, P.; Jiang, J.X. Identification and characterization of an amino acid transporter expressed differentially in liver. Proc. Natl. Acad. Sci. USA 2000, 97, 3230–3235. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Busque, S.M.; Sailer, M.; Stoeger, C.; Broer, S.; Daniel, H.; Rubio-Aliaga, I.; Wagner, C.A. Loss of function mutation of the Slc38a3 glutamine transporter reveals its critical role for amino acid metabolism in the liver, brain, and kidney. Pflugers Arch. 2016, 468, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Boulland, J.L.; Osen, K.K.; Levy, L.M.; Danbolt, N.C.; Edwards, R.H.; Storm-Mathisen, J.; Chaudhry, F.A. Cell-specific expression of the glutamine transporter SN1 suggests differences in dependence on the glutamine cycle. Eur. J. Neurosci. 2002, 15, 1615–1631. [Google Scholar] [CrossRef] [PubMed]
- Boulland, J.L.; Rafiki, A.; Levy, L.M.; Storm-Mathisen, J.; Chaudhry, F.A. Highly differential expression of SN1, a bidirectional glutamine transporter, in astroglia and endothelium in the developing rat brain. Glia 2003, 41, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.B. Regulated trafficking of neurotransmitter transporters: Common notes but different melodies. J. Neurochem. 2002, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Melikian, H.E. Neurotransmitter transporter trafficking: Endocytosis, recycling, and regulation. Pharmacol. Ther. 2004, 104, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, D.P.; Blakely, R.D. Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters. Pharmacol. Rev. 2016, 68, 888–953. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C: Poised to signal. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E395–E402. [Google Scholar] [CrossRef] [PubMed]
- Shimohama, S.; Saitoh, T.; Gage, F.H. Changes in protein kinase C isozymes in the rat hippocampal formation following hippocampal lesion. Hippocampus 1993, 3, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hillman, D.E. Immunohistochemical localization of protein kinase C delta during postnatal development of the cerebellum. Brain Res. Dev. Brain Res. 1994, 80, 19–25. [Google Scholar] [CrossRef]
- Masliah, E.; Cole, G.; Shimohama, S.; Hansen, L.; DeTeresa, R.; Terry, R.D.; Saitoh, T. Differential involvement of protein kinase C isozymes in Alzheimer’s disease. J. Neurosci. 1990, 10, 2113–2124. [Google Scholar] [PubMed]
- Barmack, N.H.; Qian, Z.; Yoshimura, J. Regional and cellular distribution of protein kinase C in rat cerebellar Purkinje cells. J. Comp. Neurol. 2000, 427, 235–254. [Google Scholar] [CrossRef]
- Todo, T.; Shitara, N.; Nakamura, H.; Takakura, K.; Tomonaga, M.; Ikeda, K. Astrocytic localization of the immunoreactivity for protein kinase C isozyme (type III) in human brain. Brain Res. 1990, 517, 351–353. [Google Scholar] [CrossRef]
- Porter, J.T.; McCarthy, K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997, 51, 439–455. [Google Scholar] [CrossRef]
- Balkrishna, S.; Broer, A.; Kingsland, A.; Broer, S. Rapid downregulation of the rat glutamine transporter SNAT3 by a caveolin-dependent trafficking mechanism in Xenopus laevis oocytes. Am. J. Physiol. Cell Physiol. 2010, 299, C1047–C1057. [Google Scholar] [CrossRef] [PubMed]
- Nissen-Meyer, L.S.; Popescu, M.C.; Hamdani el, H.; Chaudhry, F.A. Protein kinase C-mediated phosphorylation of a single serine residue on the rat glial glutamine transporter SN1 governs its membrane trafficking. J. Neurosci. 2011, 31, 6565–6575. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk-Wegrzynowicz, M.; Lee, E.; Mingwei, N.; Aschner, M. Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway. Glia 2011, 59, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Nissen-Meyer, L.S.; Chaudhry, F.A. Protein Kinase C Phosphorylates the System N Glutamine Transporter SN1 (Slc38a3) and Regulates Its Membrane Trafficking and Degradation. Front. Endocrinol. 2013, 4, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyland, M.E. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front. Biosci. 2009, 14, 2386–2399. [Google Scholar] [CrossRef]
- Benzil, D.L.; Finkelstein, S.D.; Epstein, M.H.; Finch, P.W. Expression pattern of alpha-protein kinase C in human astrocytomas indicates a role in malignant progression. Cancer Res. 1992, 52, 2951–2956. [Google Scholar] [PubMed]
- Yoshimura, S.; Sakai, H.; Nakashima, S.; Nozawa, Y.; Shinoda, J.; Sakai, N.; Yamada, H. Differential expression of Rho family GTP-binding proteins and protein kinase C isozymes during C6 glial cell differentiation. Brain Res. Mol. Brain Res. 1997, 45, 90–98. [Google Scholar] [CrossRef]
- Masliah, E.; Yoshida, K.; Shimohama, S.; Gage, F.H.; Saitoh, T. Differential expression of protein kinase C isozymes in rat glial cell cultures. Brain Res. 1991, 549, 106–111. [Google Scholar] [CrossRef]
- Brodie, C.; Kuperstein, I.; Acs, P.; Blumberg, P.M. Differential role of specific PKC isoforms in the proliferation of glial cells and the expression of the astrocytic markers GFAP and glutamine synthetase. Brain Res. Mol. Brain Res. 1998, 56, 108–117. [Google Scholar] [CrossRef]
- Schneggenburger, R.; Forsythe, I.D. The calyx of Held. Cell Tissue Res. 2006, 326, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Renden, R.; Taschenberger, H.; Puente, N.; Rusakov, D.A.; Duvoisin, R.; Wang, L.Y.; Lehre, K.P.; von Gersdorff, H. Glutamate transporter studies reveal the pruning of metabotropic glutamate receptors and absence of AMPA receptor desensitization at mature calyx of Held synapses. J. Neurosci. 2005, 25, 8482–8497. [Google Scholar] [CrossRef] [PubMed]
- Uwechue, N.M.; Marx, M.C.; Chevy, Q.; Billups, B. Activation of glutamate transport evokes rapid glutamine release from perisynaptic astrocytes. J. Physiol. 2012, 590, 2317–2331. [Google Scholar] [CrossRef] [PubMed]
- Kirischuk, S.; Heja, L.; Kardos, J.; Billups, B. Astrocyte sodium signaling and the regulation of neurotransmission. Glia 2016, 64, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Noda, K.; Ueda, Y.; Hanaki, H.; Saido, T.C.; Ikuta, T.; Kuroki, T.; Tamaoki, T.; Hirai, S.; Osada, S.; et al. UCN-01, an anti-tumor drug, is a selective inhibitor of the conventional PKC subfamily. FEBS Lett. 1995, 359, 259–261. [Google Scholar] [CrossRef]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, C.; Okur, F.; Setiawan, I.; Broer, S.; Lang, F. Properties and regulation of glutamine transporter SN1 by protein kinases SGK and PKB. Biochem. Biophys. Res. Commun. 2003, 306, 156–162. [Google Scholar] [CrossRef]
- Sidoryk-Wegrzynowicz, M.; Lee, E.S.; Ni, M.; Aschner, M. Manganese-induced downregulation of astroglial glutamine transporter SNAT3 involves ubiquitin-mediated proteolytic system. Glia 2010, 58, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Daniels, G.M.; Amara, S.G. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J. Biol. Chem. 1999, 274, 35794–35801. [Google Scholar] [CrossRef] [PubMed]
- Melikian, H.E.; Buckley, K.M. Membrane trafficking regulates the activity of the human dopamine transporter. J. Neurosci. 1999, 19, 7699–7710. [Google Scholar] [PubMed]
- Apparsundaram, S.; Schroeter, S.; Giovanetti, E.; Blakely, R.D. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J. Pharmacol. Exp. Ther. 1998, 287, 744–751. [Google Scholar] [PubMed]
- Jayanthi, L.D.; Samuvel, D.J.; Ramamoorthy, S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J. Biol. Chem. 2004, 279, 19315–19326. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Galli, A.; Ramamoorthy, S.; Risso, S.; DeFelice, L.J.; Blakely, R.D. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J. Neurosci. 1997, 17, 45–57. [Google Scholar] [PubMed]
- Geerlings, A.; Lopez-Corcuera, B.; Aragon, C. Characterization of the interactions between the glycine transporters GLYT1 and GLYT2 and the SNARE protein syntaxin 1A. FEBS Lett. 2000, 470, 51–54. [Google Scholar] [CrossRef]
- Fornes, A.; Nunez, E.; Alonso-Torres, P.; Aragon, C.; Lopez-Corcuera, B. Trafficking properties and activity regulation of the neuronal glycine transporter GLYT2 by protein kinase C. Biochem. J. 2008, 412, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Beckman, M.L.; Bernstein, E.M.; Quick, M.W. Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J. Neurosci. 1999, 19, RC9. [Google Scholar] [PubMed]
- Bode, B.P.; Reuter, N.; Conroy, J.L.; Souba, W.W. Protein kinase C regulates nutrient uptake and growth in hepatoma cells. Surgery 1998, 124, 260–268. [Google Scholar] [CrossRef]
- Kalandadze, A.; Wu, Y.; Robinson, M.B. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J. Biol. Chem. 2002, 277, 45741–45750. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.I.; Ortega, A. Regulation of the Na+-dependent high affinity glutamate/aspartate transporter in cultured Bergmann glia by phorbol esters. J. Neurosci. Res. 1997, 50, 585–590. [Google Scholar] [CrossRef]
- Barrera, S.P.; Castrejon-Tellez, V.; Trinidad, M.; Robles-Escajeda, E.; Vargas-Medrano, J.; Varela-Ramirez, A.; Miranda, M. PKC-Dependent GlyT1 Ubiquitination Occurs Independent of Phosphorylation: Inespecificity in Lysine Selection for Ubiquitination. PLoS ONE 2015, 10, e0138897. [Google Scholar] [CrossRef] [PubMed]
- De Juan-Sanz, J.; Nunez, E.; Lopez-Corcuera, B.; Aragon, C. Constitutive endocytosis and turnover of the neuronal glycine transporter GlyT2 is dependent on ubiquitination of a C-terminal lysine cluster. PLoS ONE 2013, 8, e58863. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, A.L.; Gonzalez, M.I.; Krizman-Genda, E.N.; Susarla, B.T.; Robinson, M.B. Ubiquitination-mediated internalization and degradation of the astroglial glutamate transporter, GLT-1. Neurochem. Int. 2008, 53, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, I.M.; Garcia-Tardon, N.; Gimenez, C.; Zafra, F. PKC-dependent endocytosis of the GLT1 glutamate transporter depends on ubiquitylation of lysines located in a C-terminal cluster. Glia 2008, 56, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Sorkina, T.; Miranda, M.; Dionne, K.R.; Hoover, B.R.; Zahniser, N.R.; Sorkin, A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J. Neurosci. 2006, 26, 8195–8205. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tardon, N.; Gonzalez-Gonzalez, I.M.; Martinez-Villarreal, J.; Fernandez-Sanchez, E.; Gimenez, C.; Zafra, F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J. Biol. Chem. 2012, 287, 19177–19187. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, C.; Palmada, M.; Rajamanickam, J.; Schniepp, R.; Amara, S.; Lang, F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J. Neurochem. 2006, 97, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Susarla, B.T.; Robinson, M.B. Internalization and degradation of the glutamate transporter GLT-1 in response to phorbol ester. Neurochem. Int. 2008, 52, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yadav, R.; Gao, M.; Weng, H.R. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia 2014, 62, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tanaka, M.; Wang, Y.; Sha, S.; Furuya, K.; Chen, L.; Sokabe, M. Neurosteroid dehydroepiandrosterone enhances activity and trafficking of astrocytic GLT-1 via sigma1 receptor-mediated PKC activation in the hippocampal dentate gyrus of rats. Glia 2017, 65, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Deken, S.L.; Wang, D.; Quick, M.W. Plasma membrane GABA transporters reside on distinct vesicles and undergo rapid regulated recycling. J. Neurosci. 2003, 23, 1563–1568. [Google Scholar] [PubMed]
- Chaudhry, F.A.; Lehre, K.P.; van Lookeren Campagne, M.; Ottersen, O.P.; Danbolt, N.C.; Storm-Mathisen, J. Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 1995, 15, 711–720. [Google Scholar] [CrossRef]
- Martinez-Lozada, Z.; Guillem, A.M.; Flores-Mendez, M.; Hernandez-Kelly, L.C.; Vela, C.; Meza, E.; Zepeda, R.C.; Caba, M.; Rodriguez, A.; Ortega, A. GLAST/EAAT1-induced glutamine release via SNAT3 in Bergmann glial cells: Evidence of a functional and physical coupling. J. Neurochem. 2013, 125, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Tzingounis, A.V.; Wadiche, J.I. Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 2007, 8, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Thomas, R.C.; Schwiening, C.J. Comparison of simultaneous pH measurements made with 8-hydroxypyrene-1,3,6-trisulphonic acid (HPTS) and pH-sensitive microelectrodes in snail neurones. Pflugers Arch. 1998, 436, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, S. Molecular Insights into the Regulation of Glutamine Transport across Cellular Membranes. Ph.D. Thesis, The Australian National University, Canberra, Australia, 2013. Available online: http://library.anu.edu.au/record=b3120904 (accessed on 12 January 2018).

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Todd, A.C.; Bröer, A.; Hulme, S.R.; Bröer, S.; Billups, B. PKC-Mediated Modulation of Astrocyte SNAT3 Glutamine Transporter Function at Synapses in Situ. Int. J. Mol. Sci. 2018, 19, 924. https://doi.org/10.3390/ijms19040924
Dong W, Todd AC, Bröer A, Hulme SR, Bröer S, Billups B. PKC-Mediated Modulation of Astrocyte SNAT3 Glutamine Transporter Function at Synapses in Situ. International Journal of Molecular Sciences. 2018; 19(4):924. https://doi.org/10.3390/ijms19040924
Chicago/Turabian StyleDong, Wuxing, Alison C. Todd, Angelika Bröer, Sarah R. Hulme, Stefan Bröer, and Brian Billups. 2018. "PKC-Mediated Modulation of Astrocyte SNAT3 Glutamine Transporter Function at Synapses in Situ" International Journal of Molecular Sciences 19, no. 4: 924. https://doi.org/10.3390/ijms19040924