Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate–Adaptive Immunity of Pediatric Asthma
Abstract
1. Introduction
2. Pediatric Asthma
3. Thymic Stromal Lymphopoietin
4. TSLPR Heterocomplex
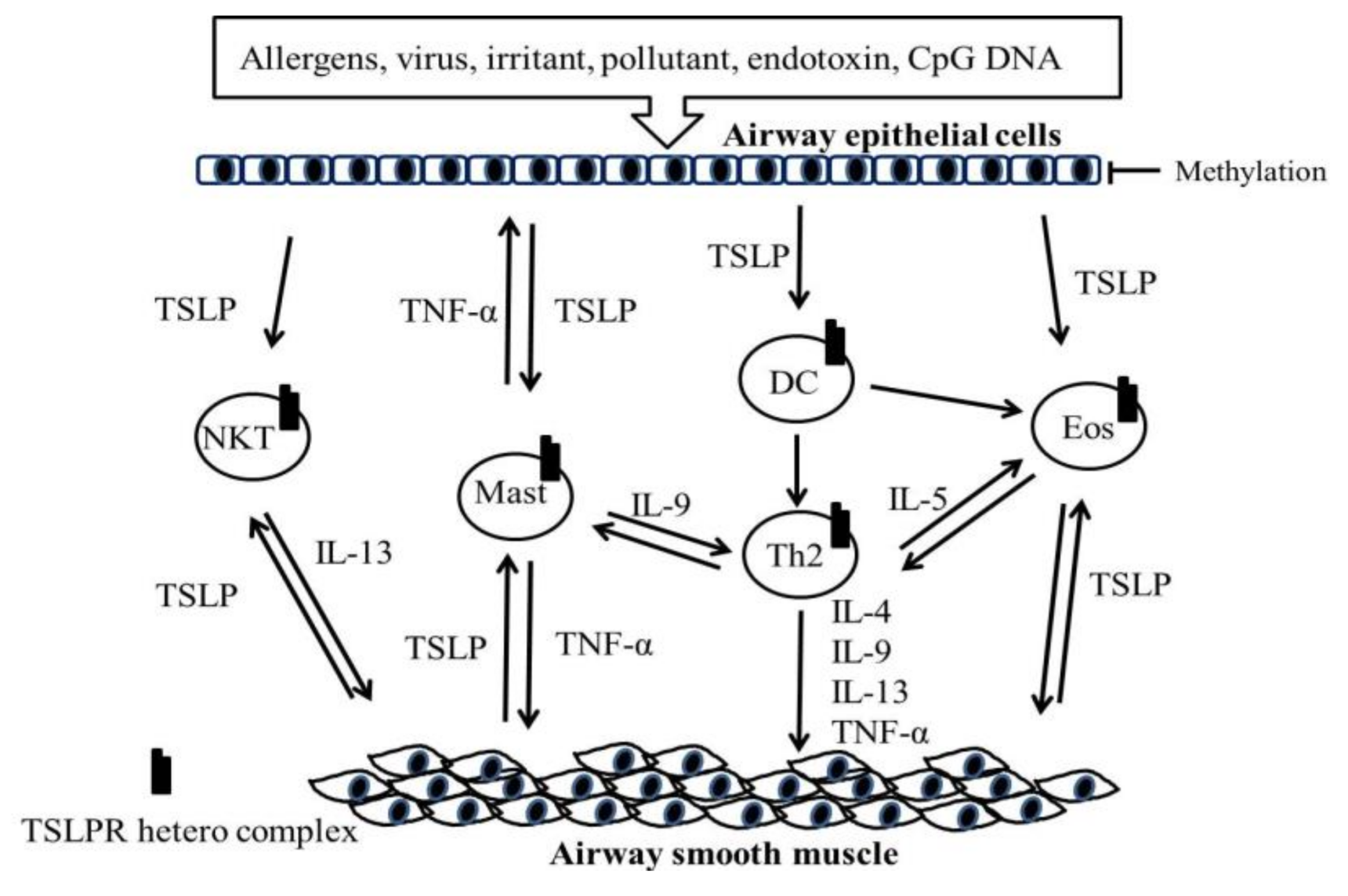
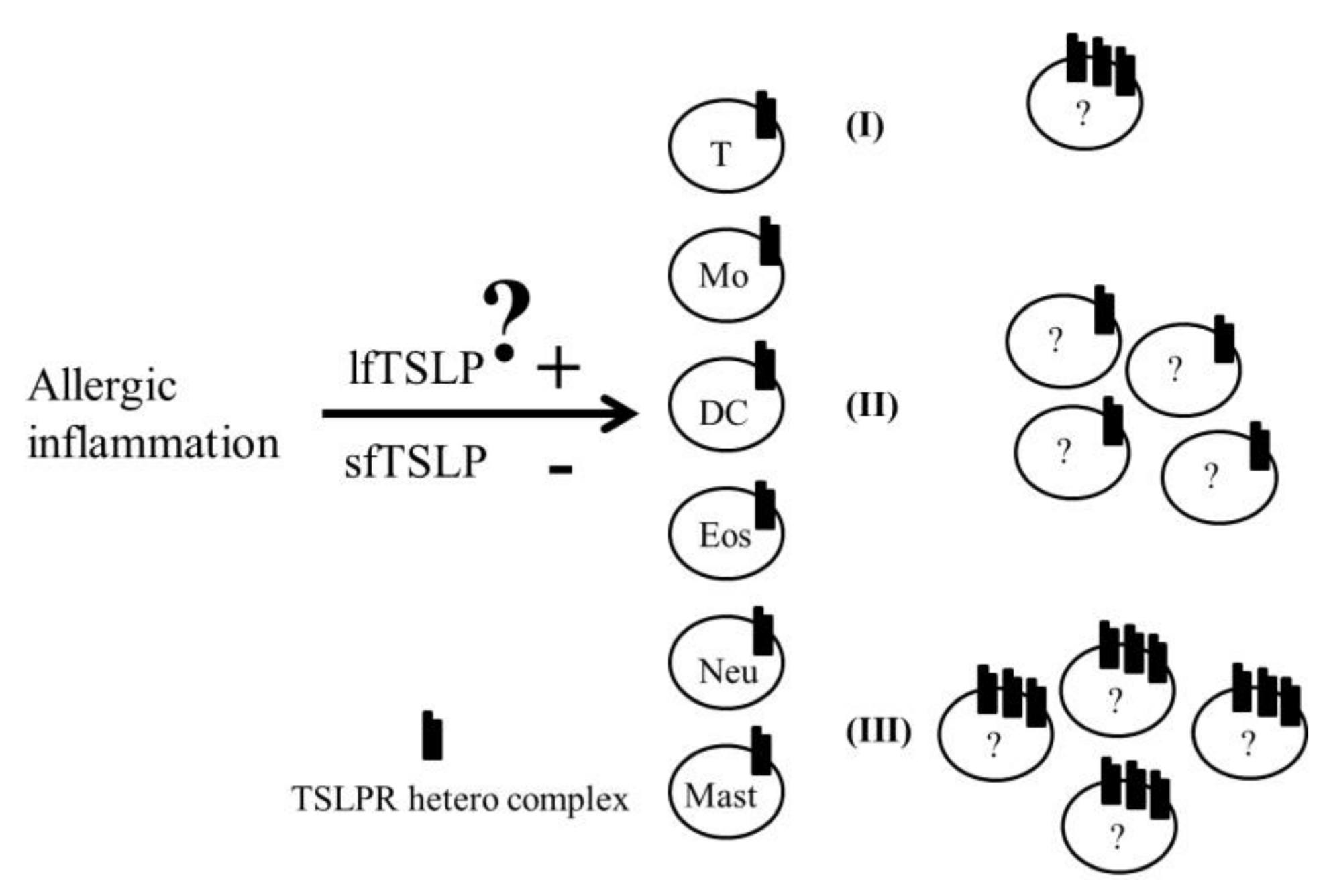
5. Role of the TSLP and TSLPR Heterocomplex in Asthma
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koczulla, A.R.; Vogelmeier, C.F.; Garn, H.; Renz, H. New concepts in asthma: Clinical phenotypes and pathophysiological mechanisms. Drug Discov. Today 2017, 22, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Mauad, T.; Bel, E.H.; Sterk, P.J. Asthma therapy and airway remodeling. J. Allergy Clin. Immunol. 2007, 120, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.; Sterk, P. Airway remodelling: The future. Eur. Respir. Soc. 2007, 30, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Royce, S.; Lim, C.; Patel, K.; Wang, B.; Samuel, C.; Tang, M. Intranasally administered serelaxin abrogates airway remodelling and attenuates airway hyperresponsiveness in allergic airways disease. Clin. Exp. Allergy 2014, 44, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Al-Ramli, W.; Hamid, Q. Remodeling in asthma. Proc. Am. Thorac. Soc. 2009, 6, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, A.S.; Nelson, D.; Thomas, J.A.; Holt, P.G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J. Exp. Med. 1994, 179, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Liu, Y.-J.; Arima, K. Cellular and molecular mechanisms of TSLP function in human allergic disorders-TSLP programs the “Th2 code” in dendritic cells. Allergol. Int. 2012, 61, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Comeau, M.; Ziegler, S. The influence of TSLP on the allergic response. Mucosal Immunol. 2010, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Hu, S.; Cheung, P.F.; Lam, C.W. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010, 43, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Liu, Y.J. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin. Exp. Allergy 2009, 39, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Saglani, S. T cells in asthma: Influences of genetics, environment, and T-cell plasticity. J. Allergy Clin. Immunol. 2013, 131, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-K.; Kim, T.S.; Hufford, M.M.; Braciale, T.J. Viral infection of the lung: Host response and sequelae. J. Allergy Clin. Immunol. 2013, 132, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Geha, R.S. Thymic stromal lymphopoietin. Ann. N. Y. Acad. Sci. 2010, 1183, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Q.; Liao, W. Screening and functional pathway analysis of genes associated with pediatric allergic asthma using a DNA microarray. Mol. Med. Rep. 2015, 11, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J. Advances in pediatric asthma in 2013: Coordinating asthma care. J. Allergy Clin. Immunol. 2014, 133, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Harpsøe, M.C.; Basit, S.; Bager, P.; Wohlfahrt, J.; Benn, C.S.; Nøhr, E.A.; Linneberg, A.; Jess, T. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: A study within the Danish National Birth Cohort. J. Allergy Clin. Immunol. 2013, 131, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Wijga, A.H.; Keil, T.; Eller, E.; Bindslev-Jensen, C.; Smit, H.A.; Weyler, J.; Dom, S.; Sunyer, J.; Mendez, M. Body mass index trajectory classes and incident asthma in childhood: Results from 8 European Birth Cohorts—A Global Allergy and Asthma European Network initiative. J. Allergy Clin. Immunol. 2013, 131, 1528–1536.e13. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-W.; Lin, S.-C. Environmental factors association between asthma and acute bronchiolitis in young children—A perspective cohort study. Eur. J. Pediatr. 2012, 171, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Dalt, L.D.; Callegaro, S.; Carraro, S.; Andreola, B.; Corradi, M.; Baraldi, E. Nasal lavage leukotrienes in infants with RSV bronchiolitis. Pediatr. Allergy Immunol. 2007, 18, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Choi, J.; Kim, H.B.; Callaway, Z.; Shin, B.M.; Kim, J.-T.; Fujisawa, T.; Koh, Y.Y. A randomized intervention of montelukast for post-bronchiolitis: Effect on eosinophil degranulation. J. Pediatr. 2010, 156, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Headley, M.B.; Loo, Y.-M.; Berlin, A.; Gale, M.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus–infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Lin, H.-W.; Chiang, B.-L. Association of croup with asthma in children: A cohort study. Medicine 2017, 96, e7667. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-J.; Söderhäll, C.; Bustamante, M.; Baïz, N.; Gruzieva, O.; Gehring, U.; Mason, D.; Chatzi, L.; Basterrechea, M.; Llop, S. DNA methylation in childhood asthma: An epigenome-wide meta-analysis. Lancet Respir. Med. 2018. [Google Scholar] [CrossRef]
- Tse, S.M.; Gold, D.R.; Sordillo, J.E.; Hoffman, E.B.; Gillman, M.W.; Rifas-Shiman, S.L.; Fuhlbrigge, A.L.; Tantisira, K.G.; Weiss, S.T.; Litonjua, A.A. Diagnostic accuracy of the bronchodilator response in children. J. Allergy Clin. Immunol. 2013, 132, 554.e5–559.e5. [Google Scholar] [CrossRef] [PubMed]
- National, A.E.; Prevention, P. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 2007, 120 (Suppl. S5), S94–S138. [Google Scholar]
- Malinovschi, A.; Fonseca, J.A.; Jacinto, T.; Alving, K.; Janson, C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J. Allergy Clin. Immunol. 2013, 132, 821.e5–827.e5. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Shim, J.Y.; Jung, Y.H.; Seo, J.H.; Young Kim, H.; Kwon, J.W.; Kim, B.J.; Kim, H.B.; Kim, W.K.; Lee, S.Y. Fraction of exhaled nitric oxide and wheezing phenotypes in preschool children. Pediatr. Pulmonol. 2013, 48, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Hoch, H.E.; Szefler, S.J. Intermittent steroid inhalation for the treatment of childhood asthma. Expert Rev. Clin. Immunol. 2016, 12, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.A.; McDonald, V.M.; Gibson, P.G. Adjusting prednisone using blood eosinophils reduces exacerbations and improves asthma control in difficult patients with asthma. Respirology 2015, 20, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Katayama, N.; Kimura, H.; Fujimura, M. A case of Churg-Strauss syndrome with methylprednisolone sodium succinate allergy. Nihon Kokyuki Gakkai Zasshi 2009, 47, 1147–1150. [Google Scholar] [PubMed]
- Eid, N.S.; O’Hagan, A.; Bickel, S.; Morton, R.; Jacobson, S.; Myers, J.A. Anti-inflammatory dosing of theophylline in the treatment of status asthmaticus in children. J. Asthma Allergy 2016, 9, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Hirota, T.; Jodo, A.I.; Hitomi, Y.; Sakashita, M.; Tsunoda, T.; Miyagawa, T.; Doi, S.; Kameda, M.; Fujita, K. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Muchao, F.P.; Torres, H.C.C.; De Lalibera, I.B.; de Souza, A.V.; Rodrigues, J.C.; Schvartsman, C.; da Silva Filho, L.V.R. Albuterol via metered-dose inhaler in children: Lower doses are effective, and higher doses are safe. Pediatr. Pulmonol. 2016, 51, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-L.; Wu, C.-C.; Wang, H.-W. Effects of bambuterol and terbutaline on isolated rat’s tracheal smooth muscle. Eur. Arch. Otorhinolaryngol. 2010, 267, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, J.Y.-L.; Wu, X.; Summer, S.; Whoriskey, J.; Saris, C.; Reagan, J.D. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 2010, 86, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Wanner, A. Inhaled corticosteroids: Effects on the airway vasculature in bronchial asthma. Eur. Respir. J. 2006, 27, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inhaled corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef] [PubMed]
- Gotshall, R.W. Exercise-induced bronchoconstriction. Drugs 2002, 62, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Kew, K.M.; Quinn, M.; Quon, B.S.; Ducharme, F.M. Increased versus stable doses of inhaled corticosteroids for exacerbations of chronic asthma in adults and children. Cochrane Libr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Raissy, H.H.; Blake, K. Does use of inhaled corticosteroid for management of asthma in children make them shorter adults? Pediatr. Allergy Immunol. Pulmonol. 2013, 26, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.; Sellahewa, L.; Pappachan, J.M. Inhaled corticosteroids and bone health. Open Respir. Med. J. 2014, 8, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Sannarangappa, V.; Jalleh, R. Inhaled corticosteroids and secondary adrenal insufficiency. Open Respir. Med. J. 2014, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Egbuonu, F.; Antonio, F.A.; Edavalath, M. Effect of inhaled corticosteroids on glycemic status. Open Respir. Med. J. 2014, 8, 101–105. [Google Scholar] [PubMed]
- Stanbrook, M.B. Adding salmeterol to fluticasone does not increase serious asthma events or reduce exacerbations in children. Ann. Intern. Med. 2016, 165, Jc58. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Chuang, Y.H.; Yang, Y.H.; Chiang, B.L. Decrease in interleukin-21 in children suffering with severe atopic dermatitis. Pediatr. Allergy Immunol. 2011, 22, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.T.; Revez, J.A.; Ferreira, M.A. Lessons from ten years of genome-wide association studies of asthma. Clin. Transl. Immunol. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.-R.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.-J.; Spits, H. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Quentmeier, H.; Drexler, H.; Fleckenstein, D.; Zaborski, M.; Armstrong, A.; Sims, J.; Lyman, S. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 2001, 15, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; Williams, D.E.; Morrissey, P.J.; Garka, K.; Foxworthe, D.; Price, V.; Friend, S.L.; Farr, A.; Bedell, M.A.; Jenkins, N.A. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 2000, 192, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.J.; Furlonger, C.; Williams, D.E.; Paige, C.J. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur. J. Immunol. 1996, 26, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J. TSLP in epithelial cell and dendritic cell cross talk. Adv. Immunol. 2009, 101, 1–25. [Google Scholar] [PubMed]
- Liu, Y.-J.; Soumelis, V.; Watanabe, N.; Ito, T.; Wang, Y.-H.; de Waal Malefyt, R.; Omori, M.; Zhou, B.; Ziegler, S.F. TSLP: An epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007, 25, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.-D.; Choi, I.-H.; Kim, H.-M. Berberine inhibits the production of thymic stromal lymphopoietin by the blockade of caspase-1/NF-κB pathway in mast cells. Int. Immunopharmacol. 2011, 11, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.; Rochman, Y.; Spolski, R.; Samsel, L.; Leonard, W.J. Thymic stromal lymphopoietin is produced by dendritic cells. J. Immunol. 2011, 187, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J. Thymic stromal lymphopoietin: Master switch for allergic inflammation. J. Exp. Med. 2006, 203, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Messaddeq, N.; Teletin, M.; Pasquali, J.-L.; Metzger, D.; Chambon, P. Retinoid X receptor ablation in adult mouse keratinocytes generates an atopic dermatitis triggered by thymic stromal lymphopoietin. Proc. Natl. Acad. Sci. USA 2005, 102, 14795–14800. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Ebihara, N.; Yokoi, N.; Kawasaki, S.; Tanioka, H.; Inatomi, T.; de Waal Malefyt, R.; Hamuro, J.; Kinoshita, S.; Murakami, A. Functional role of thymic stromal lymphopoietin in chronic allergic keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.; Xia, J.; Tan, Y.; Wang, X.; Zhang, Y.; Zhou, B.; Li, H.; Han, D. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009, 129, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Hartgring, S.; Willis, C.; Dean, C.; Broere, F.; van Eden, W.; Bijlsma, J.; Lafeber, F.; van Roon, J. Critical proinflammatory role of thymic stromal lymphopoietin and its receptor in experimental autoimmune arthritis. Arthritis Rheumatol. 2011, 63, 1878–1887. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.L.; Ziegler, S.F. Thymic stromal lymphopoietin and cancer. J. Immunol. 2014, 193, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Lin, Y.; Yang, X.; Li, B.; Liu, J.; He, R. Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor growth by promoting apoptosis of tumor cells. Oncotarget 2016, 7, 16840–16854. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Hirota, T.; Jodo, A.I.; Doi, S.; Kameda, M.; Fujita, K.; Miyatake, A.; Enomoto, T.; Noguchi, E.; Yoshihara, S. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2009, 40, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Bjerkan, L.; Schreurs, O.; Engen, S.; Jahnsen, F.; Baekkevold, E.; Blix, I.J.; Schenck, K. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol. 2015, 8, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Dermatol. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fornasa, G.; Tsilingiri, K.; Caprioli, F.; Botti, F.; Mapelli, M.; Meller, S.; Kislat, A.; Homey, B.; Di Sabatino, A.; Sonzogni, A. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J. Allergy Clin. Immunol. 2015, 136, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Hu, Y.; Liu, L.; Zou, M.; Huang, C.; Luo, L.; Yu, C.; Wan, X.; Zhao, H.; Chen, J. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci. Rep. 2016, 6, 39559. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.M.; Lasky-Su, J.; Soto-Quirós, M.E.; Avila, L.; Liang, C.; Lake, S.L.; Hudson, T.J.; Spesny, M.; Fournier, E.; Sylvia, J.S. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am. J. Respir. Crit. Care Med. 2008, 177, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Vercelli, D. Discovering susceptibility genes for asthma and allergy. Nat. Rev. Immunol. 2008, 8, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Adapa, D.; Sai, Y.; Anand, S.; Mehaboobi, S.; Aramalla, E. A Brief Review on Immune Mediated Diseases. J. Clin. Cell. Immunol. 2011, 11, 2. [Google Scholar]
- Borriello, F.; Iannone, R.; Di Somma, S.; Vastolo, V.; Petrosino, G.; Visconte, F.; Raia, M.; Scalia, G.; Loffredo, S.; Varricchi, G. Lipopolysaccharide-elicited TSLPR expression enriches a functionally discrete subset of human CD14+ CD1c+ monocytes. J. Immunol. 2017, 198, 3426–3435. [Google Scholar] [CrossRef] [PubMed]
- Kummola, L.; Ortutay, Z.; Chen, X.; Caucheteux, S.; Hämäläinen, S.; Aittomäki, S.; Yagi, R.; Zhu, J.; Pesu, M.; Paul, W.E. IL-7Rα Expression Regulates Murine Dendritic Cell Sensitivity to Thymic Stromal Lymphopoietin. J. Immunol. 2017, 198, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Liu, Y.-J. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat. Immunol. 2006, 7, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.; Yousefi, S.; Stöckle, C.; Simon, H.U.; Simon, D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy 2012, 67, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Geering, B.; Stoeckle, C.; Conus, S.; Simon, H.-U. Living and dying for inflammation: Neutrophils, eosinophils, basophils. Trends Immunol. 2013, 34, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Han, N.-R.; Oh, H.-A.; Nam, S.-Y.; Moon, P.-D.; Kim, D.-W.; Kim, H.-M.; Jeong, H.-J. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J. Investig. Dermatol. 2014, 134, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, Z.-J.; Zhao, H.-Z.; Shen, H.-P.; Cheng, Y.-P.; Luo, Z.-B.; Tang, Y.-M. Prognostic significance of cytokine receptor-like factor 2 alterations in acute lymphoblastic leukemia: A meta-analysis. World J. Pediatr. 2015, 11, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Pandey, A. Site-directed mutagenesis reveals a unique requirement for tyrosine residues in IL-7Rα and TSLPR cytoplasmic domains in TSLP-dependent cell proliferation. BMC Immunol. 2010, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Lin, C.-C.; Lin, C.G.-J.; Hsiao, Y.-H.; Wu, L.S.-H. Polymorphisms of interleukin 7 receptor are associated with mite-sensitive allergic asthma in children in Taiwan. Tzu Chi Med. J. 2010, 22, 18–23. [Google Scholar] [CrossRef]
- Shamim, Z.; Müller, K.; Svejgaard, A.; Poulsen, L.K.; Bodtger, U.; Ryder, L. Association between genetic polymorphisms in the human interleukin-7 receptor α-chain and inhalation allergy. Int. J. Immunogenet. 2007, 34, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, L.; Wu, X. A potential link between TSLP/TSLPR/STAT5 and TLR2/MyD88/NFκB-p65 in human corneal epithelial cells for Aspergillus fumigatus tolerance. Mol. Immunol. 2016, 71, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Redhu, N.; Gounni, A. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin. Exp. Allergy 2012, 42, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Durum, S.K. IL-7 and TSLP receptors: Twisted sisters. Blood 2014, 124, 4–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, X.; Ma, P.; de Paiva, C.S.; Cunningham, M.A.; Hwang, C.S.; Pflugfelder, S.C.; Li, D.-Q. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3076–3082. [Google Scholar] [CrossRef] [PubMed]
- Bjerkan, L.; Sonesson, A.; Schenck, K. Multiple functions of the new cytokine-based antimicrobial peptide thymic stromal lymphopoietin (TSLP). Pharmaceuticals 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.; Peelman, F.; Braun, H.; Lopez, J.; Van Rompaey, D.; Dansercoer, A.; Vandenberghe, I.; Pauwels, K.; Tavernier, J.; Lambrecht, B.N. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 2017, 8, 14937. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Singh, J.; Jindal, S.K. Association of interleukin 7 receptor (rs1494555 and rs6897932) gene polymorphisms with asthma in a north Indian population. Allergy Rhinol. 2015, 6, e168. [Google Scholar] [CrossRef] [PubMed]
- Sherrill, J.D.; Gao, P.-S.; Stucke, E.M.; Blanchard, C.; Collins, M.H.; Putnam, P.E.; Franciosi, J.P.; Kushner, J.P.; Abonia, J.P.; Assa’ad, A.H. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010, 126, 160–165.e3. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.-S.; Rafaels, N.M.; Mu, D.; Hand, T.; Murray, T.; Boguniewicz, M.; Hata, T.; Schneider, L.; Hanifin, J.M.; Gallo, R.L. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J. Allergy Clin. Immunol. 2010, 125, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, G.; Hu, B.; Song, Y.; Shi, Y. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin. Exp. Immunol. 2011, 164, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.L.; van Leeuwen, E.M.; Derks, I.A.; van Lier, R.A. Differential regulation of human IL-7 receptor α expression by IL-7 and TCR signaling. J. Immunol. 2008, 180, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Ashbaugh, J.J.; Brambilla, R.; Karmally, S.A.; Cabello, C.; Malek, T.R.; Bethea, J.R. IL7Rα contributes to experimental autoimmune encephalomyelitis through altered T cell responses and nonhematopoietic cell lineages. J. Immunol. 2013, 190, 4525–4534. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Vanichsarn, C.; Nadeau, K.C. TSLP directly impairs pulmonary Treg function: Association with aberrant tolerogenic immunity in asthmatic airway. Allergy Asthma Clin. Immunol. 2010, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Jacques, E.; Koussih, L.; Gounni, A.S.; Chakir, J. Thymic stromal lymphopoietin–induced human asthmatic airway epithelial cell proliferation through an IL-13–dependent pathway. J. Allergy Clin. Immunol. 2010, 125, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Uller, L.; Leino, M.; Bedke, N.; Sammut, D.; Green, B.; Lau, L.; Howarth, P.H.; Holgate, S.T.; Davies, D.E. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-β in bronchial epithelial cells from donors with asthma. Thorax 2010, 65, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-I.; Kang, I.-H.; Chun, S.-W.; Yun, K.-J.; Moon, H.-B.; Chae, S.-C. Identifying the polymorphisms in the thymic stromal lymphopoietin receptor (TSLPR) and their association with asthma. BMB Rep. 2010, 43, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.B.; Martin, L.J.; Kovacic, M.B.; Mersha, T.B.; He, H.; Pilipenko, V.; Lindsey, M.A.; Ericksen, M.B.; Bernstein, D.I.; LeMasters, G.K. Epistasis between serine protease inhibitor Kazal-type 5 (SPINK5) and thymic stromal lymphopoietin (TSLP) genes contributes to childhood asthma. J. Allergy Clin. Immunol. 2014, 134, 891.e3–899.e3. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Moro, K.; Fukunaga, K.; Suzuki, Y.; Miyata, J.; Masaki, K.; Betsuyaku, T.; Koyasu, S.; Asano, K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun. 2013, 4, 2675. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; O’byrne, P.M.; Boulet, L.-P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Oksel, C.; Custovic, A. Development of allergic sensitization and its relevance to paediatric asthma. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Huang, J.-J.; Wang, J.-Y.; Chuang, H.-C.; Chiang, B.-L.; Ye, Y.-L. Upregulated thymic stromal lymphopoietin receptor expression in children with asthma. Eur. J. Clin. Investig. 2016, 46, 511–519. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Cheng, F.-Y.; Liu, J.-J.; Ye, Y.-L. Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate–Adaptive Immunity of Pediatric Asthma. Int. J. Mol. Sci. 2018, 19, 1231. https://doi.org/10.3390/ijms19041231
Lin S-C, Cheng F-Y, Liu J-J, Ye Y-L. Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate–Adaptive Immunity of Pediatric Asthma. International Journal of Molecular Sciences. 2018; 19(4):1231. https://doi.org/10.3390/ijms19041231
Chicago/Turabian StyleLin, Sheng-Chieh, Fang-Yi Cheng, Jun-Jen Liu, and Yi-Ling Ye. 2018. "Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate–Adaptive Immunity of Pediatric Asthma" International Journal of Molecular Sciences 19, no. 4: 1231. https://doi.org/10.3390/ijms19041231
APA StyleLin, S.-C., Cheng, F.-Y., Liu, J.-J., & Ye, Y.-L. (2018). Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate–Adaptive Immunity of Pediatric Asthma. International Journal of Molecular Sciences, 19(4), 1231. https://doi.org/10.3390/ijms19041231




