Rhodobacter sphaeroides Extract Lycogen™ Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats
Abstract
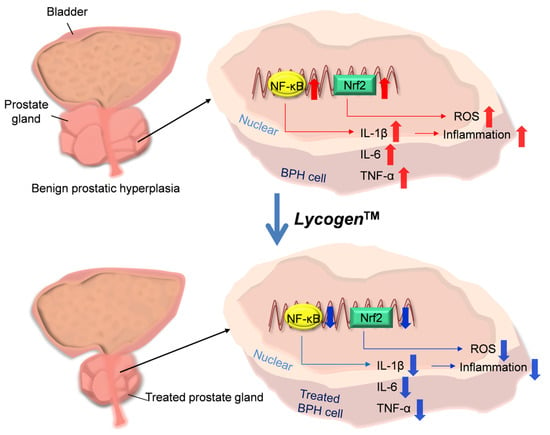
1. Introduction
2. Results
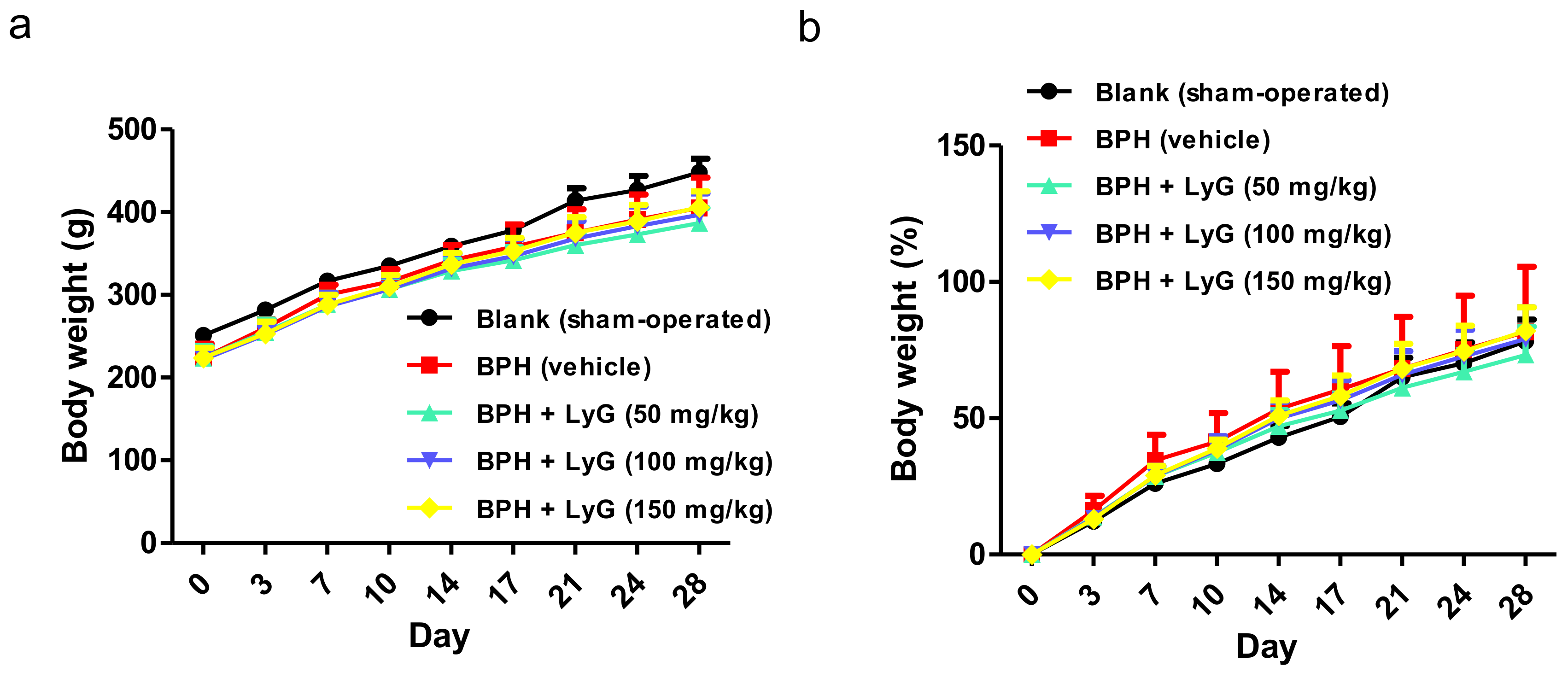
2.1. Lycogen™ Significantly Suppressed Prostate Enlargement in Testosterone Induced BPH Rats
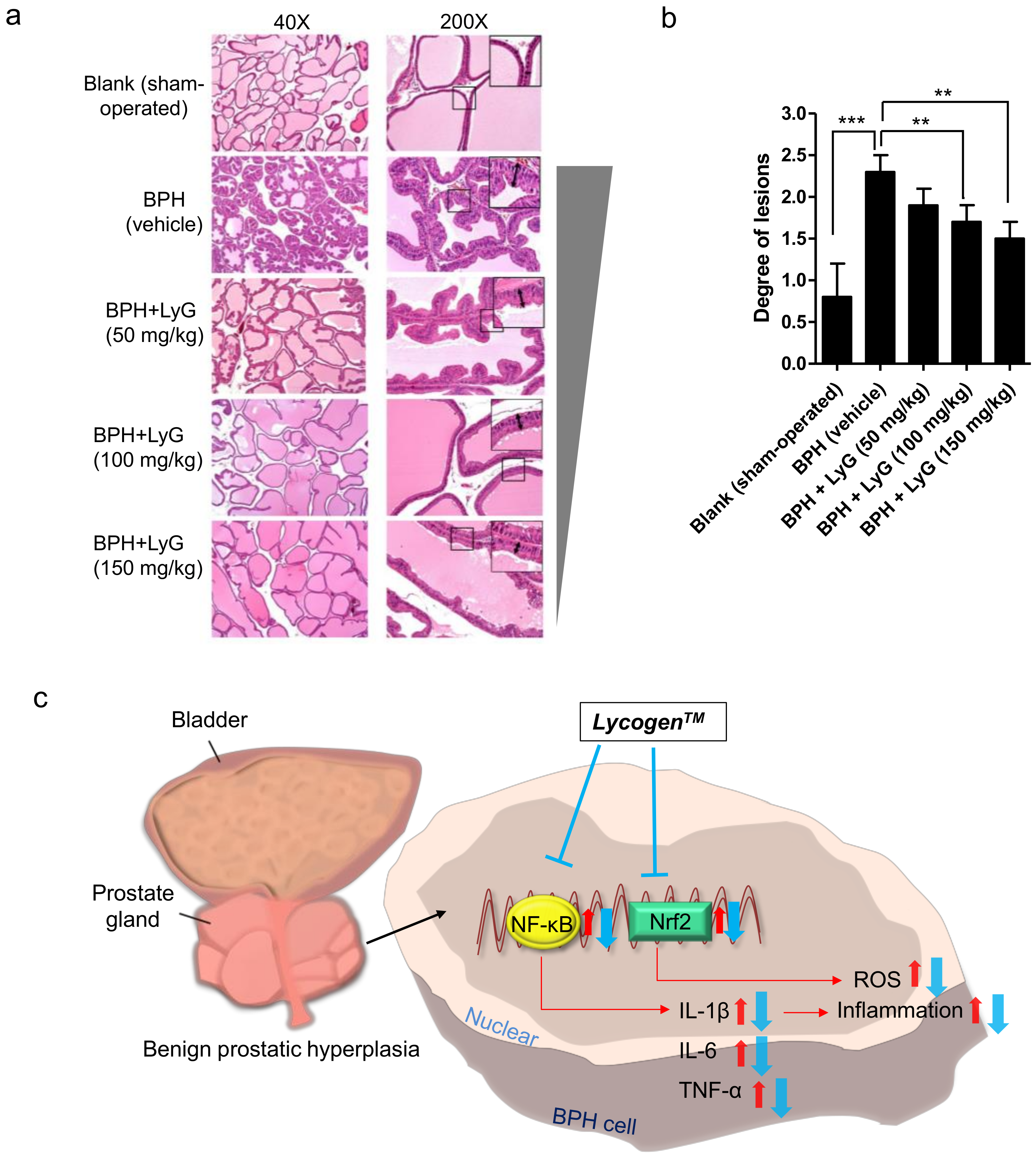
2.2. Lycogen™ Dramatically Reduced Prostate Lesions in Testosterone Induced BPH Rats
3. Discussion
4. Materials and Methods
4.1. Lycogen™ and Animals
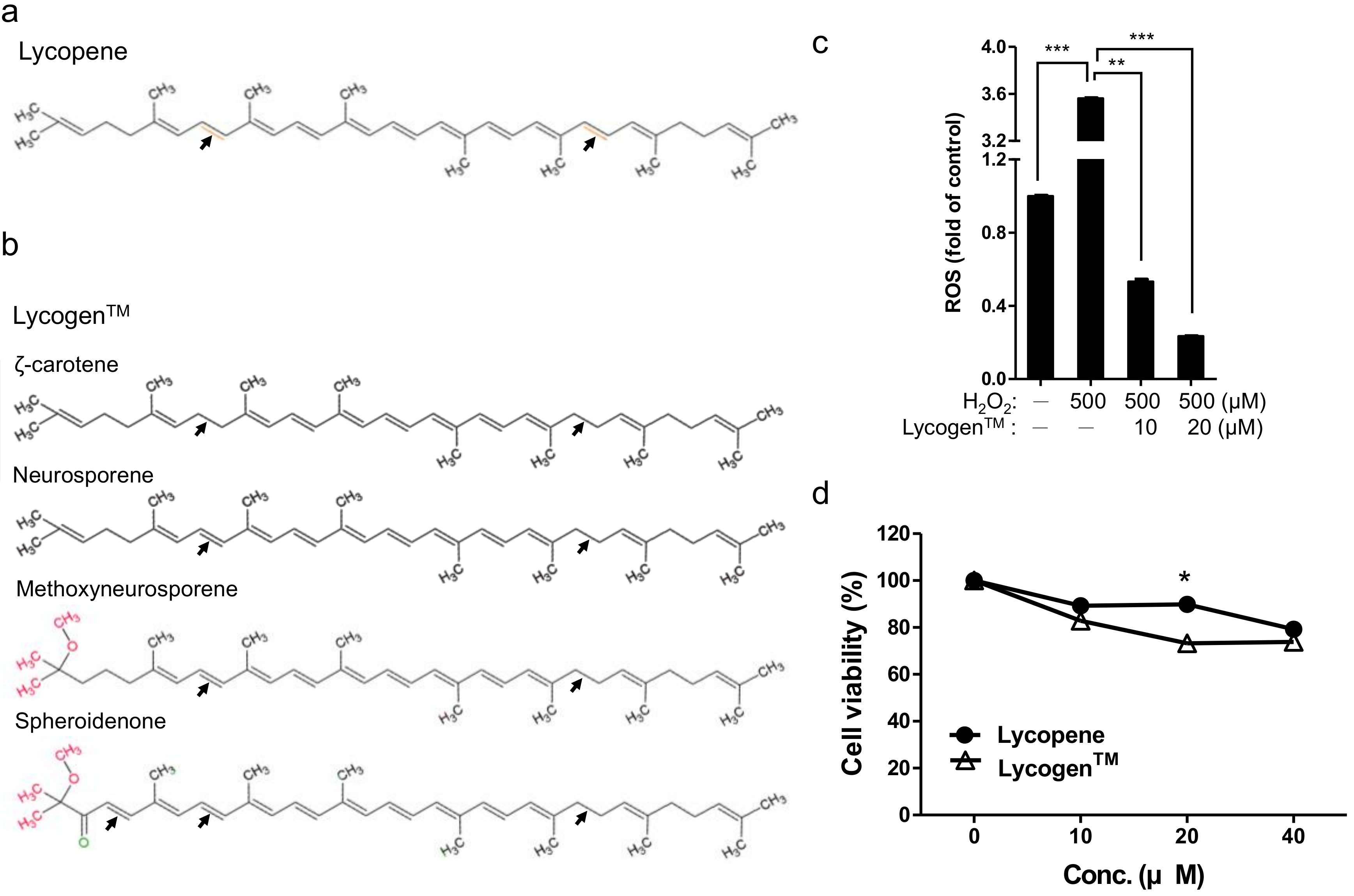
4.2. Measurement of Cell Viability
4.3. Measurement of Intracellular ROS
4.4. Establishment of Experimental BPH Model
4.5. Assessment of Prostate Index (PI) Percentage and Prostate Inhibition Percentage
Lycogen™/prostate wet weight of BPH rats) × 100]
4.6. Histological Examination
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, W.T.; Liu, W.S. Anti-Inflammatory Property of Biomaterial Carotenoids Production by Rhodobacter Sphaeroides WL-APD911. Adv. Mater. Res. 2011, 287–290, 2028–2031. [Google Scholar] [CrossRef]
- Liu, W.S.; Chen, M.C.; Chiu, K.H.; Wen, Z.H.; Lee, C.H. Amelioration of dextran sodium sulfate-induced colitis in mice by Rhodobacter sphaeroides extract. Molecules 2012, 17, 13622–13630. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.L.; Ding, Q.L.; Wu, Z.Q. Effect of baicalein on experimental prostatic hyperplasia in rats and mice. Biol. Pharm. Bull. 2004, 27, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoid biosynthesis and biotechnological application. Arch. Biochem. Biophys. 2001, 385, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yoon, S.H.; Lee, S.H.; Kim, J.Y.; Oh, D.K.; Kim, S.W. An update on microbial carotenoid production: Application of recent metabolic engineering tools. Appl. Microbiol. Biot. 2007, 77, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Ding, S.W.; Chiu, K.H.; Liu, W.S.; Lin, T.J.; Wen, Z.H. Extract from a mutant Rhodobacter sphaeroides as an enriched carotenoid source. Food Nutr. Res. 2016, 60, 29580. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.W.; Liu, J.J.; Liu, C.F.; Liu, W.S.; Lim, Y.P.; Cheng, Y.J.; Lee, C.H. An extract of Rhodobacter sphaeroides reduces cisplatin-induced nephrotoxicity in mice. Toxins 2013, 5, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Kuan, Y.D.; Chiu, K.H.; Wang, W.K.; Chang, F.H.; Liu, C.H.; Lee, C.H. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar. Drugs 2013, 11, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, M.; Oka, M.; Ueda, M.; Takagaki, K.; Tanaka, M.; Ohgi, T.; Yano, J. Eviprostat suppresses proinflammatory gene expression in the prostate of rats with partial bladder-outlet obstruction: A genome-wide DNA microarray analysis. Cytokine 2009, 47, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wein, A.; Partin, N.A.; Peters, A.C. Campbell-Walsh Urology; Saunders: Philadelphia, PA, USA, 2011; p. 4168. [Google Scholar]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Di Silverio, F.; Salciccia, S.; Autran Gomez, A.M.; Gentilucci, A.; Gentile, V. Inflammation and chronic prostatic diseases: Evidence for a link? Eur. Urol. 2007, 52, 964–972. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Presicce, F.; Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 2016, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20 (Suppl. 3), S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Marberger, M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin. Urol. 2006, 16, 25–29. [Google Scholar] [PubMed]
- Kahokehr, A.; Vather, R.; Nixon, A.; Hill, A.G. Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: Systematic review and meta-analysis of randomized controlled trials. BJU Int. 2013, 111, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L. Diagnosis and management of benign prostatic hyperplasia. Am. Fam. Physician 2008, 77, 1403–1410. [Google Scholar] [PubMed]
- Nickel, J.C.; Sander, S.; Moon, T.D. A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int. J. Clin. Pract. 2008, 62, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Pagano, E.; Laudato, M.; Griffo, M.; Capasso, R. Phytotherapy of benign prostatic hyperplasia. A minireview. Phytother. Res. 2014, 28, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, R.C.; McVary, K.T. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J. Urol. 2008, 179, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Azimi, H.; Khakshur, A.A.; Aghdasi, I.; Fallah-Tafti, M.; Abdollahi, M. A review of animal and human studies for management of benign prostatic hyperplasia with natural products: Perspective of new pharmacological agents. Inflamm. Allergy Drug Targets 2012, 11, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.D.; Blake, C.; Bu, L.; Hamaker, A.N.; Lephart, E.D. Equol an isoflavonoid: Potential for improved prostate health, in vitro and in vivo evidence. Reprod. Biol. Endocrinol. 2011, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.D.; Lephart, E.D.; Handa, R.J. The Isoflavonoid—Equol Isomer’s Neurophysiological Effects After Short-term, Low-dose Administration in Male Rats. J. Nutr. Health Food Sci. 2016, 4, 1–7. [Google Scholar]
- Lephart, E.D. Anti-oxidant and anti-aging properties of equol in prostate health (BPH). Open J. Endocr. Metab. Dis. 2014, 4, 1. [Google Scholar] [CrossRef]
- Wertz, K.; Siler, U.; Goralczyk, R. Lycopene: Modes of action to promote prostate health. Arch. Biochem. Biophys. 2004, 430, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Takkouche, B.; Caamano-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 340–345. [Google Scholar]
- Giovannucci, E. Tomato products, lycopene, and prostate cancer: A review of the epidemiological literature. J. Nutr. 2005, 135, 2030S–2031S. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Misso, M. Lycopene for the prevention and treatment of benign prostatic hyperplasia and prostate cancer: A systematic review. Maturitas 2012, 72, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.D.; Borchers, A.H.; Sundareshan, P.; Bougelet, C.; Berkman, M.R.; Nagle, R.B.; Bowden, G.T. Interleukin-1beta secreted from monocytic cells induces the expression of matrilysin in the prostatic cell line LNCaP. J. Biol. Chem. 1997, 272, 14188–14192. [Google Scholar] [CrossRef] [PubMed]
- Sezginer, E.K.; Yilmaz-Oral, D.; Lokman, U.; Nebioglu, S.; Aktan, F.; Gur, S. Effects of varying degrees of partial bladder outlet obstruction on urinary bladder function of rats: A novel link to inflammation, oxidative stress and hypoxia. Lower Urin. Tract Symp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kruslin, B.; Tomas, D.; Dzombeta, T.; Milkovic-Perisa, M.; Ulamec, M. Inflammation in Prostatic Hyperplasia and Carcinoma-Basic Scientific Approach. Front. Oncol. 2017, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.A.; Wei, J.T. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 2006, 7, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Nadler, R.B.; Koch, A.E.; Calhoun, E.A.; Campbell, P.L.; Pruden, D.L.; Bennett, C.L.; Yarnold, P.R.; Schaeffer, A.J. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J. Urol. 2000, 164, 214–218. [Google Scholar] [CrossRef]
- Chandi, G.K.; Gill, B.S. Production and characterization of microbial carotenoids as an alternative to synthetic colors: A review. Int. J. Food Prop. 2011, 14, 503–513. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Progress in Microbial Carotenoids Production. Indian J. Microbiol. 2017, 57, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Ye, V.M.; Bhatia, S.K. Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnol. Lett. 2012, 34, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Vachali, P.; Bhosale, P.; Bernstein, P.S. Microbial carotenoids. Methods Mol. Biol. 2012, 898, 41–59. [Google Scholar] [PubMed]
- Heider, S.A.; Peters-Wendisch, P.; Wendisch, V.F.; Beekwilder, J.; Brautaset, T. Metabolic engineering for the microbial production of carotenoids and related products with a focus on the rare C50 carotenoids. Appl. Microbiol. Biotechnol. 2014, 98, 4355–4368. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoid biosynthesis in microorganisms and plants. Eur. J. Biochem. 1994, 223, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Klein, A.; Hugueney, P.; Sandmann, G.; Kuntz, M. Molecular cloning and functional expression in E. coli of a novel plant enzyme mediating zeta-carotene desaturation. FEBS Lett. 1995, 372, 199–202. [Google Scholar] [CrossRef]
- Herzog, A.; Siler, U.; Spitzer, V.; Seifert, N.; Denelavas, A.; Hunziker, P.B.; Hunziker, W.; Goralczyk, R.; Wertz, K. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB J. 2005, 19, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Delves, G.H.; Chopra, M.; Lwaleed, B.A.; Cooper, A.J. Prostate cells exposed to lycopene in vitro liberate lycopene-enriched exosomes. BJU Int. 2006, 98, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. The antioxidant and biological properties of the carotenoids. Ann. N. Y. Acad. Sci. 1998, 854, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Dinkova-Kostova, A.T.; Holtzclaw, W.D. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzym. Regul. 2003, 43, 121–134. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Blade, C.; Arola, L.; Salvado, J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liang, X.; Shi, L.; Wang, L.; Chen, J.; Kang, C.; Zhu, J.; Mi, M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE 2013, 8, e79075. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Lauridsen, S.T.; Daneshvar, B.; Jakobsen, J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000, 154, 201–210. [Google Scholar] [CrossRef]
- Yang, T.-H.; Lai, Y.-H.; Lin, T.-P.; Liu, W.-S.; Kuan, L.-C.; Liu, C.-C. Chronic exposure to Rhodobacter sphaeroides extract Lycogen™ prevents UVA-induced malondialdehyde accumulation and procollagen I down-regulation in human dermal fibroblasts. Int. J. Mol. Sci. 2014, 15, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Bowen, P.E. Effects of lycopene and tomato paste extracts on DNA and lipid oxidation in LNCaP human prostate cancer cells. BioFactors 2005, 23, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solis, C.; Pedraza-Chaverri, J.; Torres-Ramos, M.; Jimenez-Farfan, D.; Cruz Salgado, A.; Serrano-Garcia, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid.-Based Complement. Altern. Med. 2013, 2013, 705121. [Google Scholar] [CrossRef] [PubMed]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Sharifi, R.; Ghosh, L.; Kim, H.S.; Christov-Tzelkov, K.; van Breemen, R. Tomato sauce supplementation and prostate cancer: Lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp. Biol. Med. 2002, 227, 886–893. [Google Scholar] [CrossRef]
- Obermuller-Jevic, U.C.; Olano-Martin, E.; Corbacho, A.M.; Eiserich, J.P.; van der Vliet, A.; Valacchi, G.; Cross, C.E.; Packer, L. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. J. Nutr. 2003, 133, 3356–3360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a guardian of redox signalling. Acta Biochim. Pol. 2012, 59, 21–25. [Google Scholar] [PubMed]
- Yeliseev, A.A.; Kaplan, S. Anaerobic carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1: H2O is a source of oxygen for the 1-methoxy group of spheroidene but not for the 2-oxo group of spheroidenone. FEBS Lett. 1997, 403, 10–14. [Google Scholar] [CrossRef]
- Scolnik, P.A.; Walker, M.A.; Marrs, B.L. Biosynthesis of carotenoids derived from neurosporene in Rhodopseudomonas capsulata. J. Biol. Chem. 1980, 255, 2427–2432. [Google Scholar] [PubMed]
- Chung, K.S.; An, H.J.; Cheon, S.Y.; Kwon, K.R.; Lee, K.H. Bee venom suppresses testosterone-induced benign prostatic hyperplasia by regulating the inflammatory response and apoptosis. Exp. Biol. Med. 2015, 240, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, M.Y.; Jeon, W.Y.; Lee, N.; Seo, C.S.; Shin, H.K. Inhibitory Effect of Yongdamsagan-Tang Water Extract, a Traditional Herbal Formula, on Testosterone-Induced Benign Prostatic Hyperplasia in Rats. Evid.-Based Complement. Altern. Med. 2016, 2016, 1428923. [Google Scholar] [CrossRef] [PubMed]
- Naslund, M.J.; Coffey, D.S. The differential effects of neonatal androgen, estrogen and progesterone on adult rat prostate growth. J. Urol. 1986, 136, 1136–1140. [Google Scholar] [CrossRef]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
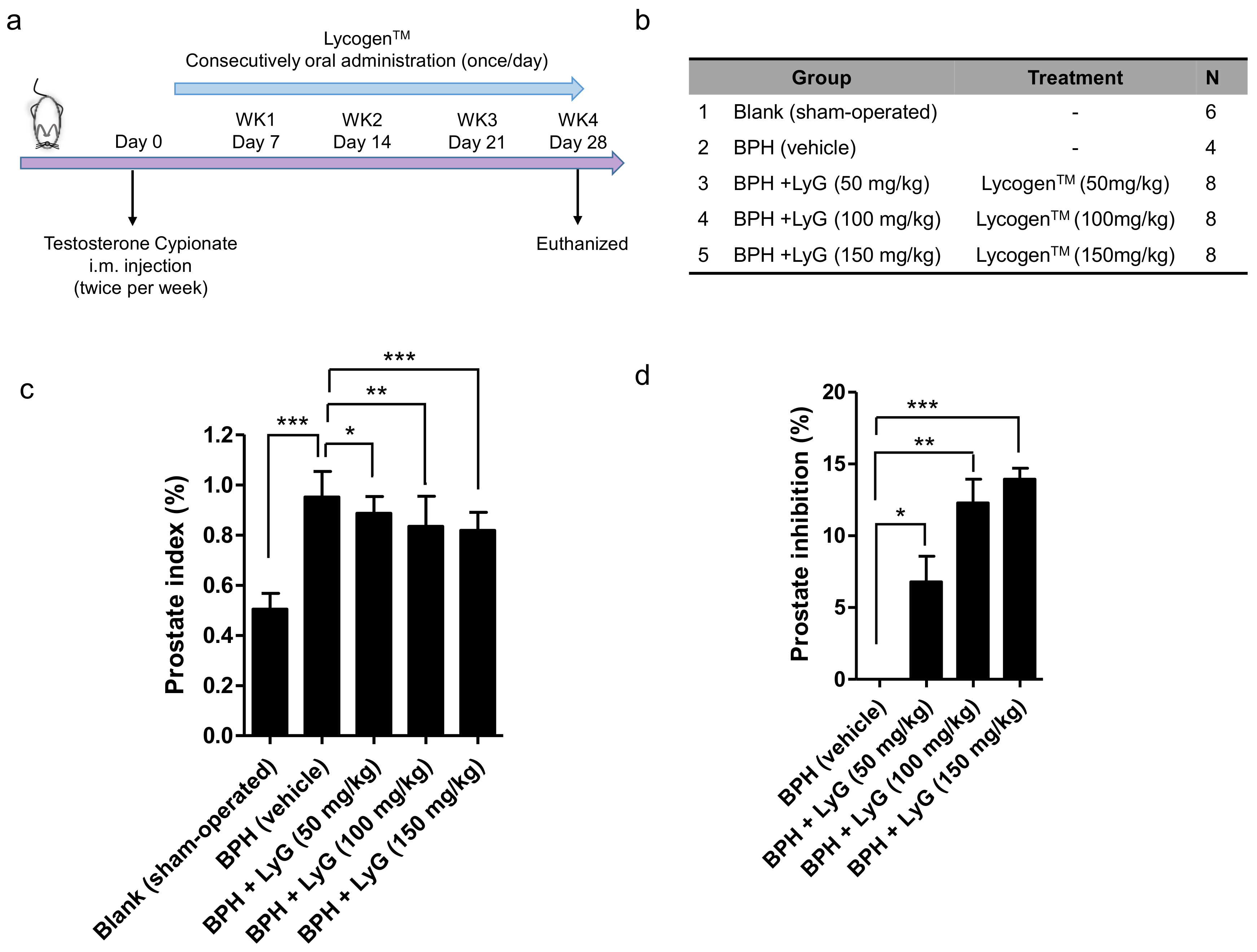
| Group | Dose (mg/kg) | PW (g) | Inhibition (%) | Prostate Index | Inhibition (%) |
|---|---|---|---|---|---|
| Blank (sham-operated) | 0 | 2.294 ± 0.088 | 0.504 ± 0.03 | ||
| BPH (vehicle) | 0 | 3.892 ± 0.239 | 0.952 ± 0.05 | ||
| LyG-50 mg/kg | 50 | 3.482 ± 0.095 | 10.5% *** | 0.887 ± 0.02 | 6.8% *** |
| LyG-100 mg/kg | 100 | 3.338 ± 0.109 | 14.2% *** | 0.835 ± 0.04 | 12.3% *** |
| LyG-150 mg/kg | 150 | 3.375 ± 0.117 | 13.3% *** | 0.819 ± 0.03 | 13.9% *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-T.; Wang, Y.-Y.; Liu, W.-S.; Cheng, C.-M.; Chiu, K.-H.; Liu, L.-L.; Liu, X.-Z.; Wen, Z.-H.; Chen, Y.-H.; Chen, T.-M. Rhodobacter sphaeroides Extract Lycogen™ Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats. Int. J. Mol. Sci. 2018, 19, 1137. https://doi.org/10.3390/ijms19041137
Wang C-T, Wang Y-Y, Liu W-S, Cheng C-M, Chiu K-H, Liu L-L, Liu X-Z, Wen Z-H, Chen Y-H, Chen T-M. Rhodobacter sphaeroides Extract Lycogen™ Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats. International Journal of Molecular Sciences. 2018; 19(4):1137. https://doi.org/10.3390/ijms19041137
Chicago/Turabian StyleWang, Chiang-Ting, Ya-Yun Wang, Wen-Sheng Liu, Chun-Ming Cheng, Kuo-Hsun Chiu, Li-Lian Liu, Xue-Zhu Liu, Zhi-Hong Wen, Ya-Huey Chen, and Tsung-Ming Chen. 2018. "Rhodobacter sphaeroides Extract Lycogen™ Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats" International Journal of Molecular Sciences 19, no. 4: 1137. https://doi.org/10.3390/ijms19041137
APA StyleWang, C.-T., Wang, Y.-Y., Liu, W.-S., Cheng, C.-M., Chiu, K.-H., Liu, L.-L., Liu, X.-Z., Wen, Z.-H., Chen, Y.-H., & Chen, T.-M. (2018). Rhodobacter sphaeroides Extract Lycogen™ Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats. International Journal of Molecular Sciences, 19(4), 1137. https://doi.org/10.3390/ijms19041137