Loss of ISWI Function in Drosophila Nuclear Bodies Drives Cytoplasmic Redistribution of Drosophila TDP-43
Abstract
1. Introduction
2. Results and Discussion
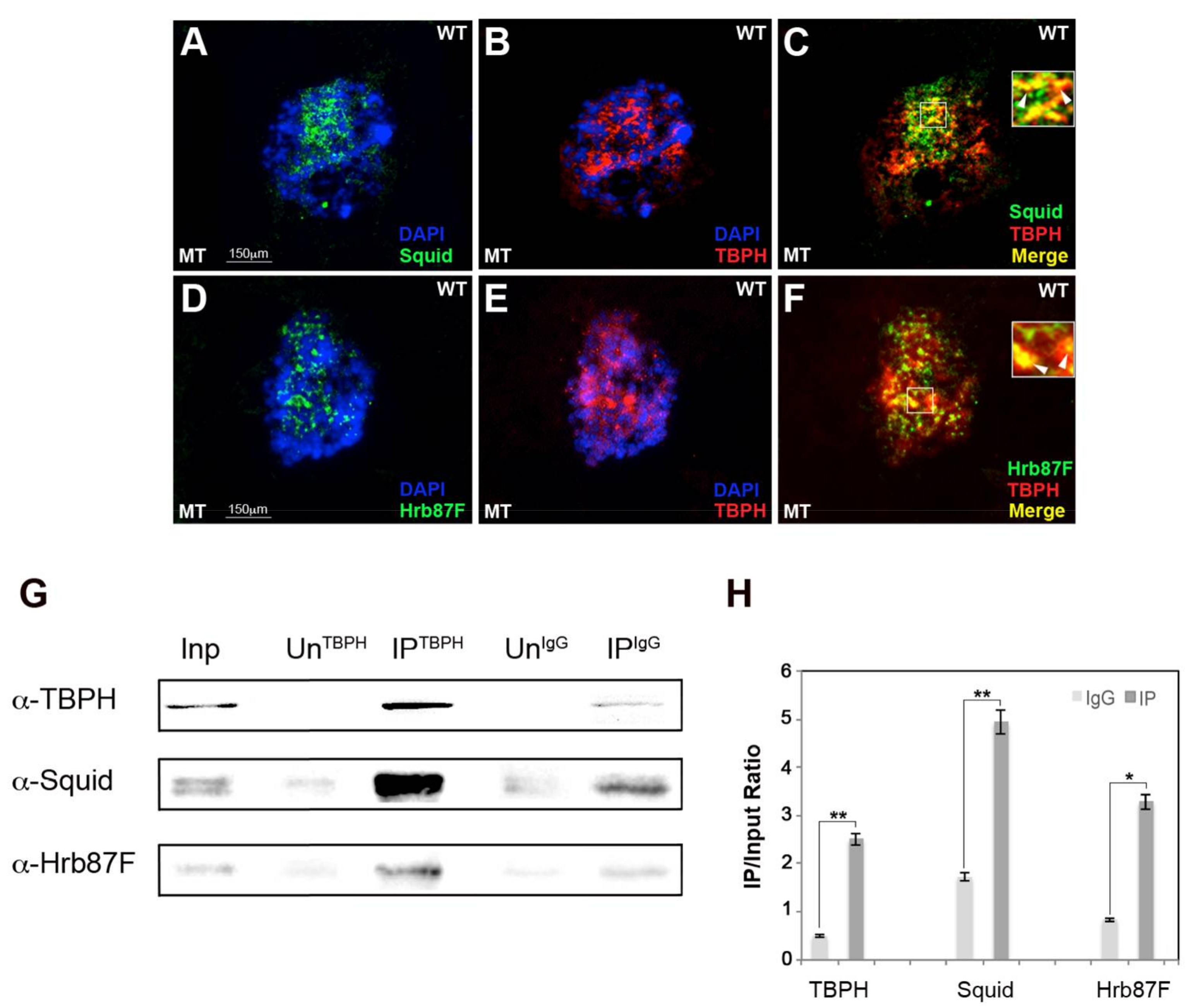
2.1. hnRNP TBPH Interacts with Squid and Hrb87F hnRNPs In Vivo
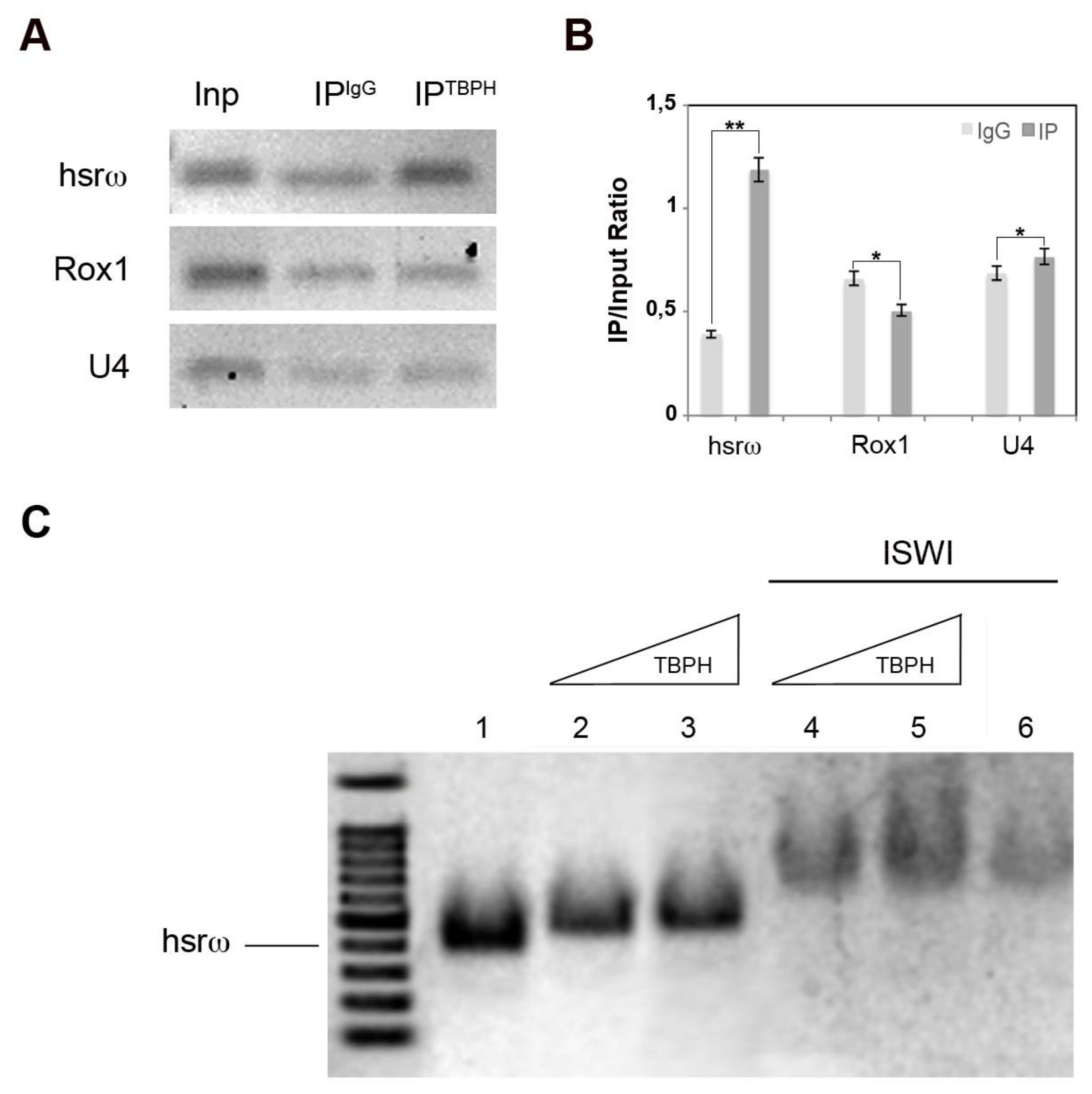
2.2. TBPH Physically and Functionally Interacts with the hsrω arcRNA In Vivo and In Vitro
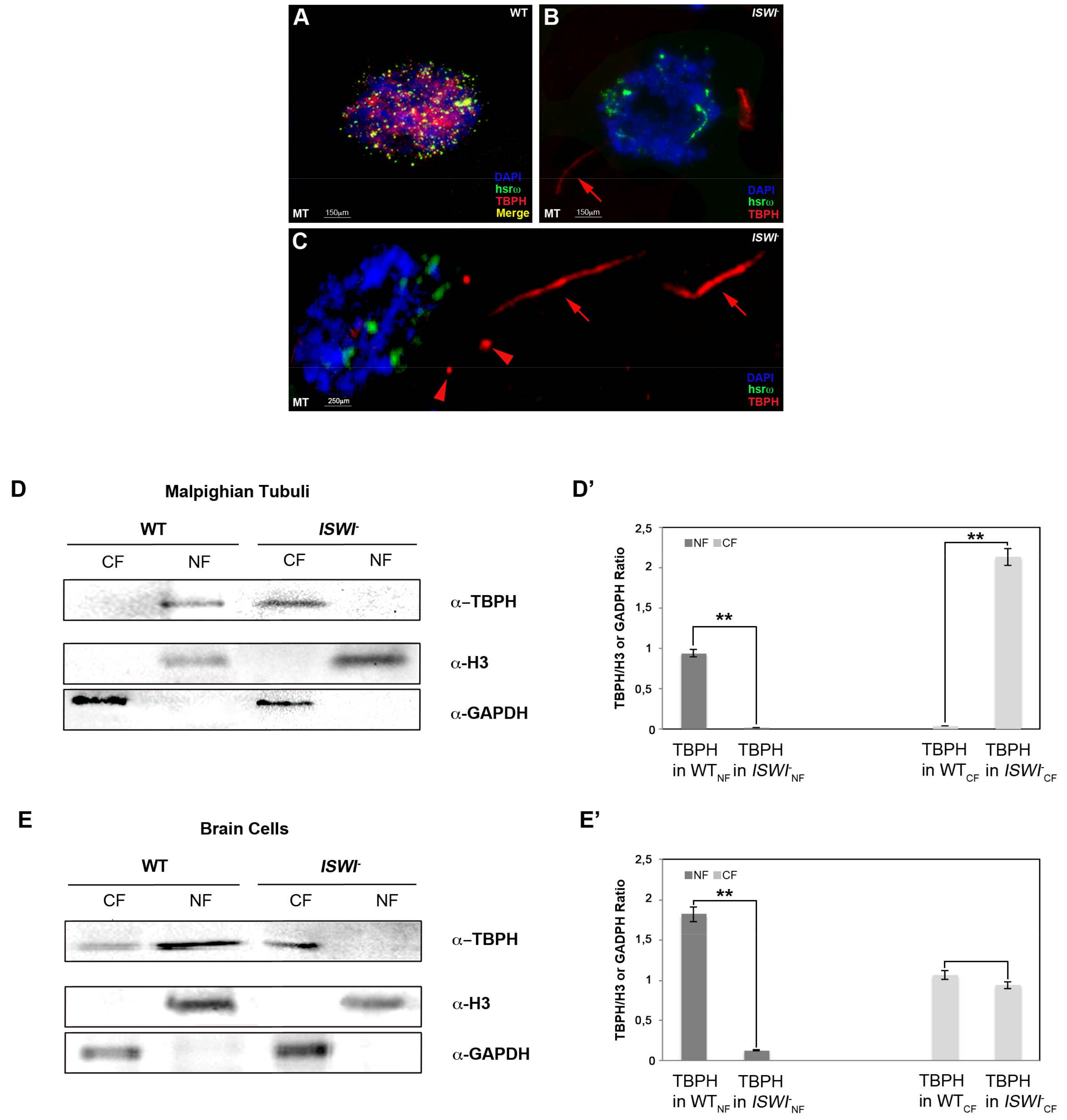
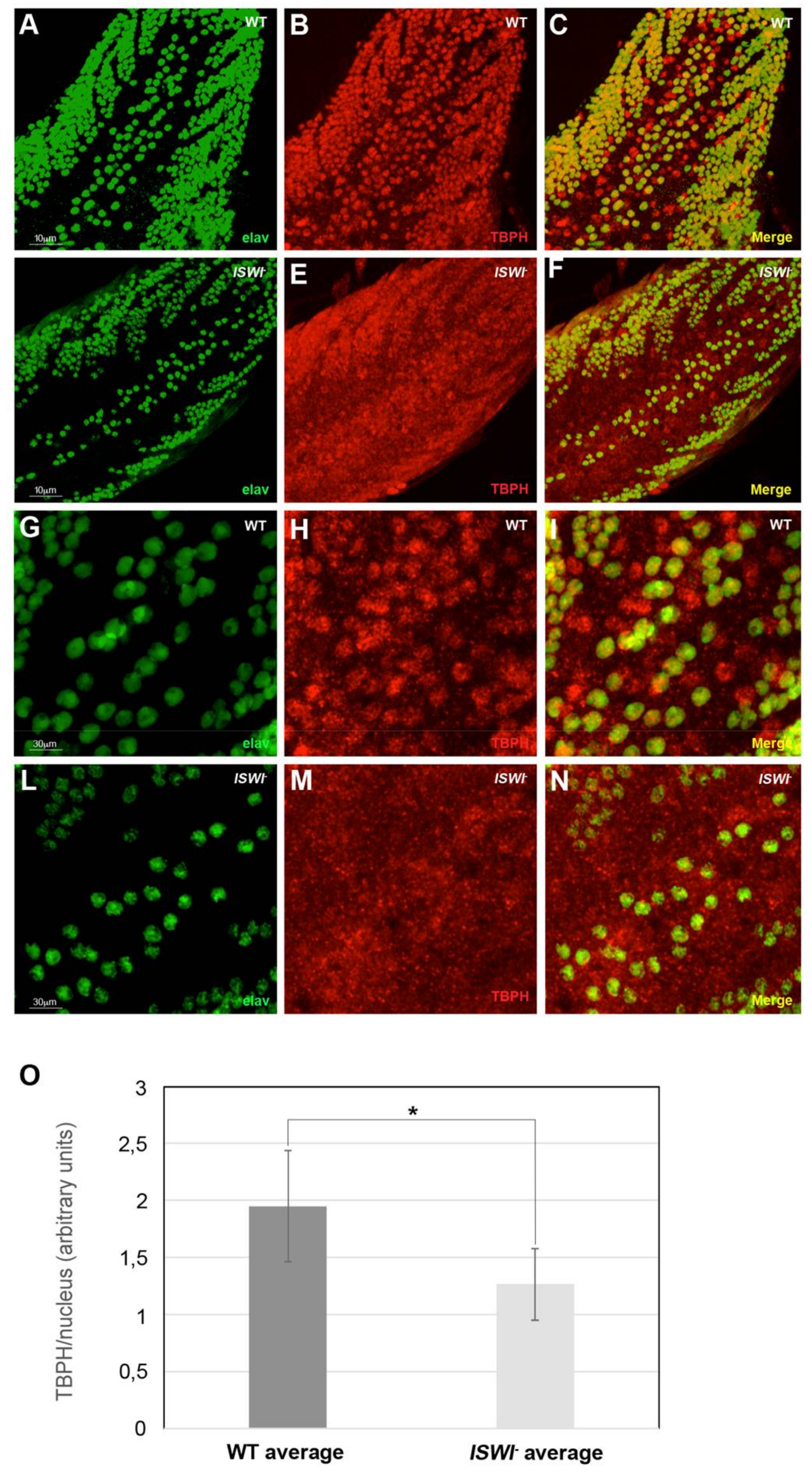
2.3. Loss of ISWI Chromatin Remodeler Function Drives TBPH Cytoplasmic Redistribution in the Omega Speckles
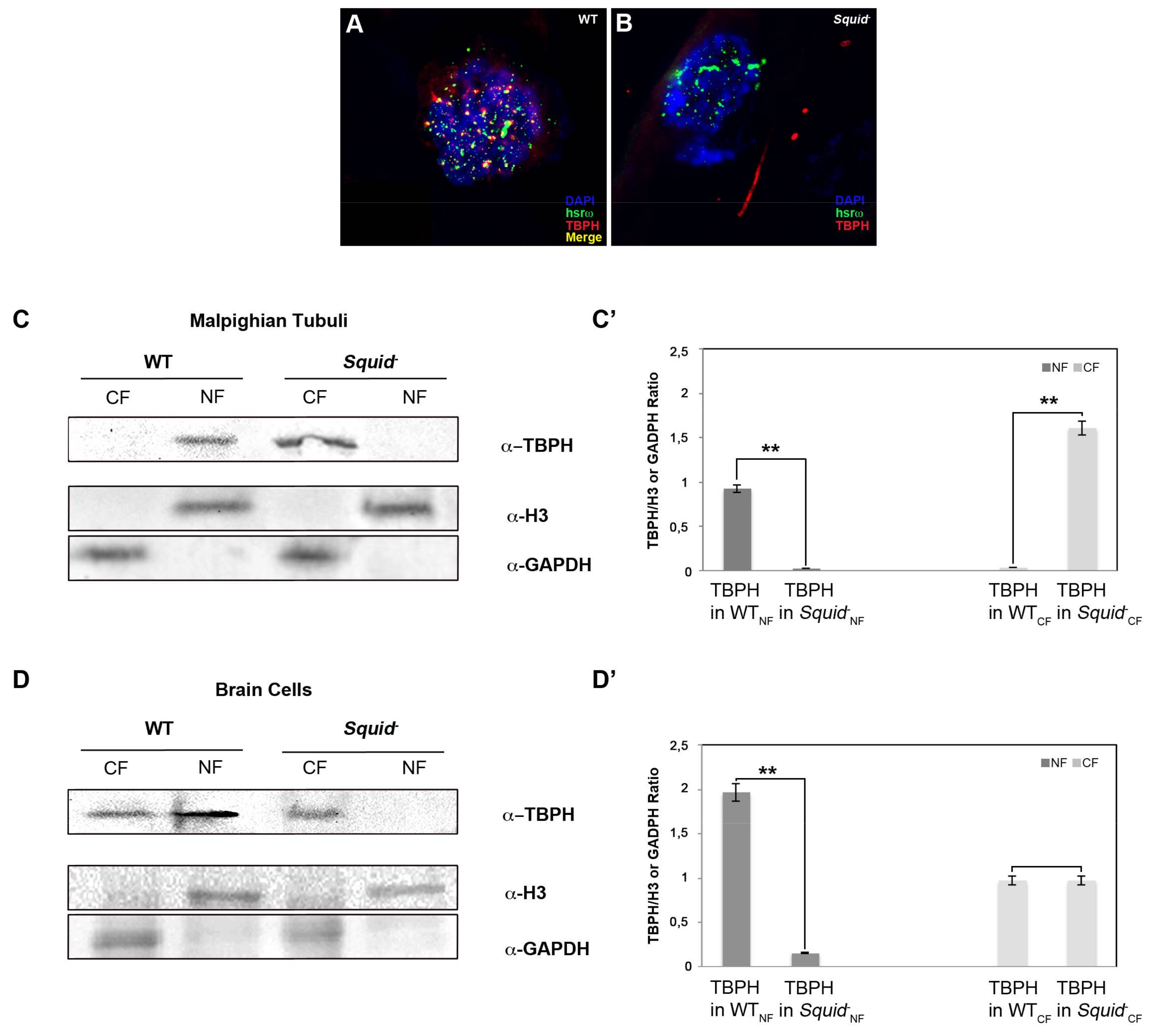
2.4. Squid hnRNP Influences TBPH Distribution between the Nucleus and Cytoplasm
3. Materials and Methods
3.1. Fly Strains and Genetic Interactions
3.2. Antibodies, Plasmid and RNA Probe
3.3. Immunofluorescence, FRISH, and Immuno-FRISH
3.4. Protein Extraction and Western Blotting
3.5. Co-Immunoprecipitation
3.6. Cross-Linking RNA Immuno-Precipitation (CLIP-RIP)
3.7. Real-Time Polymerase Chain Reaction (RT-PCR)
3.8. Protein Cloning, Expression and Purification
3.9. Gel Mobility Shift Assay
3.10. RNA Samples Preparation
3.11. Images Acquisition and Quantification
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y.; et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.A.; Wan, L.; Dreyfuss, G. RNA and disease. Cell 2009, 136, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Chen-Plotkin, A.S.; Lee, V.M.; Trojanowski, J.Q. TAR DNA-binding protein 43 in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.A. Inclusion body myositis. Curr. Opin. Rheumatol. 2011, 23, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, N.C.; Wang, Y.D.; Scarborough, E.A.; Moore, J.; Diaz, Z.; MacLea, K.S.; Freibaum, B.; Li, S.; Molliex, A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, M.; Taylor, J.P.; Parker, R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 2013, 154, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, L.L.; Corona, D.; Onorati, M.C. Emerging Roles for hnRNPs in post-transcriptional regulation: What can we learn from flies? Chromosoma 2014, 123, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F.E. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010, 7, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ritson, G.P.; Custer, S.K.; Freibaum, B.D.; Guinto, J.B.; Geffel, D.; Moore, J.; Tang, W.; Winton, M.J.; Neumann, M.; Trojanowski, J.Q.; et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J. Neurosci. 2010, 30, 7729–7739. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F.E. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001, 276, 36337–36343. [Google Scholar] [CrossRef] [PubMed]
- Sephton, C.F.; Cenik, B.; Cenik, B.K.; Herz, J.; Yu, G. TDP-43 in central nervous system development and function: Clues to TDP-43-associated neurodegeneration. Biol. Chem. 2012, 393, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Siva, K.; Denti, M.A. A network of RNA and protein interactions in Fronto Temporal Dementia. Front. Mol. Neurosci. 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Brindisi, A.; Giombi, M.; Tisminetzky, S.; Ayala, Y.M.; Baralle, F.E. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: An important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J. Biol. Chem. 2005, 280, 37572–37584. [Google Scholar] [CrossRef] [PubMed]
- Mercado, P.A.; Ayala, Y.M.; Romano, M.; Buratti, E.; Baralle, F.E. Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res. 2005, 33, 6000–6010. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F.E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. A J. Virtual Libr. 2008, 13, 867–878. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Polymenidou, M.; Cleveland, D.W. TDP-43 and FUS/TLS: Emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 2010, 19, R46–R64. [Google Scholar] [CrossRef] [PubMed]
- Gendron, T.F.; Rademakers, R.; Petrucelli, L. TARDBP mutation analysis in TDP-43 proteinopathies and deciphering the toxicity of mutant TDP-43. J. Alzheimer’s Dis. 2013, 33, S35–S45. [Google Scholar]
- Chang, C.K.; Huang, T.H. Untangling the structure of the TDP-43 N-terminal domain. FEBS J. 2016, 283, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Sambataro, F.; Pennuto, M. Post-translational Modifications and Protein Quality Control in Motor Neuron and Polyglutamine Diseases. Front. Mol. Neurosci. 2017, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.S.; Zhang, B.; Spector, D.L. Biogenesis and function of nuclear bodies. TIG 2011, 27, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, K.V.; Rajendra, T.K.; Lal, A.K.; Lakhotia, S.C. Omega speckles—A novel class of nuclear speckles containing hnRNPs associated with noncoding hsr-omega RNA in Drosophila. J. Cell Sci. 2000, 113 Pt 19, 3485–3497. [Google Scholar] [PubMed]
- Mallik, M.; Lakhotia, S.C. Pleiotropic consequences of misexpression of the developmentally active and stress-inducible non-coding hsromega gene in Drosophila. J. Biosci. 2011, 36, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Lakhotia, S.C.; Mallik, M.; Singh, A.K.; Ray, M. The large noncoding hsromega-n transcripts are essential for thermotolerance and remobilization of hnRNPs, HP1 and RNA polymerase II during recovery from heat shock in Drosophila. Chromosoma 2012, 121, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Hirose, T. Chromatin remodeling complexes in the assembly of long noncoding RNA-dependent nuclear bodies. Nucleus 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Clemson, C.M.; Hutchinson, J.N.; Sara, S.A.; Ensminger, A.W.; Fox, A.H.; Chess, A.; Lawrence, J.B. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 2009, 33, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Lakhotia, S.C. Human sat III and Drosophila hsr omega transcripts: A common paradigm for regulation of nuclear RNA processing in stressed cells. Nucleic Acids Res. 2006, 34, 5508–5514. [Google Scholar] [CrossRef] [PubMed]
- Onorati, M.C.; Lazzaro, S.; Mallik, M.; Ingrassia, A.M.; Carreca, A.P.; Singh, A.K.; Chaturvedi, D.P.; Lakhotia, S.C.; Corona, D.F. The ISWI chromatin remodeler organizes the hsromega ncRNA-containing omega speckle nuclear compartments. PLoS Genet. 2011, 7, e1002096. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, S.S.; Krebs, J.E. Functional diversity of ISWI complexes. Biochem. Cell Biol. 2004, 82, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Corona, D.F.; Siriaco, G.; Armstrong, J.A.; Snarskaya, N.; McClymont, S.A.; Scott, M.P.; Tamkun, J.W. ISWI regulates higher-order chromatin structure and histone H1 assembly in vivo. PLoS Biol. 2007, 5, e232. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Lakhotia, S.C. Dynamics of hnRNPs and omega speckles in normal and heat shocked live cell nuclei of Drosophila melanogaster. Chromosoma 2015, 124, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Diaper, D.C.; Adachi, Y.; Sutcliffe, B.; Humphrey, D.M.; Elliott, C.J.; Stepto, A.; Ludlow, Z.N.; Vanden Broeck, L.; Callaerts, P.; Dermaut, B.; et al. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Hum. Mol. Genet. 2013, 22, 1539–1557. [Google Scholar] [CrossRef] [PubMed]
- Lo Piccolo, L.; Bonaccorso, R.; Onorati, M.C. Nuclear and Cytoplasmic Soluble Proteins Extraction from a Small Quantity of Drosophila’s Whole Larvae and Tissues. Int. J. Mol. Sci. 2015, 16, 12360–12367. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrogio, A.; Buratti, E.; Stuani, C.; Guarnaccia, C.; Romano, M.; Ayala, Y.M.; Baralle, F.E. Functional mapping of the interaction between TDP-43 and hnRNP A2 in vivo. Nucleic Acids Res. 2009, 37, 4116–4126. [Google Scholar] [CrossRef] [PubMed]
- Romano, G.; Klima, R.; Buratti, E.; Verstreken, P.; Baralle, F.E.; Feiguin, F. Chronological requirements of TDP-43 function in synaptic organization and locomotive control. Neurobiol. Dis. 2014, 71, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Lo Piccolo, L.; Attardi, A.; Bonaccorso, R.; Li Greci, L.; Giurato, G.; Ingrassia, A.M.; Onorati, M.C. ISWI ATP-dependent remodeling of nucleoplasmic omega-speckles in the brain of Drosophila melanogaster. J. Genet. Genom. 2017, 44, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Berson, A.; Sartoris, A.; Nativio, R.; Van Deerlin, V.; Toledo, J.B.; Porta, S.; Liu, S.; Chung, C.Y.; Garcia, B.A.; Lee, V.M.; et al. TDP-43 Promotes Neurodegeneration by Impairing Chromatin Remodeling. Curr. Biol. 2017, 27, 3579–3590. [Google Scholar] [CrossRef] [PubMed]
- Genovese, S.; Corona, D. A New Medium to Grow Live Insects. European Patent MI2007A001420/8145 PTIT, 2007. [Google Scholar]
- Goodrich, J.S.; Clouse, K.N.; Schupbach, T. Hrb27C, Sqd and Otu cooperatively regulate gurken RNA localization and mediate nurse cell chromosome dispersion in Drosophila oogenesis. Development 2004, 131, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Burgio, G.; La Rocca, G.; Sala, A.; Arancio, W.; Di Gesu, D.; Collesano, M.; Sperling, A.S.; Armstrong, J.A.; van Heeringen, S.J.; Logie, C.; et al. Genetic identification of a network of factors that functionally interact with the nucleosome remodeling ATPase ISWI. PLoS Genet. 2008, 4, e1000089. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Deng, Z.L.; Luo, X.; Tang, N.; Song, W.X.; Chen, J.; Sharff, K.A.; Luu, H.H.; Haydon, R.C.; Kinzler, K.W.; et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2007, 2, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Niranjanakumari, S.; Lasda, E.; Brazas, R.; Garcia-Blanco, M.A. Reversible cross-linking combined with immunoprecipitation to study RNA-protein interactions in vivo. Methods 2002, 26, 182–190. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.Y.; Goldberg, A.L. Cellular defenses against unfolded proteins: A cell biologist thinks about neurodegenerative diseases. Neuron 2001, 29, 15–32. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Piccolo, L.; Bonaccorso, R.; Attardi, A.; Li Greci, L.; Romano, G.; Sollazzo, M.; Giurato, G.; Ingrassia, A.M.R.; Feiguin, F.; Corona, D.F.V.; et al. Loss of ISWI Function in Drosophila Nuclear Bodies Drives Cytoplasmic Redistribution of Drosophila TDP-43. Int. J. Mol. Sci. 2018, 19, 1082. https://doi.org/10.3390/ijms19041082
Lo Piccolo L, Bonaccorso R, Attardi A, Li Greci L, Romano G, Sollazzo M, Giurato G, Ingrassia AMR, Feiguin F, Corona DFV, et al. Loss of ISWI Function in Drosophila Nuclear Bodies Drives Cytoplasmic Redistribution of Drosophila TDP-43. International Journal of Molecular Sciences. 2018; 19(4):1082. https://doi.org/10.3390/ijms19041082
Chicago/Turabian StyleLo Piccolo, Luca, Rosa Bonaccorso, Andrea Attardi, Lorenzo Li Greci, Giulia Romano, Martina Sollazzo, Giorgio Giurato, Antonia Maria Rita Ingrassia, Fabian Feiguin, Davide F. V. Corona, and et al. 2018. "Loss of ISWI Function in Drosophila Nuclear Bodies Drives Cytoplasmic Redistribution of Drosophila TDP-43" International Journal of Molecular Sciences 19, no. 4: 1082. https://doi.org/10.3390/ijms19041082
APA StyleLo Piccolo, L., Bonaccorso, R., Attardi, A., Li Greci, L., Romano, G., Sollazzo, M., Giurato, G., Ingrassia, A. M. R., Feiguin, F., Corona, D. F. V., & Onorati, M. C. (2018). Loss of ISWI Function in Drosophila Nuclear Bodies Drives Cytoplasmic Redistribution of Drosophila TDP-43. International Journal of Molecular Sciences, 19(4), 1082. https://doi.org/10.3390/ijms19041082







