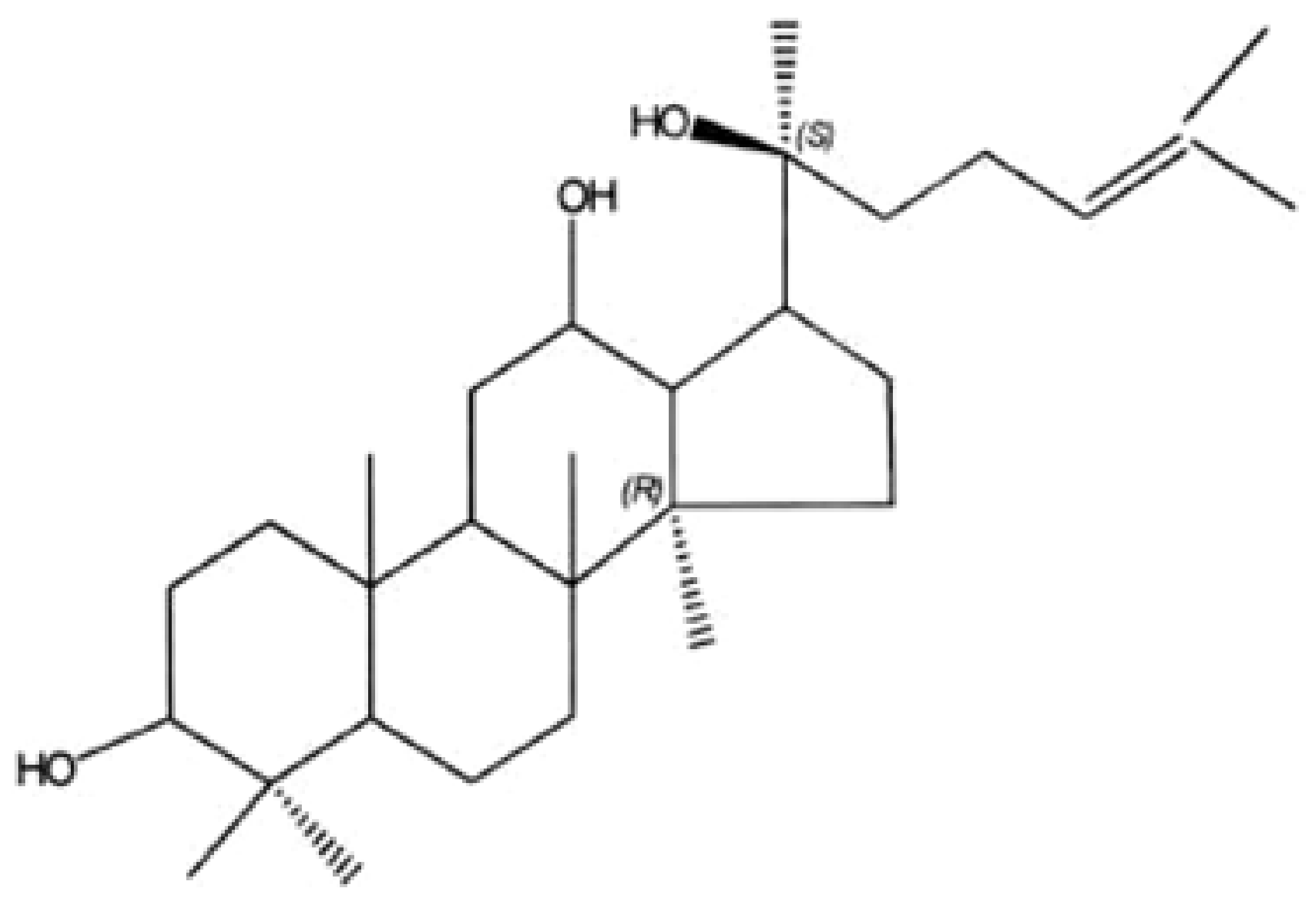
20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway
Abstract
:1. Introduction
2. Results
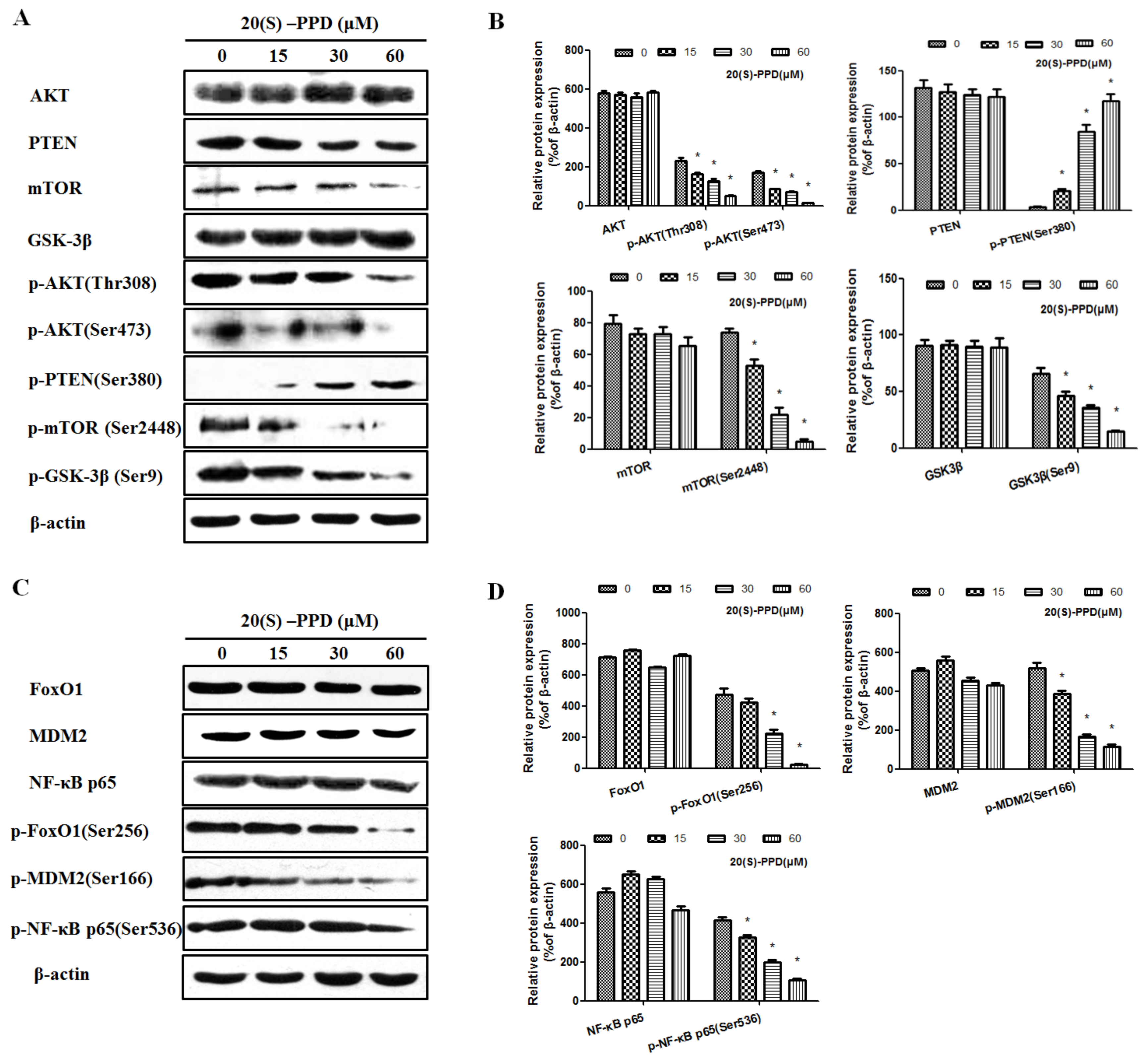
2.1. 20(S)-PPD Inhibited the PI3K/AKT/mTOR Pathway and the Downstream Protein Expression in MCF-7 Cells
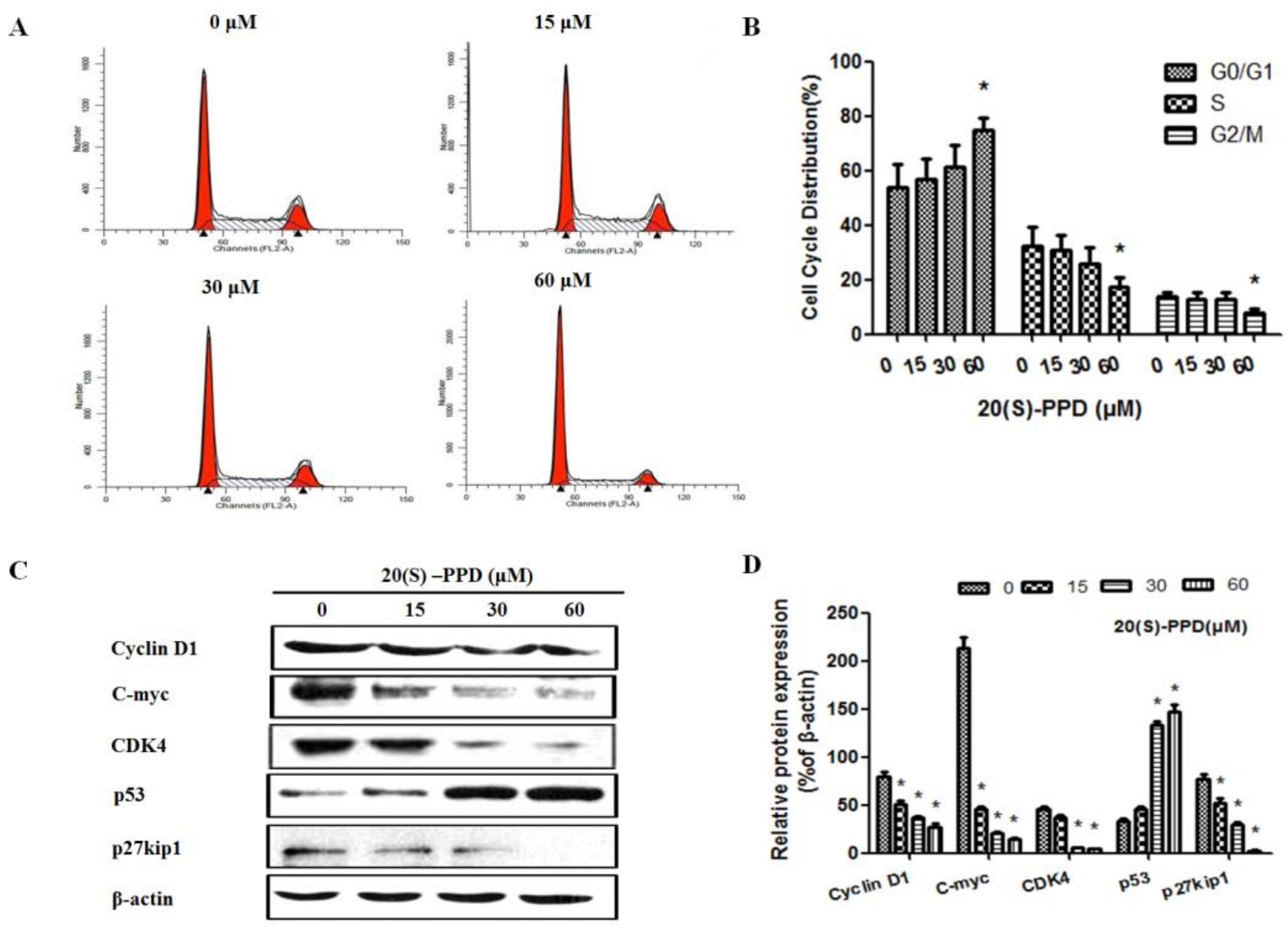
2.2. 20(S)-PPD-Induced Cell Cycle Arrest of G1/G0 Phase in MCF-7 Cells
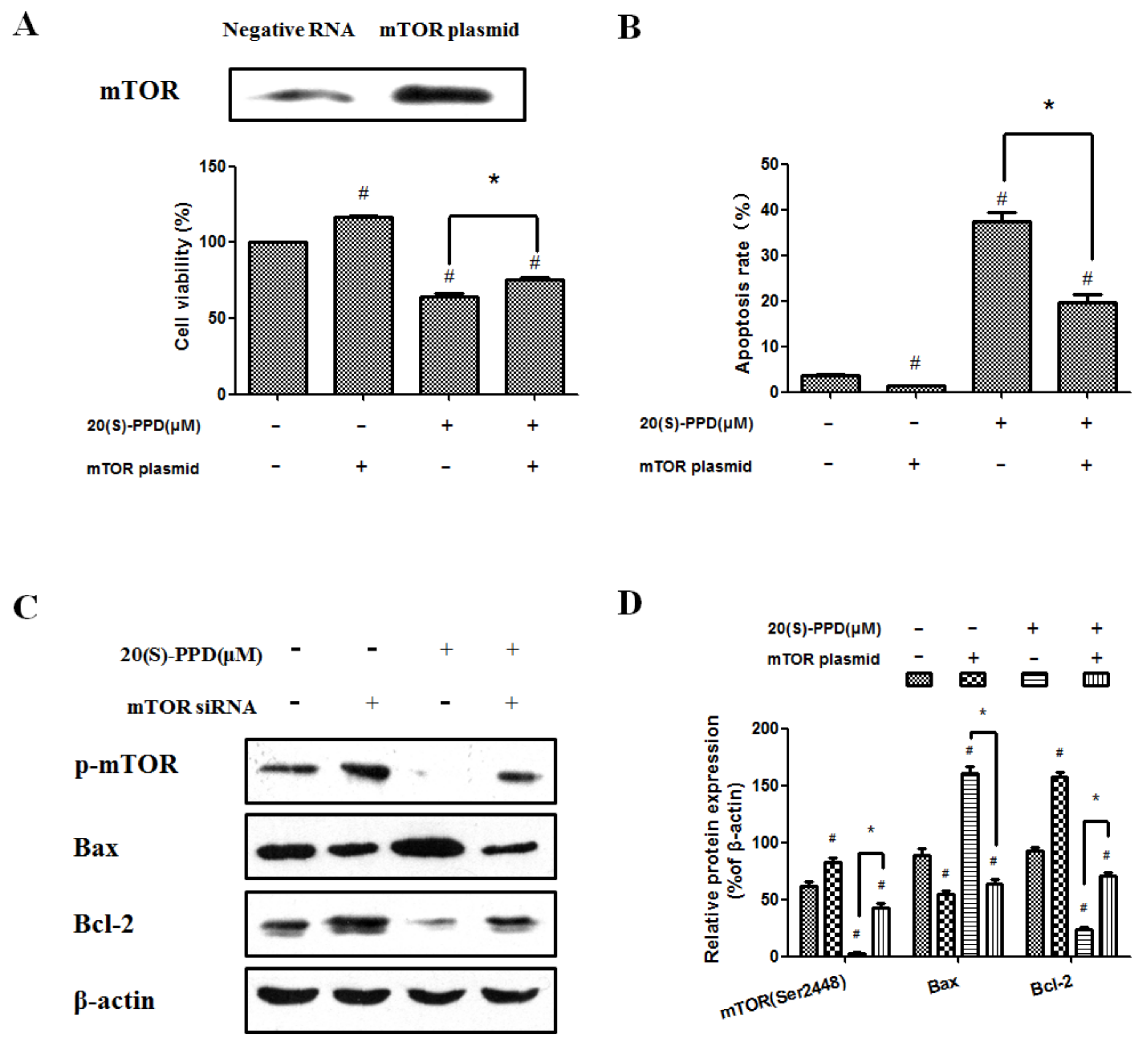
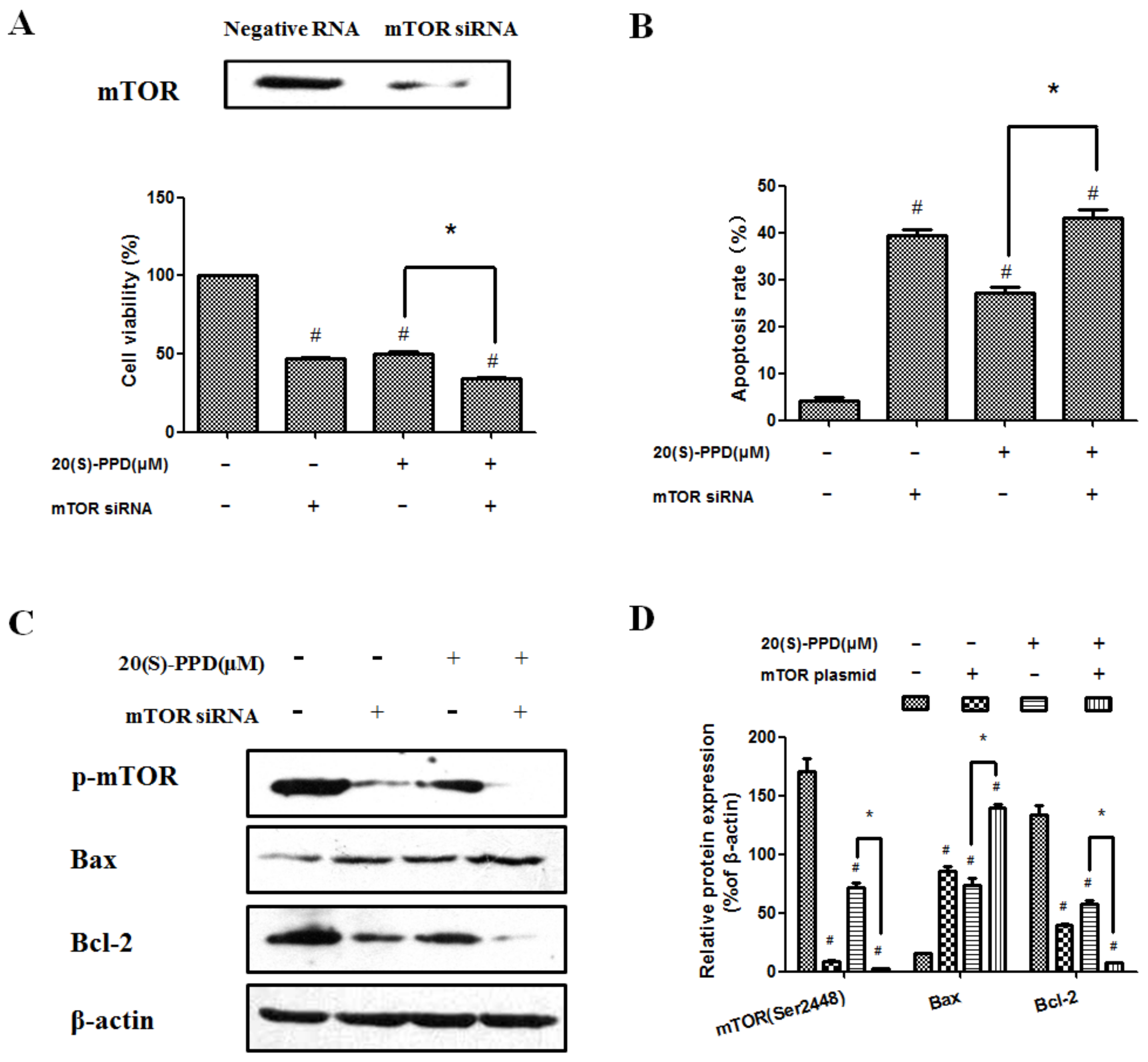
2.3. 20(S)-PPD-Induced Apoptosis Was Reversed by Transfection with mTOR Plasmid
2.4. 20(S)-PPD-Induced Apoptosis Was Promoted by Knockdown of mTOR with siRNA
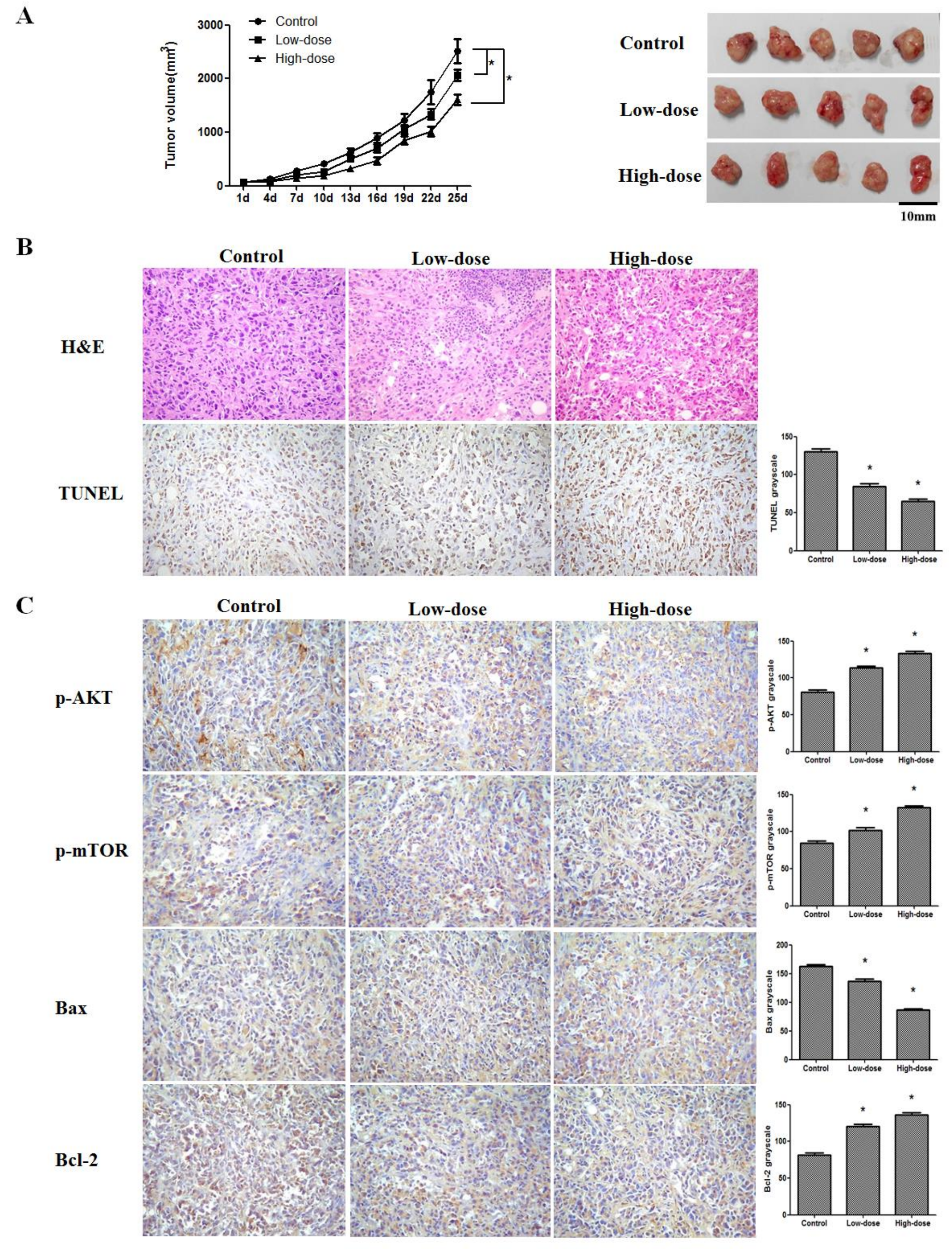
2.5. 20(S)-PPD Inhibited the Growth of MCF-7 Breast Cancer Cells in a Nude Mice Xenograft Assay
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Cell Viability Assay
4.3. Apoptosis Assessment
4.4. Western Blot Analysis
4.5. Cell Cycle Analysis
4.6. Transfection Assay
4.7. In Vivo MCF-7 Cell Xenograft Antitumor Research
4.8. Immunohistochemistry and H&E Staining
4.9. TUNEL Staining
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Nahta, R. Pharmacological strategies to overcome HER2 cross-talk and Trastuzumab resistance. Curr. Med. Chem. 2012, 19, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef] [PubMed]
- Whitman, M.; Kaplan, D.R.; Schaffhausen, B.; Cantley, L.; Roberts, T.M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature 1985, 315, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Whitman, M.; Cantley, L.C.; Erikson, R.L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc. Natl. Acad. Sci. USA 1984, 81, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist 2011, 16 (Suppl. 1), 12–19. [Google Scholar] [CrossRef] [PubMed]
- Boyault, S.; Drouet, Y.; Navarro, C.; Bachelot, T.; Lasset, C.; Treilleux, I.; Tabone, E.; Puisieux, A.; Wang, Q. Mutational characterization of individual breast tumors: TP53 and PI3K pathway genes are frequently and distinctively mutated in different subtypes. Breast Cancer Res. Treat. 2012, 132, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Manning, B.D.; Cantley, L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell 2003, 4, 257–262. [Google Scholar] [CrossRef]
- Weinstein, I.B. Addiction to oncogenes—The Achilles heal of cancer. Science 2002, 297, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Wang, C.Z.; Zhang, B.; Mehendale, S.R.; Li, X.L.; Sun, S.; Han, A.H.; Du, W.; He, T.C.; Yuan, C.S. In vitro and in vivo anticancer effects of American ginseng berry: Exploring representative compounds. Biol. Pharm. Bull. 2009, 32, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rayburn, E.R.; Hao, M.; Zhao, Y.; Hill, D.L.; Zhang, R.; Wang, H. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate 2008, 68, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, Q.; Hang, Y.; Bu, X.; Jia, W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer 2007, 109, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lee, B.H. A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicol. Appl. Pharmacol. 2004, 194, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Bu, X.; Yan, H.; Jia, W.W. 20S-protopanaxadiol-induced programmed cell death in glioma cells through caspase-dependent and -independent pathways. J. Nat. Prod. 2007, 70, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dan, H.C.; Sun, M.; Liu, Q.; Sun, X.M.; Feldman, R.I.; Hamilton, A.D.; Polokoff, M.; Nicosia, S.V.; Herlyn, M.; et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004, 64, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, R.; Xu, H.L.; Yu, X.F.; Qu, S.C.; Sui, D.Y. 20(S)-protopanaxadiol triggers mitochondrial-mediated apoptosis in human lung adenocarcinoma A549 cells via inhibiting the PI3K/Akt signaling pathway. Am. J. Chin. Med. 2013, 41, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.L.; Fu, W.W.; Xin, Y.; Li, M.W.; Wang, S.J.; Yu, X.F.; Sui, D.Y. 20(S)-Protopanaxadiol induces human breast cancer MCF-7 apoptosis through a caspase-mediated pathway. Asian Pac. J. Cancer Prev. 2014, 15, 7919–7923. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Badwe, R.; Boyle, P.; Derricks, G.; Dare, A.; Evans, T.; Eniu, A.; Jimenez, J.; Kutluk, T.; Lopes, G.; et al. Changing global policy to deliver safe, equitable and affordable care for women’s cancers. Lancet 2017, 389, 871–880. [Google Scholar] [CrossRef]
- Manthravadi, S.; Shrestha, A.; Madhusudhana, S. Impact of statin use on cancer recurrence and mortality in breast cancer: A systematic review and meta-analysis. Int. J. Cancer 2016, 139, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Montemurro, F.; Kannan, S.; Rossi, V.; Verma, C.; Baselga, J.; Scaltriti, M. AKT signaling in ERBB2-amplified breast cancer. Pharmacol. Ther. 2016, 158, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, W.; Fan, M. Emerging therapies for breast cancer. J. Hematol. Oncol. 2017, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Hui, T.; Shao, B.; Li, L.; Du, Z.; Lu, L.; Ye, L.; Li, S.; Li, Q.; Xiao, Q.; et al. Dickkopf-related protein 2 induces G0/G1 arrest and apoptosis through suppressing Wnt/β-catenin signaling and is frequently methylated in breast cancer. Oncotarget 2017, 8, 39443. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Hassan, Z.M. Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic. Biol. Med. 2012, 52, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Fry, E.A.; Taneja, P.; Inoue, K. Oncogenic and tumor-suppressive mouse models for breast cancer engaging HER2/neu. Int. J. Cancer 2017, 140, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Aleshin, A.; Slamon, D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. BCR 2016, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, H.-L.; Wang, Y.-C.; Lu, Z.-Y.; Yu, X.-F.; Sui, D.-Y. 20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 1053. https://doi.org/10.3390/ijms19041053
Zhang H, Xu H-L, Wang Y-C, Lu Z-Y, Yu X-F, Sui D-Y. 20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway. International Journal of Molecular Sciences. 2018; 19(4):1053. https://doi.org/10.3390/ijms19041053
Chicago/Turabian StyleZhang, Hong, Hua-Li Xu, Yu-Chen Wang, Ze-Yuan Lu, Xiao-Feng Yu, and Da-Yun Sui. 2018. "20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway" International Journal of Molecular Sciences 19, no. 4: 1053. https://doi.org/10.3390/ijms19041053
APA StyleZhang, H., Xu, H.-L., Wang, Y.-C., Lu, Z.-Y., Yu, X.-F., & Sui, D.-Y. (2018). 20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway. International Journal of Molecular Sciences, 19(4), 1053. https://doi.org/10.3390/ijms19041053





