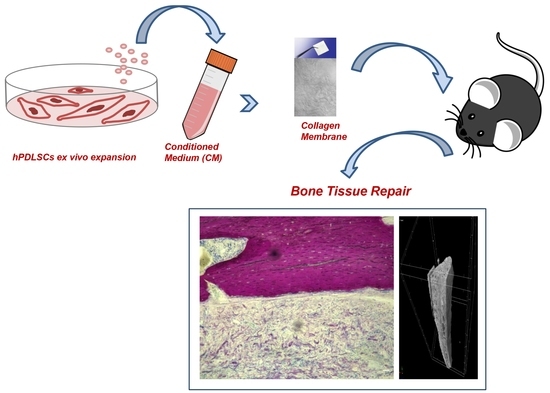
Biofunctionalized Scaffold in Bone Tissue Repair
Abstract
:1. Introduction
2. Results
2.1. Human Periodontal Ligament Stem Cells (hPDLSCs) Characterization
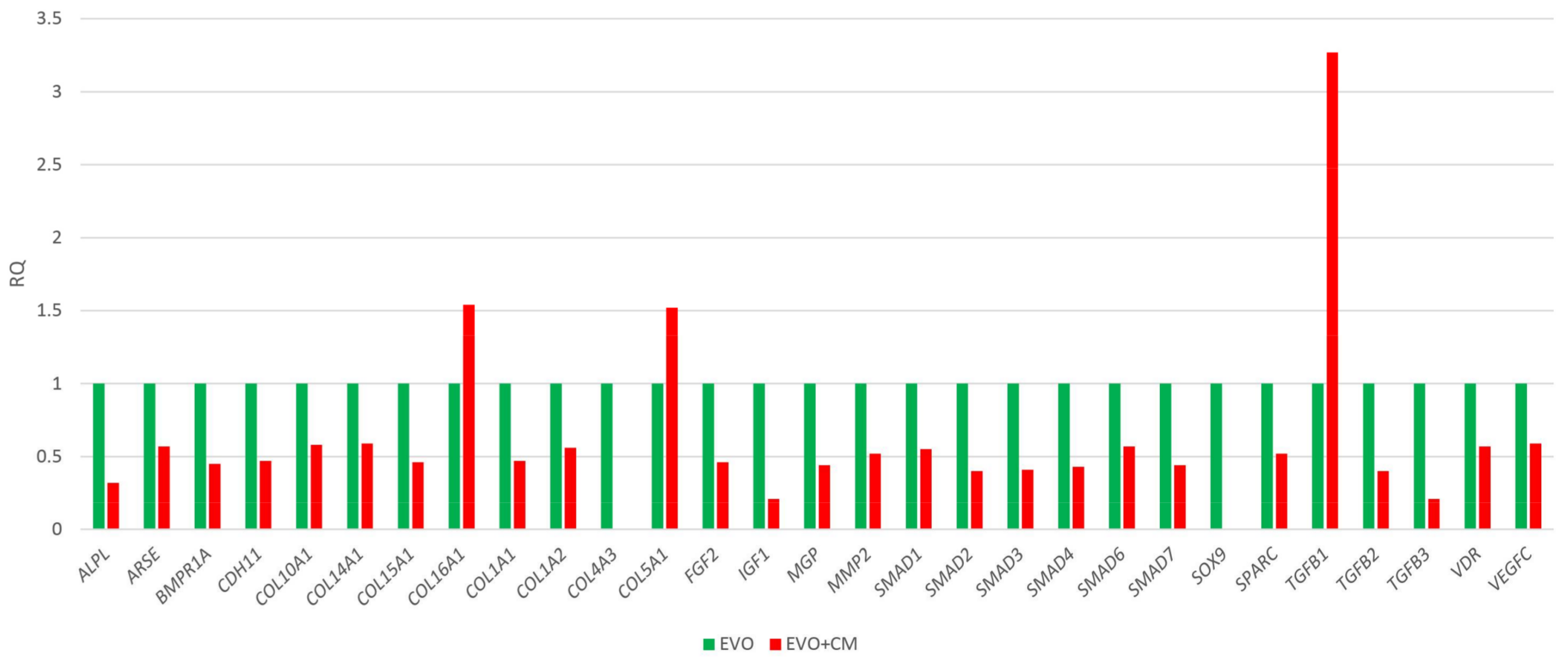
2.2. Human Osteogenesis Expression Signature
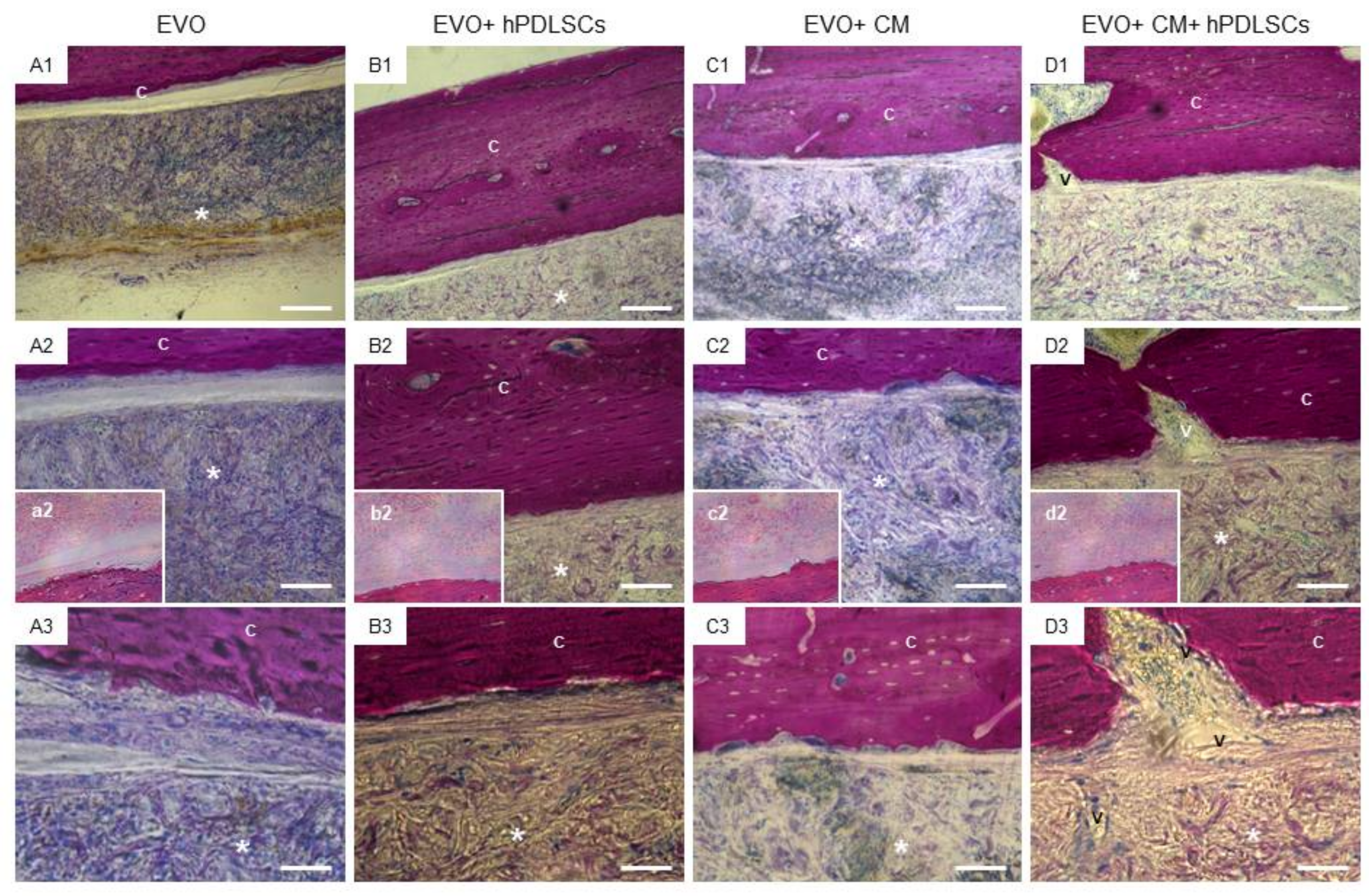
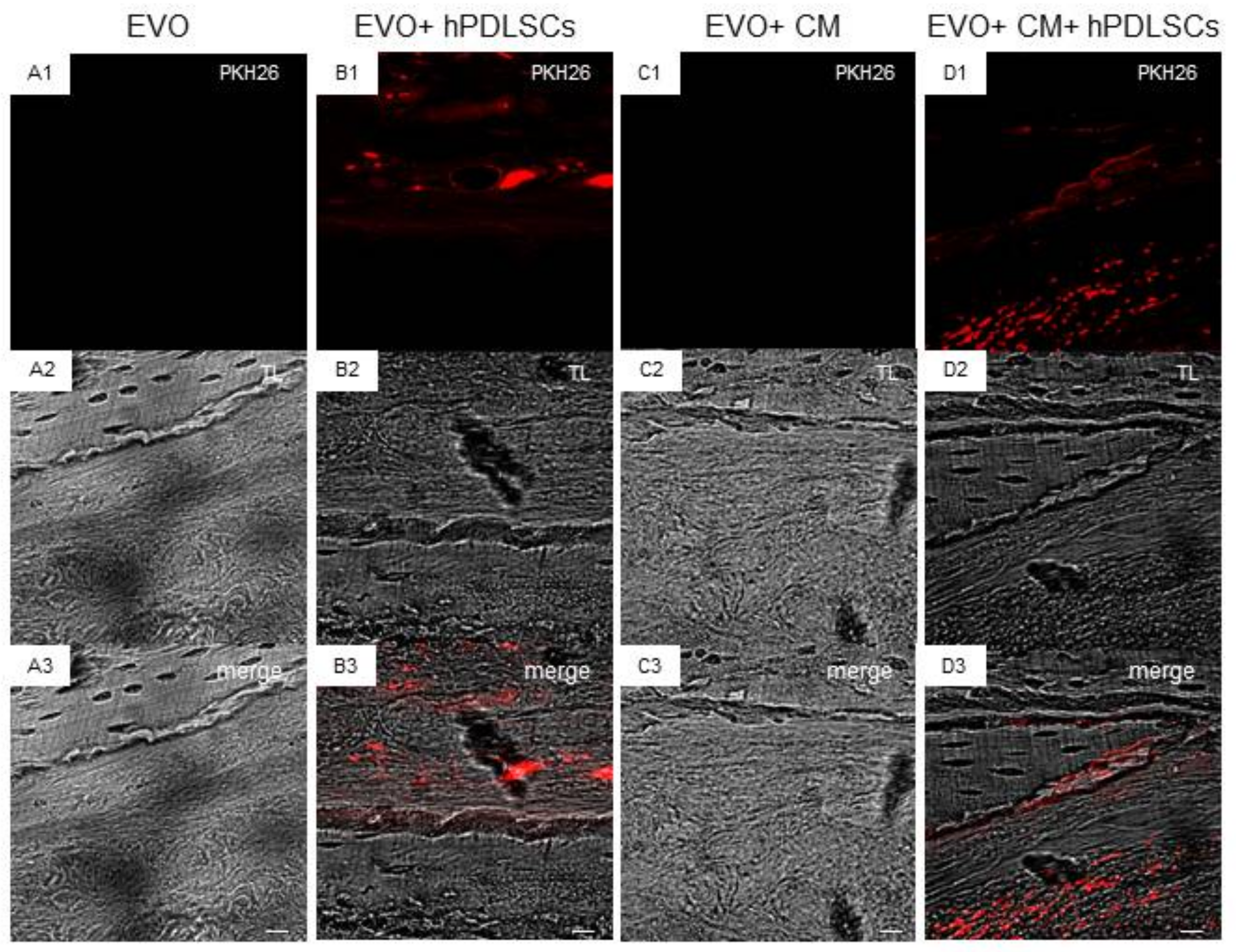
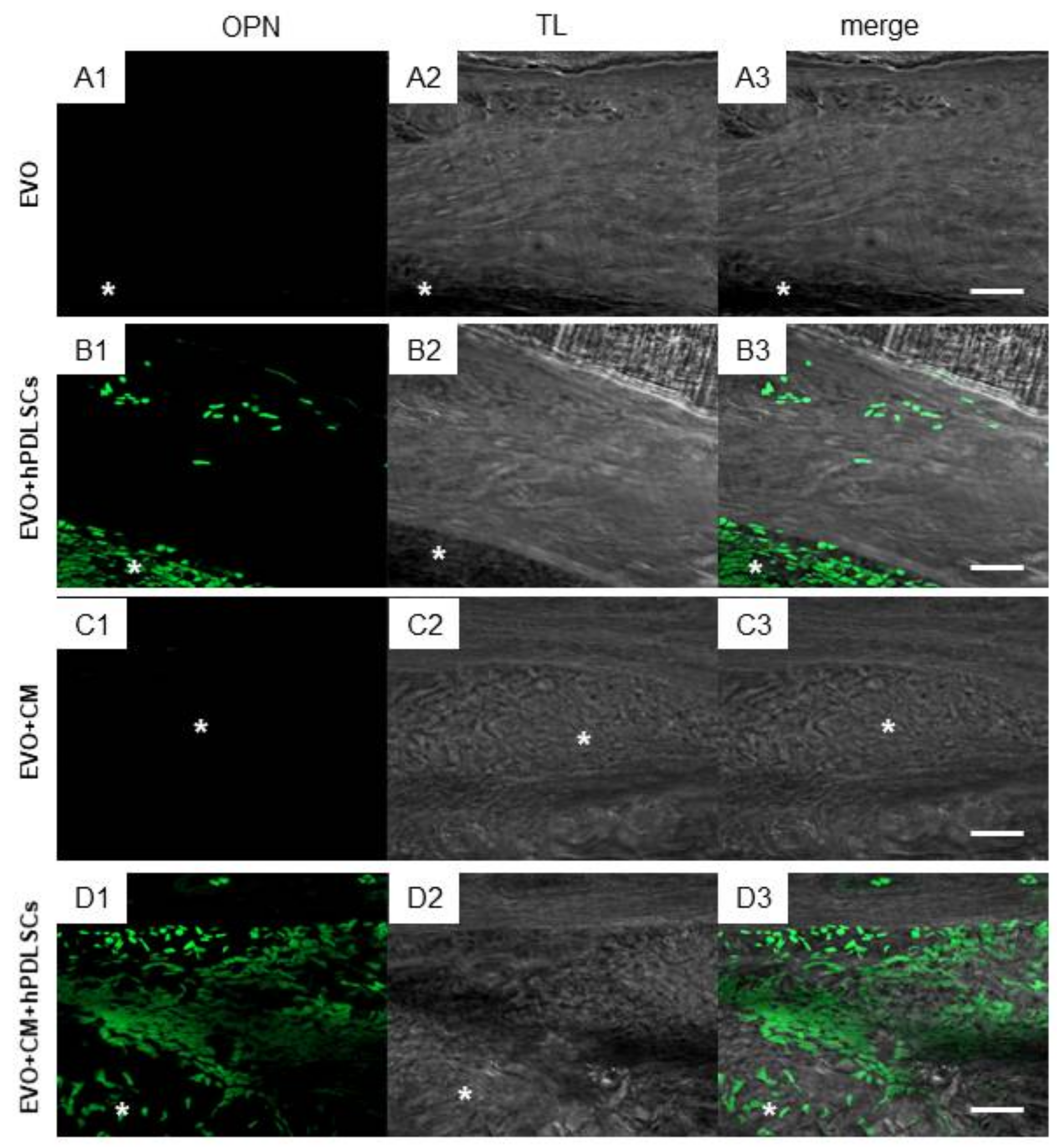
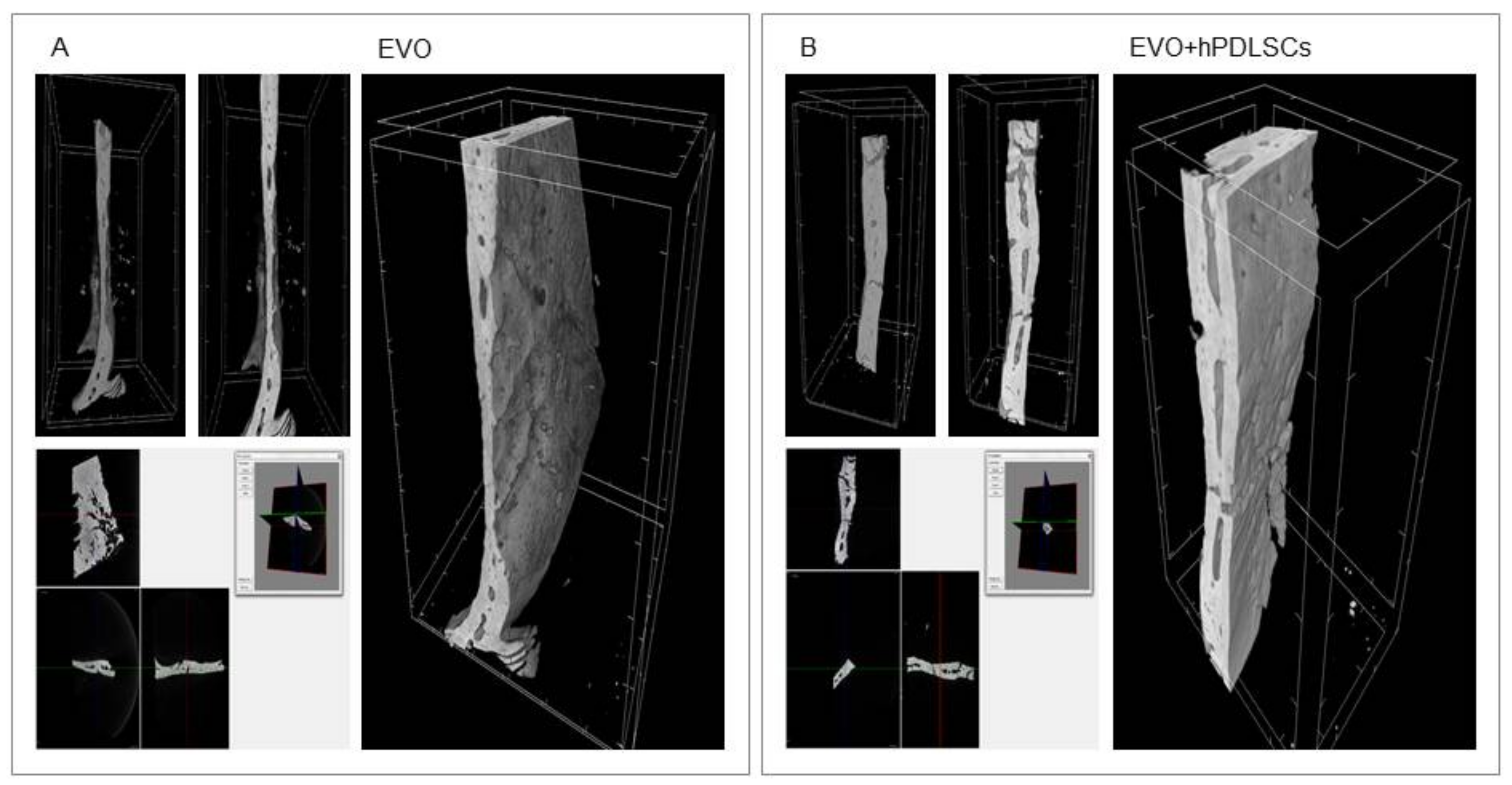
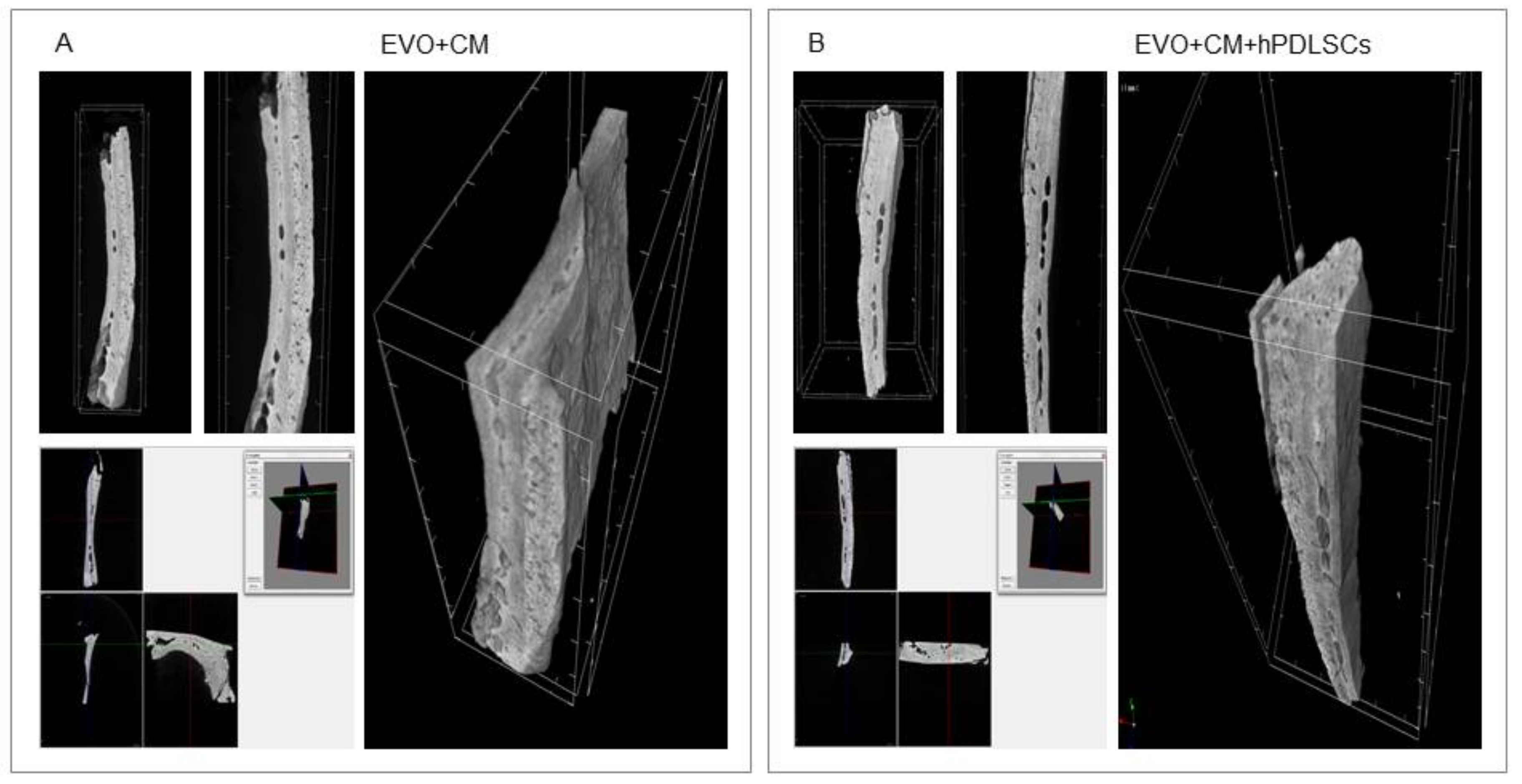
2.3. EVO In Vivo Evaluation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Characterization
4.2. (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Tetrazolium Reduction (MTT) Assay
4.3. Scaffold Material
4.4. CM Collection
4.5. RNA Extraction and TaqMan Quantitative Real-Time PCR
4.6. Statistical Analysis
4.7. Animals
4.8. Ethics Statement for Animal Use
4.9. Membrane Grafting
4.10. Experimental Design
- EVO Group (n = 4): rats subjected to the scraping of the cortical calvaria bone tissue and implant of EVO;
- EVO + hPDLSCs (n = 4): rats subjected to the scraping of the cortical calvaria bone tissue and implant of EVO + hPDLSCs;
- EVO + CM (n = 4): rats subjected to the scraping of the cortical calvaria bone tissue and implant of EVO + CM;
- EVO + CM + hPDLSCs (n = 4): rats subjected to the scraping of the cortical calvaria bone tissue and implant of EVO + CM + hPDLSCs;
4.11. Micro-CT Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| β-TCP | β-tricalcium phosphate |
| HA | Hydroxyapatite |
| PCL | Polycaprolactone |
| PGA | Polyglycolic acid |
| PLA | Poly-(lactide) |
| PLGA | Polylactic co-glycolic acid |
| MSCs | Mesenchymal stem cells |
| BMPs | Bone morphogenetic proteins |
| TGF-β3 | Transforming growth factor β3 |
| VEGF | Vascular endothelial growth factor |
| IL | Interleukin |
| SDF-1α | Stromal cell-derived factor 1α |
| MCP-1 | Monocyte chemotactic protein 1 |
| MIP-1α | Macrophage inflammatory protein 1α |
| COL | Collagen |
| FDR | False discovery rate |
| PDLSCs | Periodontal ligament stem cells |
| hPDLSCs | Human periodontal ligament stem cells |
| CM | Conditioned medium |
| hGMSCs | Human gingival mesenchymal stem cells |
| EVO | Collagen membrane Evolution |
| MTT | (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction |
| Mag | Magnification |
| SMADs | Small mothers against decapentaplegic |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GUSB | Glucuronidase β |
| 18S | 18S ribosomal RNAs |
| HPRT | Hypoxanthine Phosphoribosyltransferase |
| PKH26 | PKH26-Red Fluorescent Cell Linker Kit |
| ALP | Alkaline phosphatase |
| OPN | Osteopontin |
| CT | Computed tomography |
| SOX9 | Sex determining region Y-box 9 |
| WJ-MSCs | Wharton’s jelly of umbilical cord stem cells |
| PBS | Phosphate buffered saline |
| TIFF | Tagged image file format |
References
- Kalfas, I.H. Principles of bone healing. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ritz, U.; Gerke, R.; Gotz, H.; Stein, S.; Rommens, P.M. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen I: An in vitro study. Int. J. Mol. Sci. 2017, 18, 2568. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic materials and fabrication approaches for bone tissue engineering. Adv. Healthc. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Vijayavenkataraman, S.; Liu, H. An overview of scaffold design and fabrication technology for engineered knee meniscus. Materials 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Zhang, Z.; Elisseeff, J. Biomaterials directed in vivo osteogenic differentiation of mesenchymal cells derived from human embryonic stem cells. Tissue Eng. Part A 2013, 19, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Diomede, F.; Cardelli, P.; Bramanti, A.; Scionti, D.; Bramanti, P.; Trubiani, O.; Mazzon, E. Transcriptomic analysis of gingival mesenchymal stem cells cultured on 3d bioprinted scaffold: A promising strategy for neuroregeneration. J. Biomed. Mater. Res. Part A 2018, 106, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Scionti, D.; Merciaro, I.; Cavalcanti, M.F.; Mazzon, E.; Trubiani, O. Biotherapeutic effect of gingival stem cells conditioned medium in bone tissue restoration. Int. J. Mol. Sci. 2018, 19, 329. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S.; Shuo, Z.; Fuh, J.Y.H.; Lu, W.F. Design of three-dimensional scaffolds with tunable matrix stiffness for directing stem cell lineage specification: An in silico study. Bioengineering 2017, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular matrix scaffolds for tissue engineering and regenerative medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Tani, S.; Sano, A.; Fujioka, K. Microstructure and release characteristics of the minipellet, a collagen-based drug delivery system for controlled release of protein drugs. J. Control. Release 1999, 62, 313–324. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, F.M.; Kohler, G. Collagen as matrix for neo-organ formation by gene-transfected fibroblasts. Anticancer Res. 1997, 17, 1179–1186. [Google Scholar] [PubMed]
- Doillon, C.J.; Silver, F.H. Collagen-based wound dressing: Effects of hyaluronic acid and fibronectin on wound healing. Biomaterials 1986, 7, 3–8. [Google Scholar] [CrossRef]
- Park, J.Y.; Shim, J.H.; Choi, S.A.; Jang, J.; Kim, M.; Lee, S.H.; Cho, D.W. 3D printing technology to control bmp-2 and vegf delivery spatially and temporally to promote large-volume bone regeneration. J. Mater. Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Tani, C.; Vagnani, S.; Carli, L.; Querci, F.; Kuhl, A.A.; Spieckermann, S.; Cieluch, C.P.; Pacini, S.; Fazzi, R.; Mosca, M. Treatment with allogenic mesenchymal stromal cells in a murine model of systemic lupus erythematosus. Int. J. Stem Cells 2017, 10, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yang, H.; Liu, Y.; Liu, Q.; Wang, A.; Ding, Y.; Jin, Z. Evaluation of bmmscs-epcs sheets for repairing alveolar bone defects in ovariectomized rats. Sci. Rep. 2017, 7, 16568. [Google Scholar] [CrossRef] [PubMed]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Orsini, G.; Zini, N.; Di Iorio, D.; Piccirilli, M.; Piattelli, A.; Caputi, S. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: A morphological report. J. Biomed. Mater. Res. Part A 2008, 87, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Rajan, T.S.; Gatta, V.; D’Aurora, M.; Merciaro, I.; Marchisio, M.; Muttini, A.; Caputi, S.; Bramanti, P.; Mazzon, E.; et al. Stemness maintenance properties in human oral stem cells after long-term passage. Stem Cells Int. 2017, 2017, 5651287. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Bozkurt, B.; Hakki, E.E.; Kayis, S.A.; Turac, G.; Yilmaz, I.; Karaoz, E. Bone morphogenetic protein-2, -6, and -7 differently regulate osteogenic differentiation of human periodontal ligament stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, X.; Pei, D.; Sun, G.; Li, Y.; Zhu, C.; Qiang, C.; Sun, J.; Shi, J.; Dong, Y.; et al. The promotion function of berberine for osteogenic differentiation of human periodontal ligament stem cells via ERK-FOS pathway mediated by EGFR. Sci. Rep. 2018, 8, 2848. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Carrillo, N.; Calvo-Guirado, J.L.; Villamil, J.; Delgado-Ruiz, R.A. Osteogenic potential of platelet-rich plasma in dental stem-cell cultures. Br. J. Oral Maxillofac. Surg. 2017, 55, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, A.; Matula, Z.; Szigeti, A.; Varady, G.; Szalma, J.; Szabo, G.; Uher, F.; Sarkadi, B.; Nemet, K. In vitro characterization of human mesenchymal stem cells isolated from different tissues with a potential to promote complex bone regeneration. Stem Cells Int. 2016, 2016, 3595941. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Chen, C.; Xu, X.; Akiyama, K.; Ansari, S.; Zadeh, H.H.; Shi, S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng. Part A 2014, 20, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Diomede, F.; Ballerini, P.; Paolantonio, M.; Marchisio, M.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. The secretome of periodontal ligament stem cells from MS patients protects against eae. Sci. Rep. 2016, 6, 38743. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Iwasaki, K.; Akazawa, K.; Komaki, M.; Yokoyama, N.; Izumi, Y.; Morita, I. Conditioned medium from periodontal ligament stem cells enhances periodontal regeneration. Tissue Eng. Part A 2017, 23, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of parkinson’s disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shi, M.; Li, L.; Liu, J.; Chen, B.; Chen, Y.; An, X.; Liu, S.; Luo, R.; Long, D.; et al. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the sirt1/ampk/pgc-1alpha pathway. Clin. Sci. 2016, 130, 2181–2198. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Matsubara, K.; Ishikawa, J.; Fujio, M.; Shohara, R.; Hibi, H.; Ueda, M.; Yamamoto, A. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 2014, 61, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, W.; Osugi, M.; Kawai, T.; Ueda, M. Novel cell-free regeneration of bone using stem cell-derived growth factors. Int. J. Oral Maxillofac. Implants 2013, 28, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Ohmori, M.; Hara, K.; Fujio, M.; Ikeno, M.; Hibi, H.; Ueda, M. An experimental study on guided bone regeneration using a polylactide-co-glycolide membrane-immobilized conditioned medium. Int. J. Oral Maxillofac. Implants 2015, 30, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Katagiri, W.; Osugi, M.; Kawai, T.; Sugimura, Y.; Hibi, H.; Nakamura, S.; Ueda, M. Evaluation of the therapeutic effects of conditioned media from mesenchymal stem cells in a rat bisphosphonate-related osteonecrosis of the jaw-like model. Bone 2015, 74, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Trubiani, O.; Diomede, F.; Piattelli, A.; Bramanti, P.; Mazzon, E. Conditioned medium of periodontal ligament mesenchymal stem cells exert anti-inflammatory effects in lipopolysaccharide-activated mouse motoneurons. Exp. Cell Res. 2016, 349, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Soundara Rajan, T.; Giacoppo, S.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int. J. Immunopathol. Pharmacol. 2017, 30, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Baik, H.S.; Park, J.; Lee, K.J.; Chung, C. Local application of periodontal ligament stromal cells promotes soft tissue regeneration. Oral Dis. 2014, 20, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Jang, J.; Kang, S.; Bhang, S.H.; Jeong, G.J.; Shin, H.; Kim, D.W.; Kim, B.S. Mesenchymal stem cell-conditioned medium enhances osteogenic and chondrogenic differentiation of human embryonic stem cells and human induced pluripotent stem cells by mesodermal lineage induction. Tissue Eng. Part A 2014, 20, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Vanecek, V.; Klima, K.; Kohout, A.; Foltan, R.; Jirousek, O.; Sedy, J.; Stulik, J.; Sykova, E.; Jendelova, P. The combination of mesenchymal stem cells and a bone scaffold in the treatment of vertebral body defects. Eur. Spine J. 2013, 22, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Sumanasinghe, R.D.; Osborne, J.A.; Loboa, E.G. Mesenchymal stem cell-seeded collagen matrices for bone repair: Effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J. Biomed. Mater. Res. Part A 2009, 88, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Park, M.; Kim, Y.H.; Woo, S.Y.; Ryu, K.H. Rna sequencing reveals a transcriptomic portrait of human mesenchymal stem cells from bone marrow, adipose tissue, and palatine tonsils. Sci. Rep. 2017, 7, 17114. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Long, J.; Yu, J.; Du, J.; Ma, P.; Ma, Y.; Yang, D.; Fan, Z. Analysis of differentiation potentials and gene expression profiles of mesenchymal stem cells derived from periodontal ligament and Wharton’s jelly of the umbilical cord. Cells Tissues Organs 2013, 197, 209–223. [Google Scholar] [CrossRef]
- Ballerini, P.; Diomede, F.; Petragnani, N.; Cicchitti, S.; Merciaro, I.; Cavalcanti, M.; Trubiani, O. Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines. Cytokine 2017, 96, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Zini, N.; Gatta, V.; Fulle, S.; Merciaro, I.; D’Aurora, M.; La Rovere, R.M.; Traini, T.; Pizzicannella, J.; Ballerini, P.; et al. Human periodontal ligament stem cells cultured onto cortico-cancellous scaffold drive bone regenerative process. Eur. Cells Mater. 2016, 32, 181–201. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. Part B 2011, 17, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jiang, S.; Hantash, B.M. Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng. Part A 2010, 16, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Shakir, S.; MacIsaac, Z.M.; Naran, S.; Smith, D.M.; Bykowski, M.R.; Cray, J.J.; Craft, T.K.; Wang, D.; Weiss, L.; Campbell, P.G.; et al. Transforming growth factor beta 1 augments calvarial defect healing and promotes suture regeneration. Tissue Eng. Part A 2015, 21, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 inhibits bone resorption: A potential therapeutic strategy in periodontitis and other bone loss diseases. BioMed Res. Int. 2014, 2014, 284836. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ye, X.; Yu, X.; Xu, Q.; Pan, K.; Lu, S.; Yang, P. Co-culture with periodontal ligament stem cells enhanced osteoblastic differentiation of MC3T3-E1 cells and osteoclastic differentiation of RAW264.7 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 14596–14607. [Google Scholar] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Calvo Guirado, J.L.; Ramirez Fernandez, M.P.; Negri, B.; Delgado Ruiz, R.A.; Mate Sanchez de-Val, J.E.; Gomez-Moreno, G. Experimental model of bone response to collagenized xenografts of porcine origin (OsteoBiol® mp3): A radiological and histomorphometric study. Clin. Implant Dent. Relat. Res. 2013, 15, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Malmstrom, H.; Ren, Y.F. Porous collagen-hydroxyapatite scaffolds with mesenchymal stem cells for bone regeneration. J. Oral Implantol. 2015, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, B.; Cui, Z.; Cui, S.; Zhang, T.; Wang, X.; Liu, D.; Yang, R.; Jiang, N.; Zhou, Y.; et al. Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells. Sci. Rep. 2017, 7, 10519. [Google Scholar] [CrossRef] [PubMed]
- Cianci, E.; Recchiuti, A.; Trubiani, O.; Diomede, F.; Marchisio, M.; Miscia, S.; Colas, R.A.; Dalli, J.; Serhan, C.N.; Romano, M. Human periodontal stem cells release specialized proresolving mediators and carry immunomodulatory and prohealing properties regulated by lipoxins. Stem Cells Transl. Med. 2016, 5, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ekwueme, E.C.; Shah, J.V.; Mohiuddin, M.; Ghebes, C.A.; Crispim, J.F.; Saris, D.B.F.; Fernandes, H.A.M.; Freeman, J.W. Cross-talk between human tenocytes and bone marrow stromal cells potentiates extracellular matrix remodeling in vitro. J. Cell. Biochem. 2016, 117, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Cobo, T.; Viloria, C.G.; Solares, L.; Fontanil, T.; Gonzalez-Chamorro, E.; De Carlos, F.; Cobo, J.; Cal, S.; Obaya, A.J. Role of periostin in adhesion and migration of bone remodeling cells. PLoS ONE 2016, 11, e0147837. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Caputi, S.; Merciaro, I.; Frisone, S.; D’Arcangelo, C.; Piattelli, A.; Trubiani, O. Pro-inflammatory cytokine release and cell growth inhibition in primary human oral cells after exposure to endodontic sealer. Int. Endod. J. 2014, 47, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front. Physiol. 2016, 7, 559. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, M.F.; Maria, D.A.; de Isla, N.; Leal-Junior, E.C.; Joensen, J.; Bjordal, J.M.; Lopes-Martins, R.A.; Diomede, F.; Trubiani, O.; Frigo, L. Evaluation of the proliferative effects induced by low-level laser therapy in bone marrow stem cell culture. Photomed. Laser Surg. 2015, 33, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Trubiani, O.; Guarnieri, S.; Ferrero, E.; Di Primio, R. Cd38 is constitutively expressed in the nucleus of human hematopoietic cells. J. Cell. Biochem. 2008, 105, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, O.; Orsini, T.; Gambadoro, A.; Chiani, F.; Tocchini-Valentini, G.P. Three-dimensional microct imaging of murine embryonic development from immediate post-implantation to organogenesis: Application for phenotyping analysis of early embryonic lethality in mutant animals. Mamm. Genome 2017. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diomede, F.; D’Aurora, M.; Gugliandolo, A.; Merciaro, I.; Orsini, T.; Gatta, V.; Piattelli, A.; Trubiani, O.; Mazzon, E. Biofunctionalized Scaffold in Bone Tissue Repair. Int. J. Mol. Sci. 2018, 19, 1022. https://doi.org/10.3390/ijms19041022
Diomede F, D’Aurora M, Gugliandolo A, Merciaro I, Orsini T, Gatta V, Piattelli A, Trubiani O, Mazzon E. Biofunctionalized Scaffold in Bone Tissue Repair. International Journal of Molecular Sciences. 2018; 19(4):1022. https://doi.org/10.3390/ijms19041022
Chicago/Turabian StyleDiomede, Francesca, Marco D’Aurora, Agnese Gugliandolo, Ilaria Merciaro, Tiziana Orsini, Valentina Gatta, Adriano Piattelli, Oriana Trubiani, and Emanuela Mazzon. 2018. "Biofunctionalized Scaffold in Bone Tissue Repair" International Journal of Molecular Sciences 19, no. 4: 1022. https://doi.org/10.3390/ijms19041022
APA StyleDiomede, F., D’Aurora, M., Gugliandolo, A., Merciaro, I., Orsini, T., Gatta, V., Piattelli, A., Trubiani, O., & Mazzon, E. (2018). Biofunctionalized Scaffold in Bone Tissue Repair. International Journal of Molecular Sciences, 19(4), 1022. https://doi.org/10.3390/ijms19041022