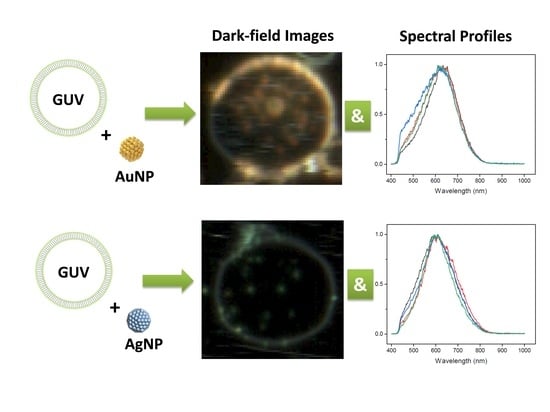
Probing Interactions between AuNPs/AgNPs and Giant Unilamellar Vesicles (GUVs) Using Hyperspectral Dark-field Microscopy
Abstract
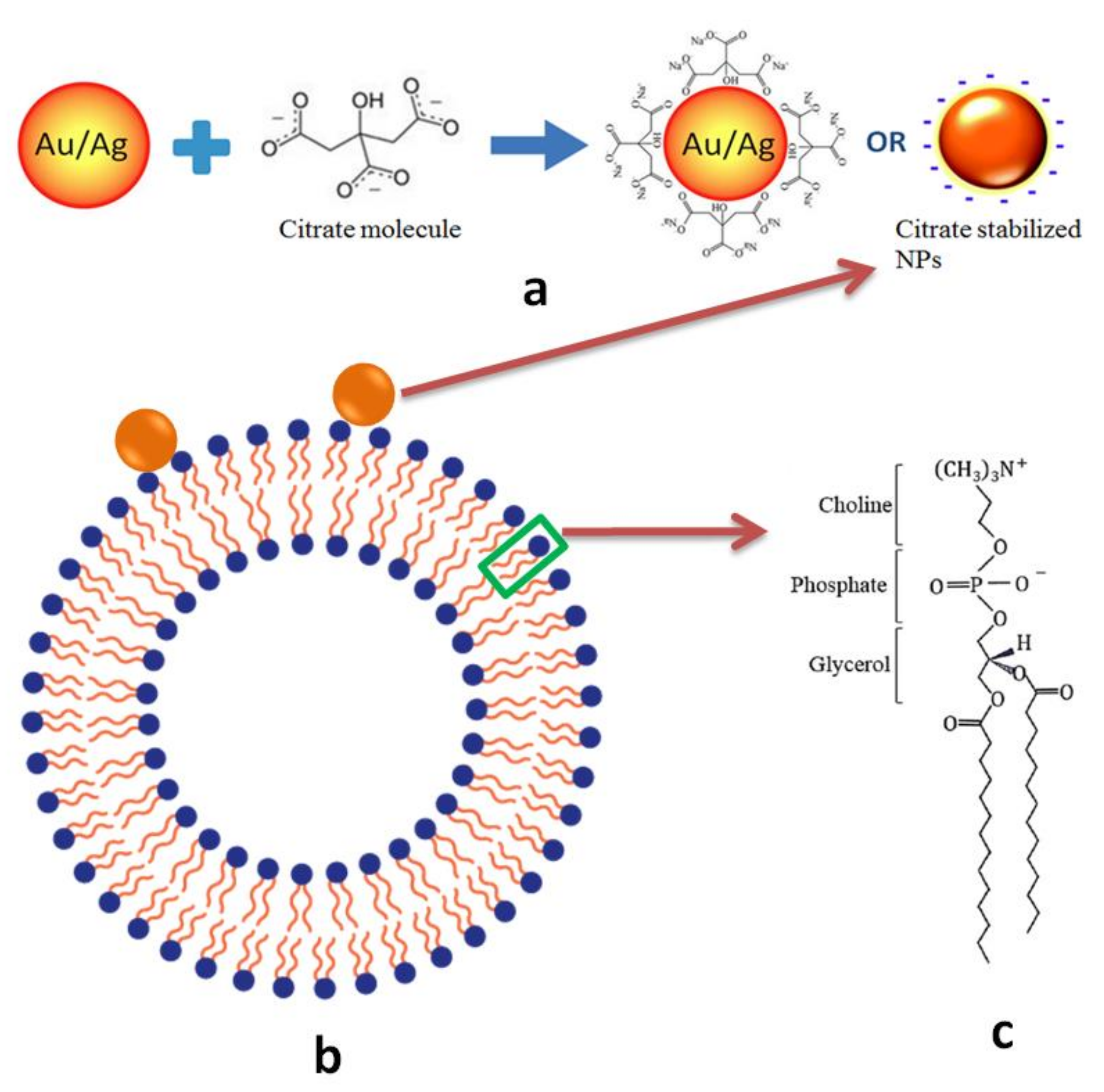
1. Introduction
2. Results
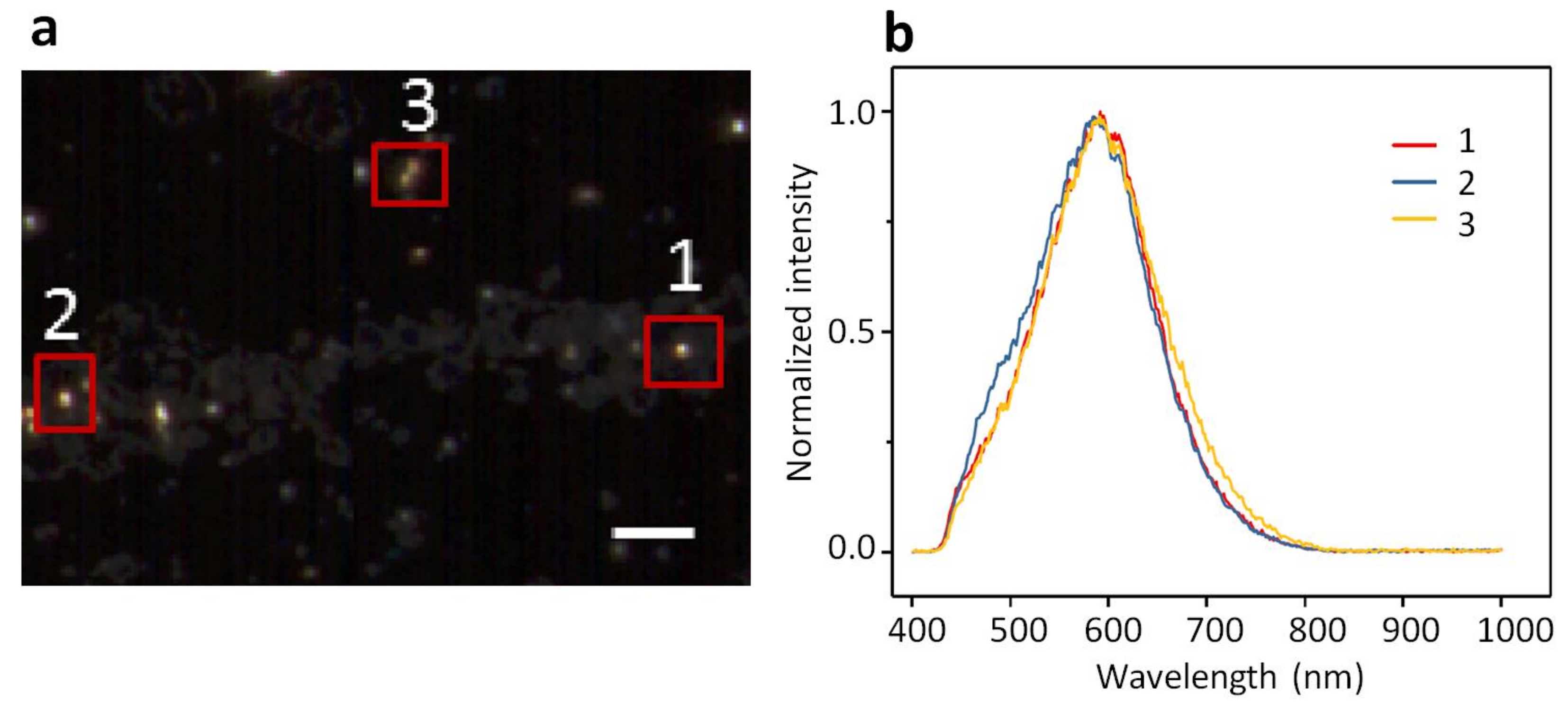
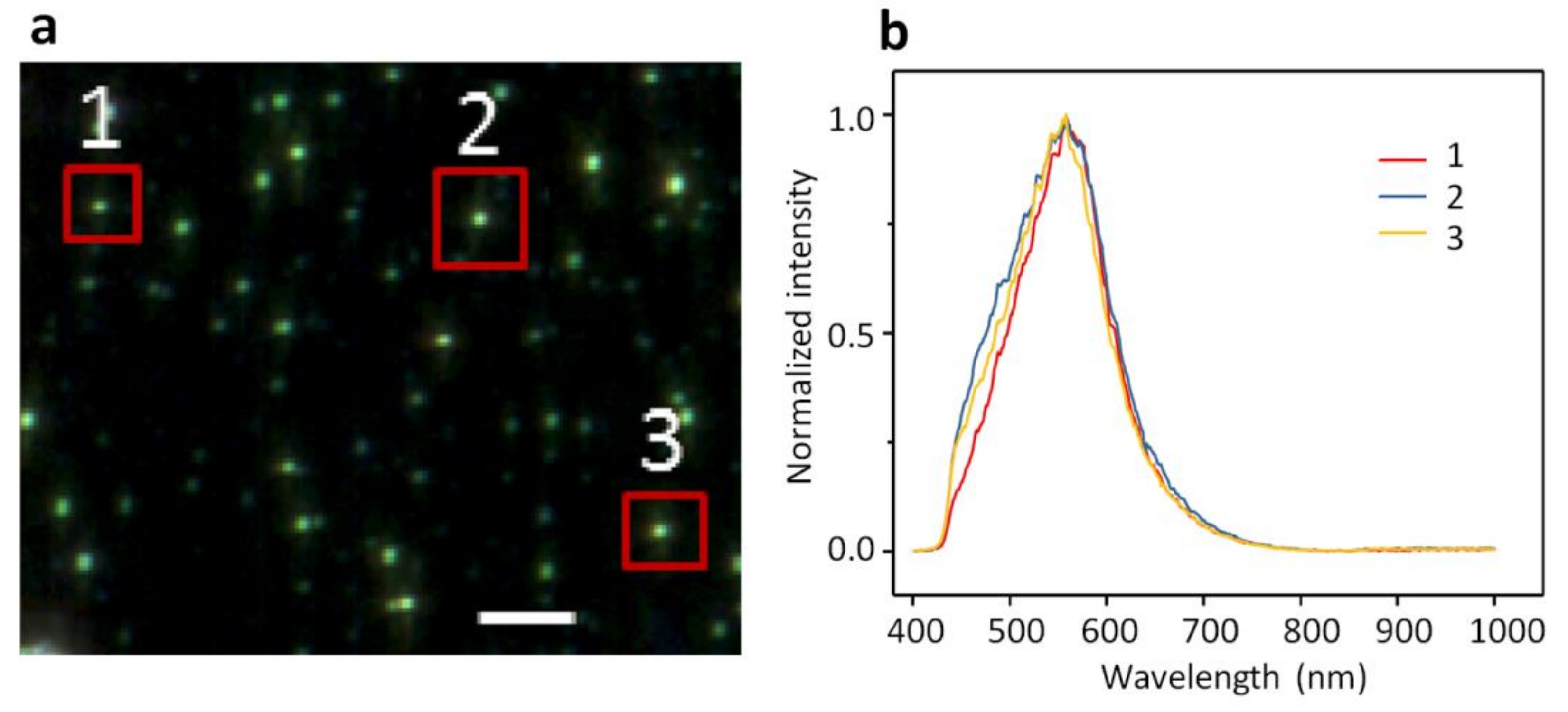
2.1. Dark-Field Microscopy (DFM) Images and Spectral Profiles of AuNPs and AgNPs
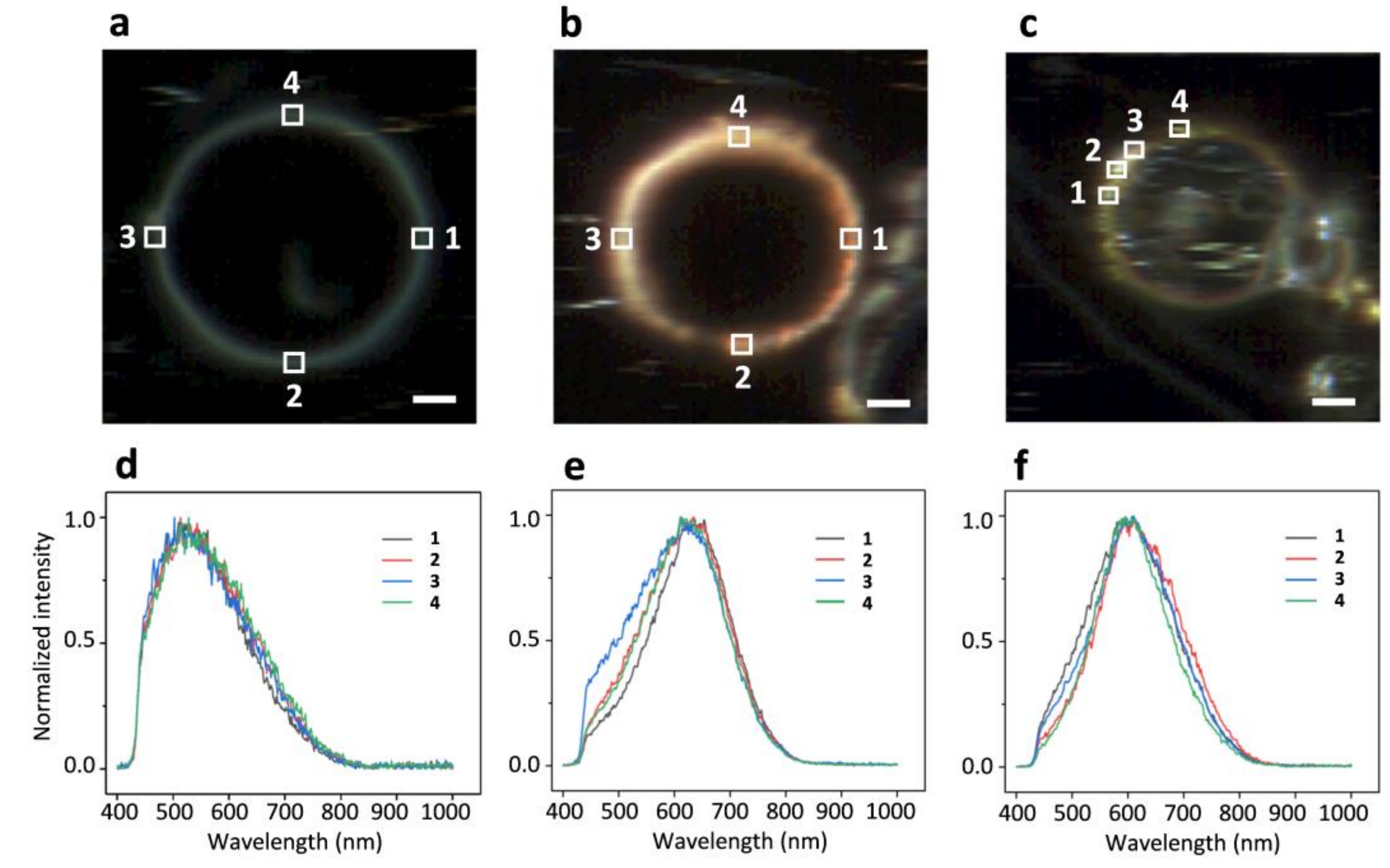
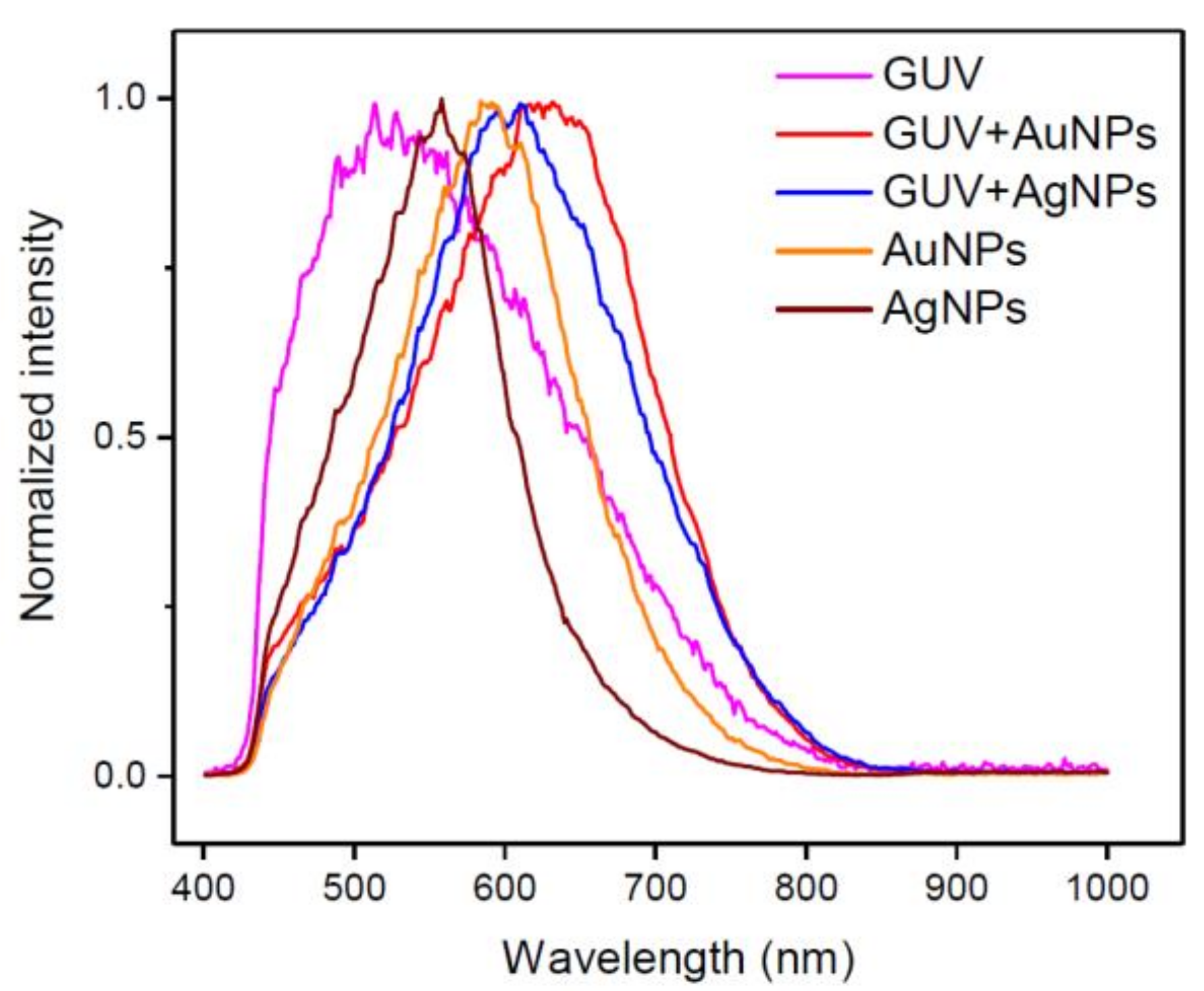
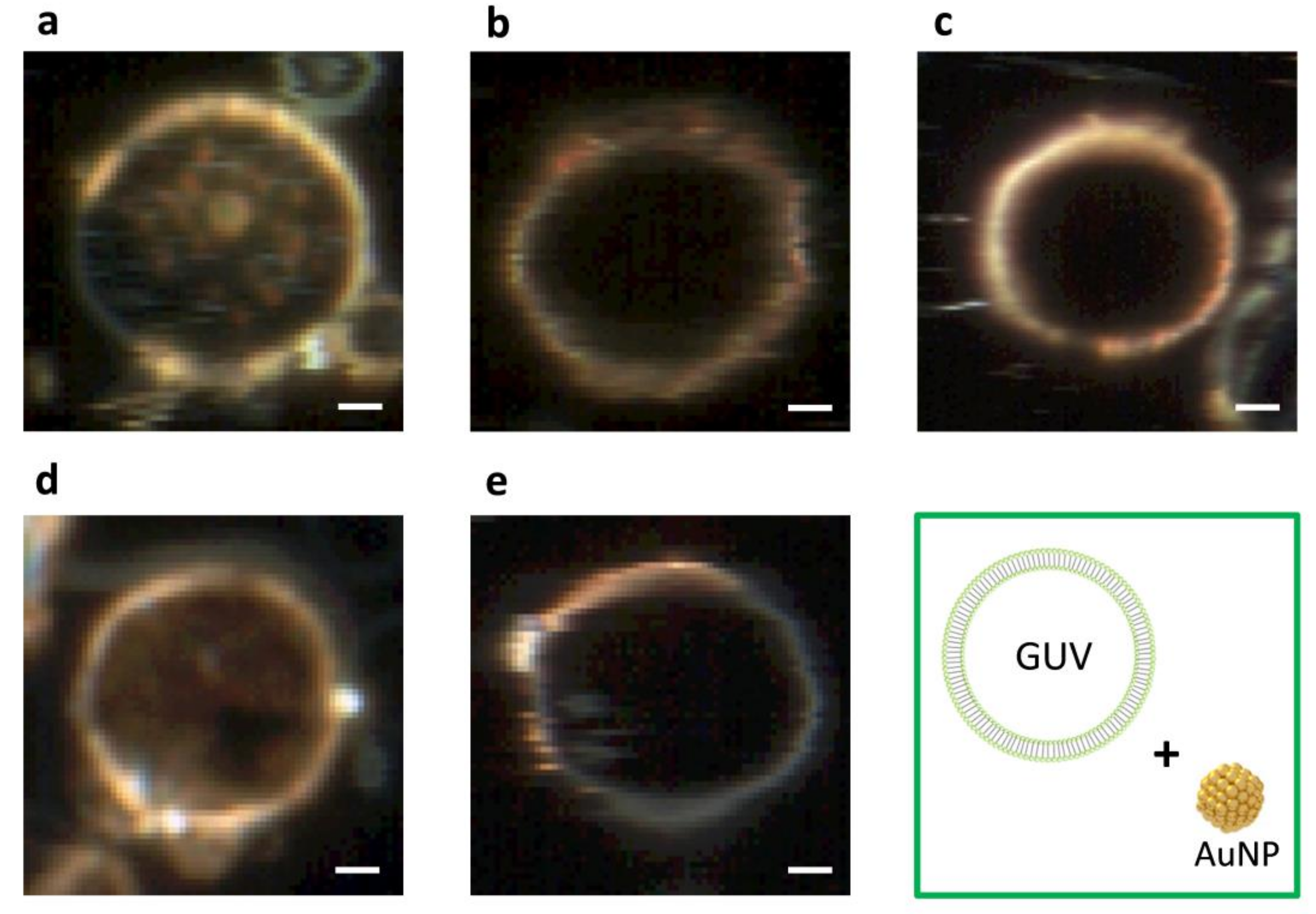
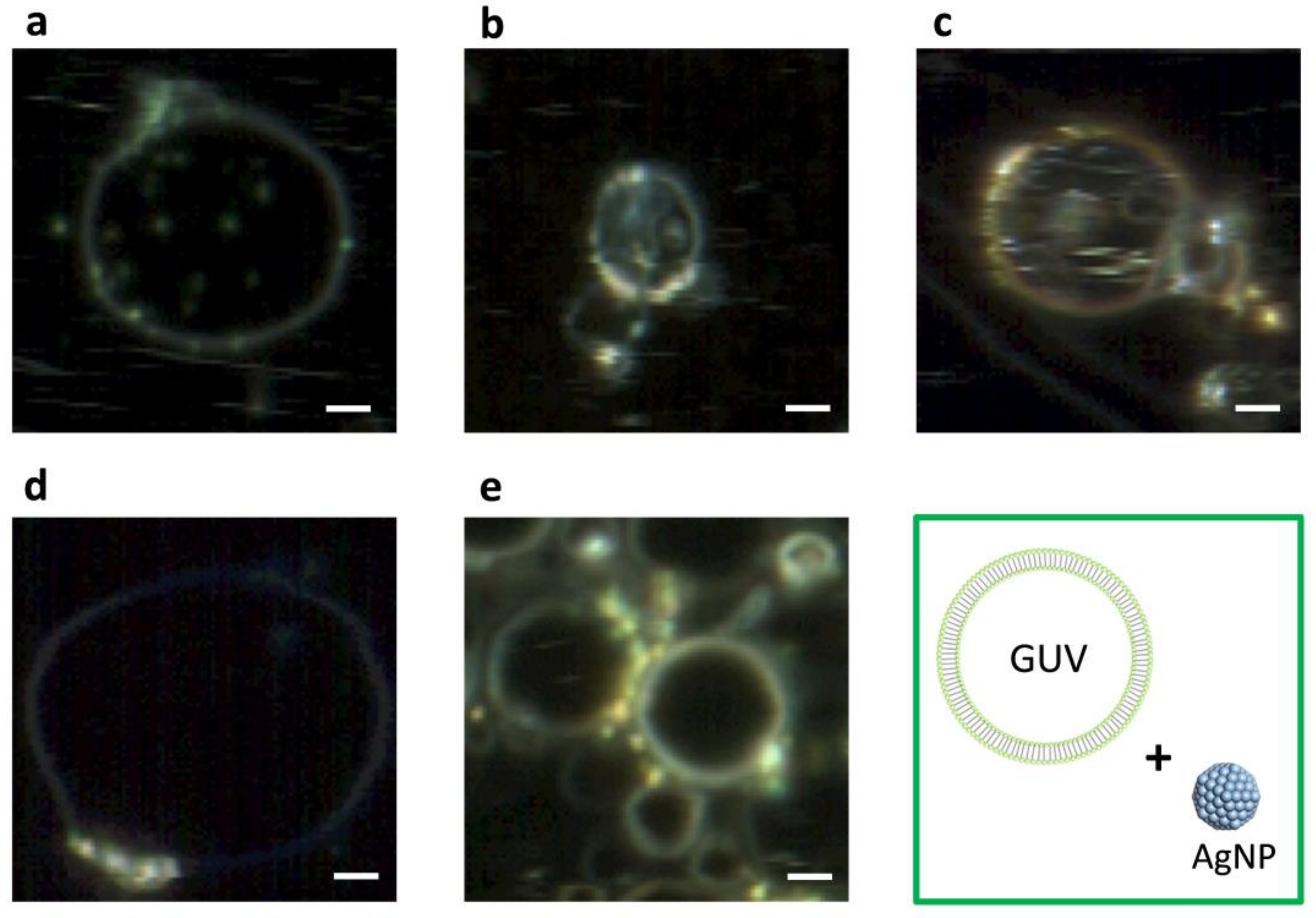
2.2. DFM Images and Spectral Profiles of GUVs Interacting with AuNPs or AgNPs
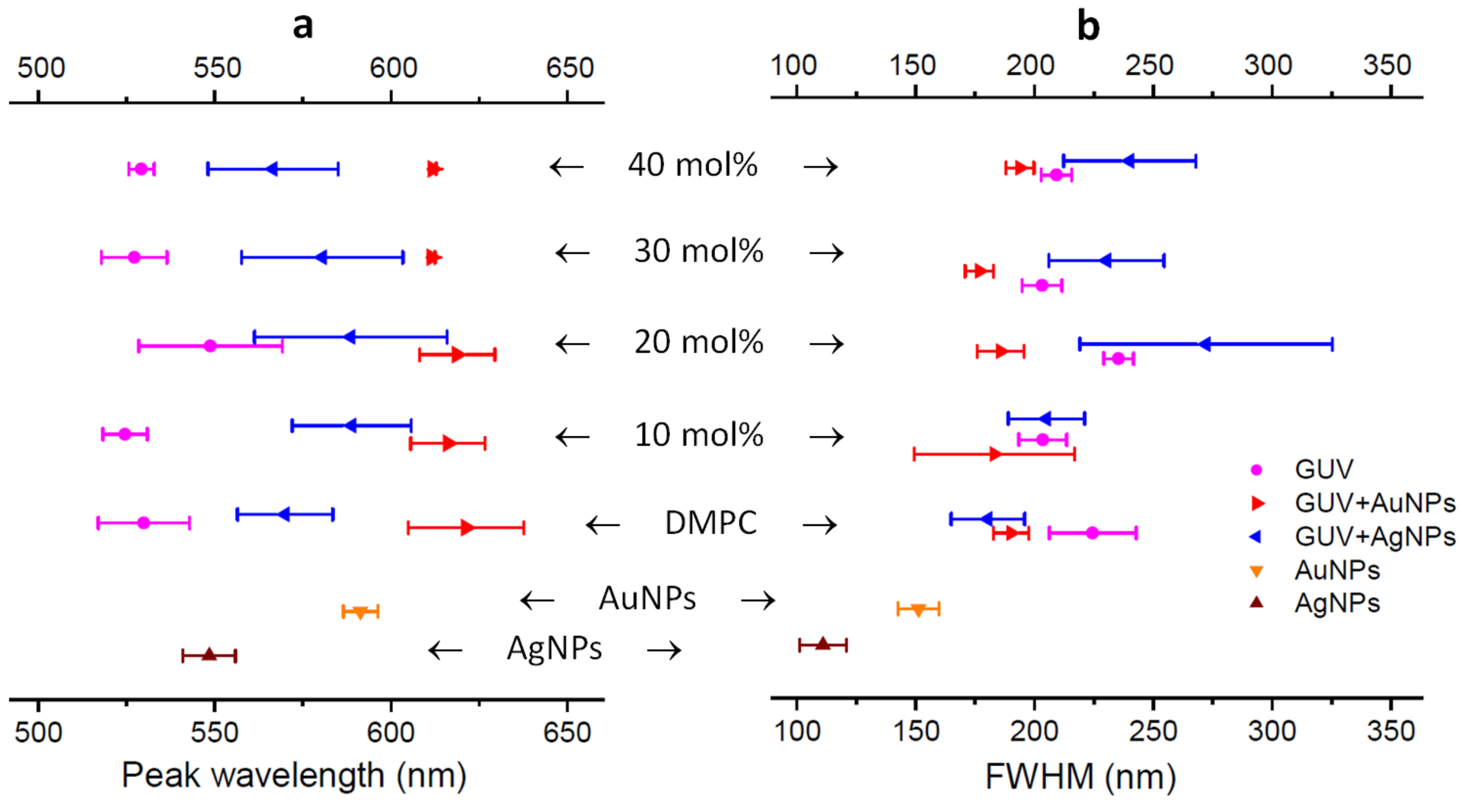
2.3. DFM Images of GUVs of Varied Composition Interacting with AuNPs or AgNPs
3. Discussion
4. Materials and Methods
4.1. Colloidal AuNPs and AgNPs
4.2. Electroformation of GUVs at Various Molar Concentration of Cholesterol vs. DMPC
4.3. Hyperspectral Dark-Field Microscopy Imaging
4.4. Analysis of Peak Wavelength and FWHM
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| AgNPs | Silver nanoparticles |
| CHOL | Cholesterol |
| DFM | Dark-field microscopy |
| DMPC | Dimyristoyl phosphatidylcholine |
| FWHM | Full width at half maximum |
| GUVs | Giant unilamellar vesicles |
| HSI | Hyperspectral imaging |
| SWIR | Short wave infrared |
| VNIR | Visible near infrared |
References
- Connor, E.; Mwamuka, J.; Gole, A.; Murphy, C.; Wyatt, M. Gold Nanoparticles Are Taken Up by Human Cells but Do Not Cause Acute Cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Papasani, M.R.; Wang, G.; Hill, R.A. Gold nanoparticles: The importance of physiological principles to devise strategies for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional Gold Nanoparticles for Diagnosis and Therapy of Disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Li, J.-C.; Chen, S.-H.; Liu, T.-Y.; Kuo, C.-H.; Huang, W.-T.; Lin, C.-S. An optical biosensing platform for proteinase activity using gold nanoparticles. Biomaterials 2010, 31, 6087–6095. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, K.-J.; Wu, T.-H.; Wang, H.; Lin, W.-Y.; Ohashi, M.; Chiou, P.-Y.; Tseng, H.-R. Photothermal Effects of Supramolecularly Assembled Gold Nanoparticles for the Targeted Treatment of Cancer Cells. Angew. Chem. Int. Ed. 2010, 49, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.; Bae, K.H.; Park, T.G. Heparin immobilized gold nanoparticles for targeted detection and apoptotic death of metastatic cancer cells. Biomaterials 2010, 31, 6530–6536. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K.; et al. Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B 2013, 106, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef] [PubMed]
- Fenz, S.F.; Sengupta, K. Giant vesicles as cell models. Integr. Biol. 2012, 4, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Resh, M.D. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1999, 1451, 1–16. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Thomas, L.; Byers, H.R.; Vink, J.; Stamenkovic, I. CD44H regulates tumor cell migration on hyaluronate-coated substrate. J. Cell Biol. 1992, 118, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Günthert, U.; Hofmann, M.; Rudy, W.; Reber, S.; Zöller, M.; Hauβmann, I.; Matzku, S.; Wenzel, A.; Ponta, H.; Herrlich, P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65, 13–24. [Google Scholar] [CrossRef]
- Murai, T.; Maruyama, Y.; Mio, K.; Nishiyama, H.; Suga, M.; Sato, C. Low Cholesterol Triggers Membrane Microdomain-dependent CD44 Shedding and Suppresses Tumor Cell Migration. J. Biol. Chem. 2011, 286, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Novo, C.; Funston, A.; Wang, H.; Staleva, H.; Zou, S.; Mulvaney, P.; Xia, Y.; Hartland, G.V. Dark-field microscopy studies of single metal nanoparticles: Understanding the factors that influence the linewidth of the localized surface plasmon resonance. J. Mater. Chem. 2008, 18, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Tahiliani, S.; Neu-Baker, N.M.; Brenner, S.A. Hyperspectral microscopy as an analytical tool for nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.M.; Li, H.; Faruque, F.; Williams, W.; Lai, K.; Hughson, M.; Bigler, S.; Beach, J.; Johnson, W. Use of hyperspectral imaging to distinguish normal, precancerous, and cancerous cells. Cancer Cytopathol. 2008, 114, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Edwards, L.W.; Fu, X.; Badman, D.L.; Huo, S.; Jin, A.J.; Lu, Q. Effects of gold nanoparticles on lipid packing and membrane pore formation. Appl. Phys. Lett. 2016, 109, 263106. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Choudhary, P.; Schurr, R.N.; Bhattacharya, P.; Brown, J.M.; Ke, P.C. Interaction of lipid vesicle with silver nanoparticle-serum albumin protein corona. Appl. Phys. Lett. 2012, 100, 013703. [Google Scholar] [CrossRef] [PubMed]
- Podila, R.; Chen, R.; Ke, P.C.; Brown, J.; Rao, A. Effects of surface functional groups on the formation of nanoparticle-protein corona. Appl. Phys. Lett. 2012, 101, 263701. [Google Scholar] [CrossRef] [PubMed]
- Nordlander, P.; Oubre, C.; Prodan, E.; Li, K.; Stockman, M.I. Plasmon Hybridization in Nanoparticle Dimers. Nano Lett. 2004, 4, 899–903. [Google Scholar] [CrossRef]
- Xiao, X.; Montaño, G.A.; Edwards, T.L.; Allen, A.; Achyuthan, K.E.; Polsky, R.; Wheeler, D.R.; Brozik, S.M. Surface Charge Dependent Nanoparticle Disruption and Deposition of Lipid Bilayer Assemblies. Langmuir 2012, 28, 17396–17403. [Google Scholar] [CrossRef] [PubMed]
- Montis, C.; Maiolo, D.; Alessandri, I.; Bergese, P.; Berti, D. Interaction of nanoparticles with lipid membranes: a multiscale perspective. Nanoscale 2014, 6, 6452–6457. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, E.; Martinez-Seara, H.; Gurtovenko, A.A.; Javanainen, M.; Häkkinen, H.; Vattulainen, I.; Akola, J. Cationic Au nanoparticle binding with plasma membrane-like lipid bilayers: Potential mechanism for spontaneous permeation to cells revealed by atomistic simulations. J. Phys. Chem. C 2014, 118, 11131–11141. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phospholipid synthesis and transport in mammalian cells. Traffic 2015, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.M.; Tamm, L.K. Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes. Biophys. J. 2004, 86, 2965–2979. [Google Scholar] [CrossRef]
- Almeida, P.F.; Vaz, W.L.; Thompson, T.E. Percolation and diffusion in three-component lipid bilayers: Effect of cholesterol on an equimolar mixture of two phosphatidylcholines. Biophys. J. 1993, 64, 399–412. [Google Scholar] [CrossRef]
- Toma, H.E.; Zamarion, V.M.; Toma, S.H.; Araki, K. The coordination chemistry at gold nanoparticles. J. Braz. Chem. Soc. 2010, 21, 1158–1176. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Mikelj, M.; Praper, T.; Demic, R.; Hodnik, V.; Turk, T.; Anderluh, G. Electroformation of giant unilamellar vesicles from erythrocyte membranes under low-salt conditions. Anal. Biochem. 2013, 435, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.I.; Soléau, S.; Méléard, P.; Faucon, F.; Bothorel, P.H.F. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. In Trends in Colloid and Interface Science VI; Helm, C., Lцsche, M., Mцhwald, H., Eds.; Steinkopff: Darmstadt, Germany, 1992; Volume 89, pp. 127–131. ISBN 978-3-7985-0913-9. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, A.; Huan, K.; Cooks, T.; Boukari, H.; Lu, Q. Probing Interactions between AuNPs/AgNPs and Giant Unilamellar Vesicles (GUVs) Using Hyperspectral Dark-field Microscopy. Int. J. Mol. Sci. 2018, 19, 1014. https://doi.org/10.3390/ijms19041014
Bhat A, Huan K, Cooks T, Boukari H, Lu Q. Probing Interactions between AuNPs/AgNPs and Giant Unilamellar Vesicles (GUVs) Using Hyperspectral Dark-field Microscopy. International Journal of Molecular Sciences. 2018; 19(4):1014. https://doi.org/10.3390/ijms19041014
Chicago/Turabian StyleBhat, Anupama, Kewei Huan, Tiana Cooks, Hacene Boukari, and Qi Lu. 2018. "Probing Interactions between AuNPs/AgNPs and Giant Unilamellar Vesicles (GUVs) Using Hyperspectral Dark-field Microscopy" International Journal of Molecular Sciences 19, no. 4: 1014. https://doi.org/10.3390/ijms19041014
APA StyleBhat, A., Huan, K., Cooks, T., Boukari, H., & Lu, Q. (2018). Probing Interactions between AuNPs/AgNPs and Giant Unilamellar Vesicles (GUVs) Using Hyperspectral Dark-field Microscopy. International Journal of Molecular Sciences, 19(4), 1014. https://doi.org/10.3390/ijms19041014