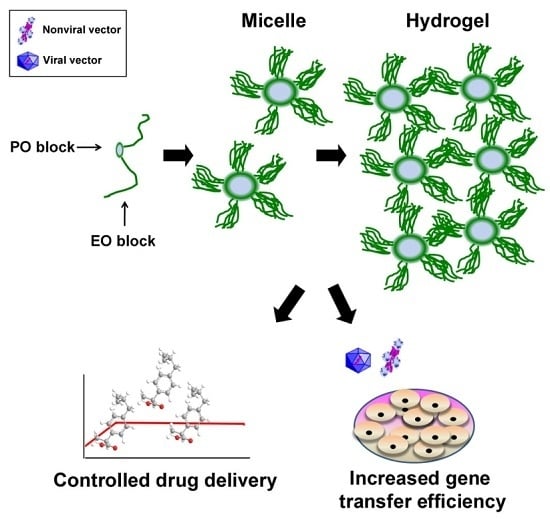
PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine—An Overview
Abstract
1. PEO-PPO-PEO Tri-Block Copolymers
1.1. General Aspects
1.2. PEO-PPO-PEO-Based Micellar Systems
1.3. PEO-PPO-PEO-Based Hydrogels
2. PEO-PPO-PEO Tri-Block Copolymers as Micellar Nanocarriers for Drug Delivery
3. PEO-PPO-PEO Tri-Block Copolymers for Passive Micellar Targeting
4. PEO-PPO-PEO Tri-Block Copolymers for Human Gene Therapy
4.1. Gene Transfer Vectors: Current Limitations
4.2. PEO-PPO-PEO Copolymers: Applications for Nonviral Gene Transfer
4.3. PEO-PPO-PEO Copolymers: Applications for Viral Gene Transfer
5. Conclusive Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvarez-Lorenzo, C.; Rey-Rico, A.; Sosnik, A.; Taboada, P.; Concheiro, A. Poloxamine-based nanomaterials for drug delivery. Front. Biosci. 2010, 2, 424–440. [Google Scholar] [CrossRef]
- Sosnik, A.; Sefton, M.V. Methylation of poloxamine for enhanced cell adhesion. Biomacromolecules 2006, 7, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Sosnik, A.; Concheiro, A. Peo-ppo block copolymers for passive micellar targeting and overcoming multidrug resistance in cancer therapy. Curr. Drug Targets 2011, 12, 1112–1130. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Rodriguez, A.; Vacanti, M.; Ibarra, C.; Arevalo, C.; Vacanti, C.A. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J. Biomater. Sci. Polym. Ed. 1998, 9, 475–487. [Google Scholar] [PubMed]
- Singh-Joy, S.D.; McLain, V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxicol. 2008, 27 (Suppl. S2), 93–128. [Google Scholar] [PubMed]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
- Chiappetta, D.A.; Sosnik, A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur. J. Pharm. Biopharm. 2007, 66, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic pluronic block copolymers. J. Control. Release 2004, 94, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D.; Zhou, Z.; Booth, C. Poly(ethylene oxide) based copolymers: Solubilisation capacity and gelation. Expert Opin. Drug Deliv. 2007, 4, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, J.; Alvarez-Lorenzo, C.; Taboada, P.; Sosnik, A.; Sandez-Macho, I.; Concheiro, A. Self-associative behavior and drug-solubilizing ability of poloxamine (tetronic) block copolymers. Langmuir 2008, 24, 10688–10697. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Intelligent drug delivery systems: Polymeric micelles and hydrogels. Mini Rev. Med. Chem. 2008, 8, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Pec, E.A.; Wout, Z.G.; Johnston, T.P. Biological activity of urease formulated in poloxamer 407 after intraperitoneal injection in the rat. J. Pharm. Sci. 1992, 81, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A. Micelle formation by oxyethylene-oxypropylene polymers. Pharm. Acta Helv. 1972, 47, 304–308. [Google Scholar] [PubMed]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide )-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Bae, Y.H.; Yin, H. Stability issues of polymeric micelles. J. Control. Release 2008, 131, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Iglesias, R.; Bromberg, L.; Temchenko, M.; Hatton, T.A.; Concheiro, A.; Alvarez-Lorenzo, C. Solubilization and stabilization of camptothecin in micellar solutions of pluronic-g-poly(acrylic acid) copolymers. J. Control. Release 2004, 97, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Degrossi, J.; Teves, S.; D’Aquino, M.; Bregni, C.; Sosnik, A. Triclosan-loaded poloxamine micelles for enhanced topical antibacterial activity against biofilm. Eur. J. Pharm. Biopharm. 2008, 69, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, I.; Blanco-Fernandez, B.; Alvarado, N.; Leiva, A.; Radic, D.; Alvarez-Lorenzo, C.; Concheiro, A. Encapsulation of antioxidant gallate derivatives in biocompatible poly(epsilon-caprolactone)-b-pluronic-b-poly(epsilon-caprolactone) micelles. Langmuir 2016, 32, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Z.; Shi, J.; Wang, F.; Luan, Y. Co-delivery of docetaxel and chloroquine via peo-ppo-pcl/tpgs micelles for overcoming multidrug resistance. Int. J. Pharm. 2015, 495, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Habas, J.P.; Pavie, E.; Perreur, C.; Lapp, A.; Peyrelasse, J. Nanostructure in block copolymer solutions: Rheology and small-angle neutron scattering. Phys. Rev. E Stat. Nonlinear Soft Matter. Phys. 2004, 70, 061802. [Google Scholar] [CrossRef] [PubMed]
- Perreur, C.; Habas, J.P.; Peyrelasse, J.; Francois, J.; Lapp, A. Rheological and small-angle neutron scattering studies of aqueous solutions of branched peo-ppo-peo copolymers. Phys. Rev. E Stat. Nonlinear Soft Matter. Phys. 2001, 63, 031505. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Gonzalez-Lopez, J.; Fernandez-Tarrio, M.; Sandez-Macho, I.; Concheiro, A. Tetronic micellization, gelation and drug solubilization: Influence of ph and ionic strength. Eur. J. Pharm. Biopharm. 2007, 66, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Silva, M.; Couceiro, J.; Concheiro, A.; Alvarez-Lorenzo, C. Osteogenic efficiency of in situ gelling poloxamine systems with and without bone morphogenetic protein-2. Eur. Cells Mater. 2011, 21, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, C.; Rodriguez-Evora, M.; Reyes, R.; Simoes, S.; Concheiro, A.; Evora, C.; Alvarez-Lorenzo, C.; Delgado, A. Bone critical defect repair with poloxamine-cyclodextrin supramolecular gels. Int. J. Pharm. 2015, 495, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Tobiyama, T.; Takada, M.; Attwood, D. Percutaneous absorption of indomethacin from pluronic f127 gels in rats. J. Pharm. Pharmacol. 1995, 47, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tarrio, M.; Yanez, F.; Immesoete, K.; Alvarez-Lorenzo, C.; Concheiro, A. Pluronic and tetronic copolymers with polyglycolyzed oils as self-emulsifying drug delivery systems. AAPS PharmSciTech 2008, 9, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Marcos, X.; Perez-Casas, S.; Llovo, J.; Concheiro, A.; Alvarez-Lorenzo, C. Poloxamer-hydroxyethyl cellulose-alpha-cyclodextrin supramolecular gels for sustained release of griseofulvin. Int. J. Pharm. 2016, 500, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Chekhonin, V.P.; Alakhov, V.; Batrakova, E.V.; Lebedev, A.S.; Melik-Nubarov, N.S.; Arzhakov, S.A.; Levashov, A.V.; Morozov, G.V.; Severin, E.S.; et al. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles. Micelles as microcontainers for drug targeting. FEBS Lett. 1989, 258, 343–345. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, S.; Liu, Y.; Luo, X.; Di, D.; Song, Y.; Liu, X.; Deng, Y. Polysialic acid and pluronic f127 mixed polymeric micelles of docetaxel as new approach for enhanced antitumor efficacy. Drug Dev. Ind. Pharm. 2017, 43, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Xia, W.T.; Xu, J.; Xu, H.L.; Lu, C.T.; Zhao, Y.Z.; Wu, X.Q. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17beta-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int. J. Nanomed. 2017, 12, 5643–5657. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Bernabeu, E.; Gonzalez, L.; Lagomarsino, E.; Zubillaga, M.; Moretton, M.A.; Chiappetta, D.A. Mixed micelles for encapsulation of doxorubicin with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines versus doxil((r)). Biomed. Pharmacother. 2017, 95, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/tpgs mixed micelles as promising carriers for cyclosporine ocular delivery. Mol. Pharm. 2018, 15, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, D.A.; Hocht, C.; Taira, C.; Sosnik, A. Efavirenz-loaded polymeric micelles for pediatric anti-hiv pharmacotherapy with significantly higher oral bioavailability [corrected]. Nanomedicine 2010, 5, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Prakash Jain, J.; Domb, A.J.; Kumar, N. Exploiting epr in polymer drug conjugate delivery for tumor targeting. Curr. Pharm. Des. 2006, 12, 4785–4796. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Togawa, H.; Kataoka, K. Physicochemical properties and nuclease resistance of antisense-oligodeoxynucleotides entrapped in the core of polyion complex micelles composed of poly(ethylene glycol)-poly(l-lysine) block copolymers. Eur. J. Pharm. Sci. 2001, 13, 35–42. [Google Scholar] [CrossRef]
- Kwon, G.S. Polymeric micelles for delivery of poorly water-soluble compounds. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 357–403. [Google Scholar] [CrossRef]
- Husseini, G.A.; Myrup, G.D.; Pitt, W.G.; Christensen, D.A.; Rapoport, N.Y. Factors affecting acoustically triggered release of drugs from polymeric micelles. J. Control. Release 2000, 69, 43–52. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, L.; Li, Y.; Wu, H. Recent progress of crosslinking strategies for polymeric micelles with enhanced drug delivery in cancer therapy. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, L.; Wang, X.; Tu, P. Comparison of hyaluronic acid-based micelles and polyethylene glycol-based micelles on reversal of multidrug resistance and enhanced anticancer efficacy in vitro and in vivo. Drug Deliv. 2018, 25, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Sass, P.M.; Grasso, L. Advances in targeted therapeutic agents. Expert Opin. Drug Discov. 2010, 5, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Cucchiarini, M. Controlled release strategies for raav-mediated gene delivery. Acta Biomater. 2016, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Huang, L. Gene therapy progress and prospects: Nonviral vectors. Gene Ther. 2002, 9, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Cucchiarini, M. Recent tissue engineering-based advances for effective raav-mediated gene transfer in the musculoskeletal system. Bioengineered 2016, 7, 175–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breyer, B.; Jiang, W.; Cheng, H.; Zhou, L.; Paul, R.; Feng, T.; He, T.C. Adenoviral vector-mediated gene transfer for human gene therapy. Curr. Gene Ther. 2001, 1, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.J.; Gay, E.E. The molecular genetics of lentiviral vectors—Current and future perspectives. Curr. Gene Ther. 2001, 1, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Noh, M.J.; Lee, K.H. Current advances in retroviral gene therapy. Curr. Gene Ther. 2011, 11, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R. Herpes simplex virus-based vectors. Int. J. Exp. Pathol. 2004, 85, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.; Berns, K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008, 21, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- De Laporte, L.; Cruz Rea, J.; Shea, L.D. Design of modular non-viral gene therapy vectors. Biomaterials 2006, 27, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, W.; Ma, N.; Steinhoff, G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013, 14, 46–60. [Google Scholar] [PubMed]
- Mumper, R.J.; Duguid, J.G.; Anwer, K.; Barron, M.K.; Nitta, H.; Rolland, A.P. Polyvinyl derivatives as novel interactive polymers for controlled gene delivery to muscle. Pharm. Res. 1996, 13, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Guerin, N.; Paradis, G.; Proulx, R.; Chistyakova, L.; Kabanov, A.; Alakhov, V. A combination of poloxamers increases gene expression of plasmid DNA in skeletal muscle. Gene Ther. 2000, 7, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Alakhov, V.Y. Pluronic block copolymers in drug delivery: From micellar nanocontainers to biological response modifiers. Crit. Rev. Ther. Drug Carr. Syst. 2002, 19, 1–72. [Google Scholar] [CrossRef]
- Sriadibhatla, S.; Yang, Z.; Gebhart, C.; Alakhov, V.Y.; Kabanov, A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol. Ther. 2006, 13, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, J.; Huang, L.; Liu, D. Effect of non-ionic surfactants on the formation of DNA/emulsion complexes and emulsion-mediated gene transfer. Pharm. Res. 1996, 13, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.; Chang, S.F.; Hsiao, F.C. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (peo-ppo-peo) polymeric micelles. Gene Ther. 2001, 8, 999–1004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pitard, B.; Pollard, H.; Agbulut, O.; Lambert, O.; Vilquin, J.T.; Cherel, Y.; Abadie, J.; Samuel, J.L.; Rigaud, J.L.; Menoret, S.; et al. A nonionic amphiphile agent promotes gene delivery in vivo to skeletal and cardiac muscles. Hum. Gene Ther. 2002, 13, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Chillon, M.; Aran, J.M.; Cruzado, J.M.; Torras, J.; Grinyo, J.M.; Fillat, C. Intramuscular sp1017-formulated DNA electrotransfer enhances transgene expression and distributes hhgf to different rat tissues. J. Gene Med. 2004, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, I.; Maksimova, I.; Lukanidin, E.; Alakhov, V.; Kabanov, A. Enhancement of the polycation-mediated DNA uptake and cell transfection with pluronic p85 block copolymer. FEBS Lett. 1996, 389, 278–280. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Lemieux, P.; Vinogradov, S.V.; Gebhart, C.L.; Guerin, N.; Paradis, G.; Bronich, T.K.; Alakhov, V.Y.; Kabanov, A.V. Evaluation of polyether-polyethyleneimine graft copolymers as gene transfer agents. Gene Ther. 2000, 7, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, C.L.; Sriadibhatla, S.; Vinogradov, S.; Lemieux, P.; Alakhov, V.; Kabanov, A.V. Design and formulation of polyplexes based on pluronic-polyethyleneimine conjugates for gene transfer. Bioconjug. Chem. 2002, 13, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.H. Effect of pluronic-block copolymers on the reduction of serum-mediated inhibition of gene transfer of polyethyleneimine-DNA complexes. Biotechnol. Appl. Biochem. 2003, 37, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.; Kim, H.D.; Kim, J.S. Pluronic-grafted poly-(l)-lysine as a new synthetic gene carrier. J. Biomed. Mater. Res. A 2003, 66, 854–859. [Google Scholar] [CrossRef] [PubMed]
- March, K.L.; Madison, J.E.; Trapnell, B.C. Pharmacokinetics of adenoviral vector-mediated gene delivery to vascular smooth muscle cells: Modulation by poloxamer 407 and implications for cardiovascular gene therapy. Hum. Gene Ther. 1995, 6, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.J.; Pastore, C.J.; Aubailly, N.; Kearney, M.; Chen, D.; Perricaudet, M.; Steg, P.G.; Isner, J.M. Improved efficiency of arterial gene transfer by use of poloxamer 407 as a vehicle for adenoviral vectors. Gene Ther. 1997, 4, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, E.; Maillard, L.; Rivard, A.; Fabre, J.E.; Couffinhal, T.; Kearney, M.; Branellec, D.; Feldman, L.J.; Walsh, K.; Isner, J.M. Effects of poloxamer 407 on transfection time and percutaneous adenovirus-mediated gene transfer in native and stented vessels. Hum. Gene Ther. 1998, 9, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Maillard, L.; Van Belle, E.; Tio, F.O.; Rivard, A.; Kearney, M.; Branellec, D.; Steg, P.G.; Isner, J.M.; Walsh, K. Effect of percutaneous adenovirus-mediated gax gene delivery to the arterial wall in double-injured atheromatous stented rabbit iliac arteries. Gene Ther. 2000, 7, 1353–1361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Liu, S.; Li, C.Y.; Yuan, F. A novel method for viral gene delivery in solid tumors. Cancer Res. 2005, 65, 7541–7545. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strappe, P.M.; Hampton, D.W.; Cachon-Gonzalez, B.; Fawcett, J.W.; Lever, A. Delivery of a lentiviral vector in a pluronic f127 gel to cells of the central nervous system. Eur. J. Pharm. Biopharm. 2005, 61, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Hofig, I.; Atkinson, M.J.; Mall, S.; Krackhardt, A.M.; Thirion, C.; Anastasov, N. Poloxamer synperonic f108 improves cellular transduction with lentiviral vectors. J. Gene Med. 2012, 14, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Driessens, G.; Nuttin, L.; Gras, A.; Maetens, J.; Mievis, S.; Schoore, M.; Velu, T.; Tenenbaum, L.; Preat, V.; Bruyns, C. Development of a successful antitumor therapeutic model combining in vivo dendritic cell vaccination with tumor irradiation and intratumoral gm-csf delivery. Cancer Immunol. Immunother. 2011, 60, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Jia, S.Q.; Zheng, S.P.; Ding, W. Celastrol enhances aav1-mediated gene expression in mice adipose tissues. Gene Ther. 2011, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. Peo-ppo-peo micelles as effective raav-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Rey-Rico, A.; Madry, H.; Landin, M.; Cucchiarini, M. Effective genetic modification and differentiation of hmscs upon controlled release of raav vectors using alginate/poloxamer composite systems. Int. J. Pharm. 2015, 496, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Rial-Hermida, I.; Taboada, P.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. Peo-ppo-peo carriers for raav-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl. Mater. Interfaces 2016, 8, 20600–20613. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. Raav-mediated overexpression of tgf-beta via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int. J. Nanomed. 2017, 12, 6985–6996. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.L.; Bou-Gharios, G.; Partridge, T.A. Non-viral gene delivery in skeletal muscle: A protein factory. Gene Ther. 2003, 10, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Kabanov, V.A. DNA complexes with polycations for the delivery of genetic material into cells. Bioconjug. Chem. 1995, 6, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01-06. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, D.K.; Gosule, L.C.; Schellman, A. DNA condensation with polyamines. II. Electron microscopic studies. J. Mol. Biol. 1978, 121, 327–337. [Google Scholar] [CrossRef]
- Zhang, J.; Sen, A.; Cho, E.; Lee, J.S.; Webb, K. Poloxamine/fibrin hybrid hydrogels for non-viral gene delivery. J. Tissue Eng. Regen. Med. 2017, 11, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Progress and prospects: Genetic treatments for disorders of bones and joints. Gene Ther. 2009, 16, 944–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rey-Rico, A.; Cucchiarini, M. Smart and controllable raav gene delivery carriers in progenitor cells for human musculoskeletal regenerative medicine with a focus on the articular cartilage. Curr. Gene Ther. 2017, 17, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Houchin, T.L.; Shea, L.D. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev. Med. Devices 2004, 1, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Babicz, H.; Madry, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Cucchiarini, M. Supramolecular polypseudorotaxane gels for controlled delivery of raav vectors in human mesenchymal stem cells for regenerative medicine. Int. J. Pharm. 2017, 531, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Alakhov, V.Y.; Kabanov, A.V. Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Expert Opin. Investig. Drugs 1998, 7, 1453–1473. [Google Scholar] [CrossRef] [PubMed]
- Hartikka, J.; Sukhu, L.; Buchner, C.; Hazard, D.; Bozoukova, V.; Margalith, M.; Nishioka, W.K.; Wheeler, C.J.; Manthorp, M.; Sawdey, M. Electroporation-facilitated delivery of plasmid DNA in skeletal muscle: Plasmid dependence of muscle damage and effect of poloxamer 188. Mol. Ther. 2001, 4, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guan, J. Thermosensitive hydrogels for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Yoon, J.J.; Lee, D.S.; Park, T.G. Photo-crosslinkable, thermo-sensitive and biodegradable pluronic hydrogels for sustained release of protein. J. Biomater. Sci. Polym. Ed. 2004, 15, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Grossman, P.M.; Mendelsohn, F.; Henry, T.D.; Hermiller, J.B.; Litt, M.; Saucedo, J.F.; Weiss, R.J.; Kandzari, D.E.; Kleiman, N.; Anderson, R.D.; et al. Results from a phase II multicenter, double-blind placebo-controlled study of del-1 (vlts-589) for intermittent claudication in subjects with peripheral arterial disease. Am. Heart J. 2007, 153, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Wloch, M.K.; Smith, L.R.; Boutsaboualoy, S.; Reyes, L.; Han, C.; Kehler, J.; Smith, H.D.; Selk, L.; Nakamura, R.; Brown, J.M.; et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J. Infect. Dis. 2008, 197, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
| Nonviral Systems | Copolymers | Genes | Targets | Administration | Observations | References |
|---|---|---|---|---|---|---|
| pDNA | SP1017: Pluronic® L61 + F127 | lacZ, luc | muscle | i.m. (rat) | 10-fold increased trangene expression | [58] |
| lacZ | increased transgene expression after electroporation | [64] | ||||
| PE6400 | lacZ | muscle | cranial muscle (mouse) | long-term expression similar to electrotransfer | [63] | |
| Pluronic® F68 and F127 | luc | n.s. | in vitro BL-6 cells | increased activity in transfecting cells in the presence of 20% serum | [61] | |
| Pluronic® P85 and L61 | luc, GFP | n.s. | in vitro NIH3T3, C2C12 and Cl66 cells | increased transgene expression | [60] | |
| PEO-PPO-PEO copolymers average MW 8400 | lacZ | Eye | ocular (rabbit, mouse) | higher transgene expression at 2 and 3 days | [62] | |
| Polycation DNA and poly(N-ethyl4-vinylpyridinium) | Pluronic® P85 | CAT | n.s. | in vitro NIH 3T3, MDCK, and Jurkat cell lines | enhanced transfection | [65] |
| P123-g-PEI(2K)polyplexe | Pluronic® P123 | luc | n.s. | in vitro Cos-7 cells, i.v. (mouse) | more uniform distribution of transgene, significant improvement of gene expression in liver | [66] |
| n.s. | in vitro prostate cancer cells (PC-3) | optimization of polyplexe size | [67] | |||
| PEI-DNA complex | Pluronic® F68, F127, P105, P94, L122, L61 | lacZ | n.s. | in vitro NIH/3T3 cells | Pluronic® with higher HLB showed marked improvement of gene expression levels in serum media compared with PEI-DNA complexes alone | [68] |
| PEI-DNA complex or pDNA | Tetronic® 904 | GFP | n.s. | in vitro N2A cells | sustained transgene expression for over 2 weeks | [88] |
| PLL-g-Pluronic® | Pluronic® F127 | lacZ | n.s. | in vitro HeLa cells | higher transfection efficiency with polymer:DNA at 1:1 | [69] |
| Viral Systems | Copolymers | Genes | Targets | Administration | Observations | References |
|---|---|---|---|---|---|---|
| Adenovirus | Pluronic® F127 | lacZ | cardiovascular | in vitrovascular smooth muscle cells | high pericellular concentrations of vector and 10- to 100-fold increase of transduction | [70] |
| Pluronic® F127 | lacZ | vascular | in vitrovascular smooth muscle cells; in vivo balloon injured carotid arteries (rat) | improved gene transfer efficiencies | [71] | |
| Pluronic® F127 | lacZ, luc | vascular | in vivo percutaneous administration in iliac arteries (rabbit) | increased efficacy of percutaneous gene transfer and reduced transfection time | [72] | |
| Pluronic® F127 | gax | vascular | in vivo external iliac artery with channel balloon catheter | gax overexpression inhibits neointimal hyperplasia and lumen loss in atheromatous stented rabbit iliac arteries | [73] | |
| Pluronic® F127 | GFP, luc | solid tumors | in vivo intratumoral infusion (mouse) | blocked convection of viral vectors in the interstitial space and the lumen of microvessels in the vicinity of the infusion site | [74] | |
| Lentivirus | Pluronic® F127 | GFP | CNS | in vivo injection to the thalamus (rat) | increased transduction of astrocytes at injection site | [75] |
| Pluronic® F108 | GFP, luc | n.s. | in vitro HEK293T, KARPAS-299, SUDHL-1, SR-786, SUP-M2, and PANC-1 cell lines | specific contribution to efficiency of each adjuvant; polybrene: charge protector and poloxamer synperonic F108: membrane modulator | [76] |
| Viral Systems | Copolymers | Genes | Targets | Administration | Observations | References |
|---|---|---|---|---|---|---|
| rAAV | Pluronic® F127 | GM-CSF | solid tumors | in vivo intratumoral infusion (mouse) | higher efficiency by combining DC, local tumor irradiation and controlled supply of recombinant mGM-CSF with Pluronic® | [77] |
| Pluronic® F68 | lacZ | adipose tissue | in vivo inguinal (mouse) | increased transgene expression after 4 weeks | [78] | |
| Pluronic® F127 | lacZ | cartilage | in vitro hMSCs | controlled release of rAAV for high efficiencies over time and gene expression levels similar to those achieved by direct vector application | [80] | |
| Pluronic® F68, Tetronic® 908 | RFP, lacZ, and SOX9 | cartilage | in vitro hMSCs | encapsulation of rAAV in polymeric micelles for effective, durable, and safe modification of hMSCs; restoration of hMSC transduction in conditions of gene transfer inhibition; effective chondrogenesis | [79] | |
| Pluronic® F68, Tetronic® 908 | lacZ | cartilage | in vitro hOACs in situ human osteochondral model | micellar encapsulation for increased stability and bioactivity of rAAV; high levels of safe transgene expression in vitro and in experimental osteochondral defects in situ | [81] | |
| Pluronic® F68, Tetronic® 908 | TGF-β | cartilage | in vitro hOACs in situ human osteochondral model | increased levels of transgene expression compared with free vector treatment; high proteoglycan deposition and increased cell numbers in hOACs in vitro; high deposition of type-II collagen and reduced hypertrophy in osteochondral defects models in situ | [82] | |
| Pluronic® F68, Tetronic® 908 | lacZ | cartilage | in vitro hMSCs | high concentrations of rAAV; sustained levels of transgene expression over time | [92] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey-Rico, A.; Cucchiarini, M. PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine—An Overview. Int. J. Mol. Sci. 2018, 19, 775. https://doi.org/10.3390/ijms19030775
Rey-Rico A, Cucchiarini M. PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine—An Overview. International Journal of Molecular Sciences. 2018; 19(3):775. https://doi.org/10.3390/ijms19030775
Chicago/Turabian StyleRey-Rico, Ana, and Magali Cucchiarini. 2018. "PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine—An Overview" International Journal of Molecular Sciences 19, no. 3: 775. https://doi.org/10.3390/ijms19030775
APA StyleRey-Rico, A., & Cucchiarini, M. (2018). PEO-PPO-PEO Tri-Block Copolymers for Gene Delivery Applications in Human Regenerative Medicine—An Overview. International Journal of Molecular Sciences, 19(3), 775. https://doi.org/10.3390/ijms19030775