Alisol B 23-Acetate Inhibits IgE/Ag-Mediated Mast Cell Activation and Allergic Reaction
Abstract
1. Introduction
2. Results
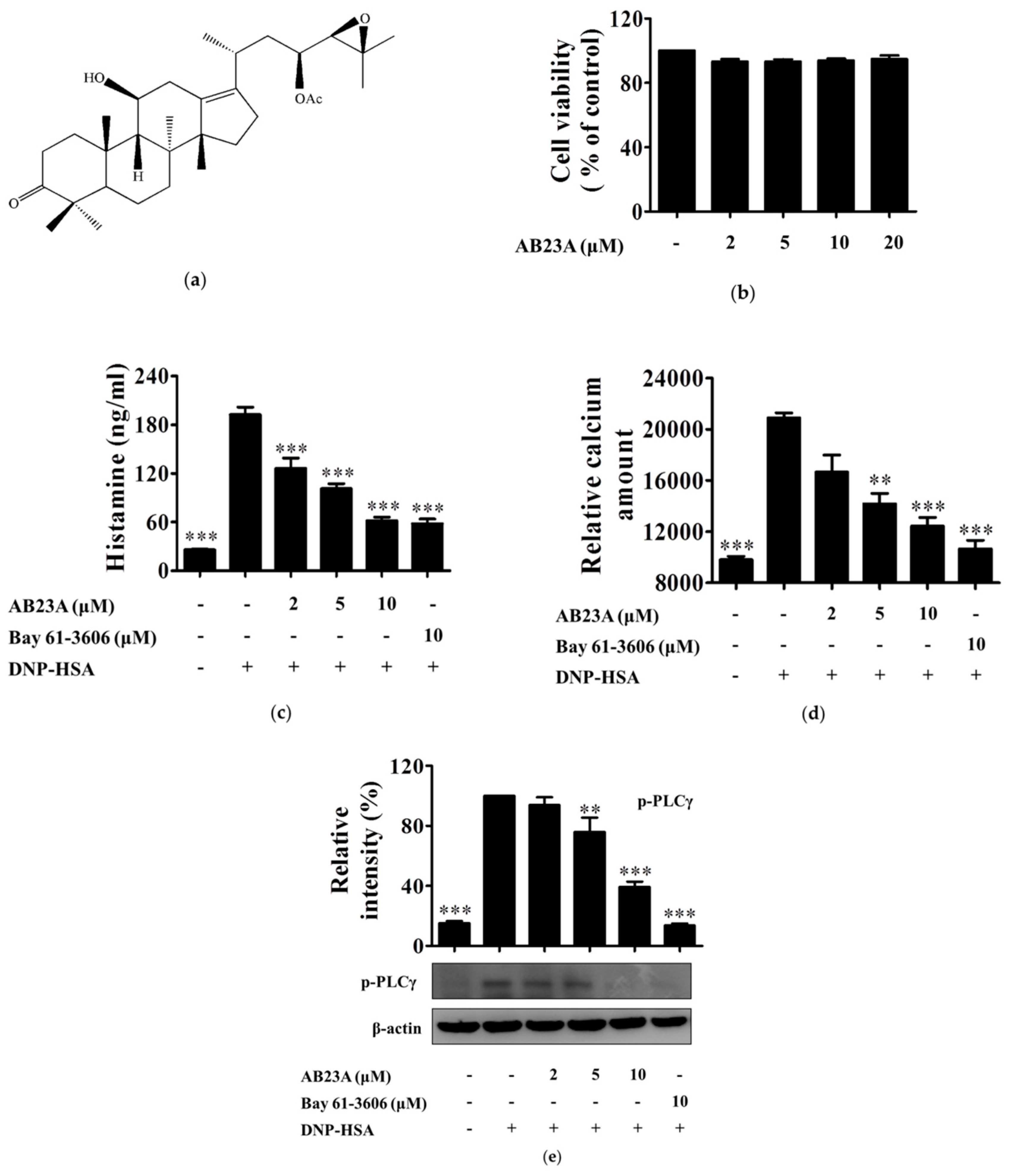
2.1. AB23A Suppresses Histamine Release and Ca2+ Mobilization in IgE/Ag-Stimulated BMMCs by Inhibiting PLCγ Phosphorylation
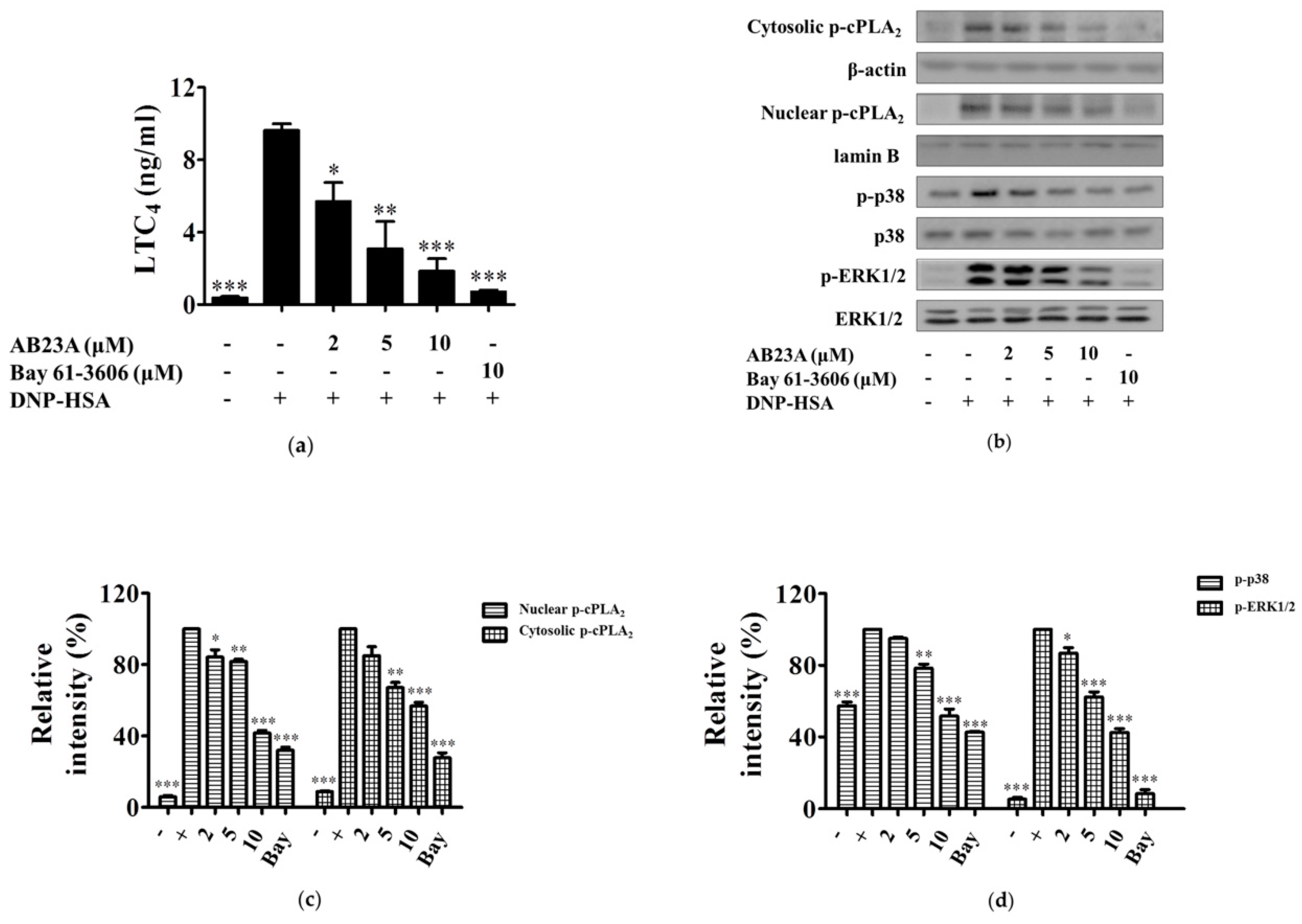
2.2. AB23A Inhibits LTC4 Generation via Blocking the Phosphorylation of p38 and ERK and Translocation of cPLA2 into the Nuclear Envelope
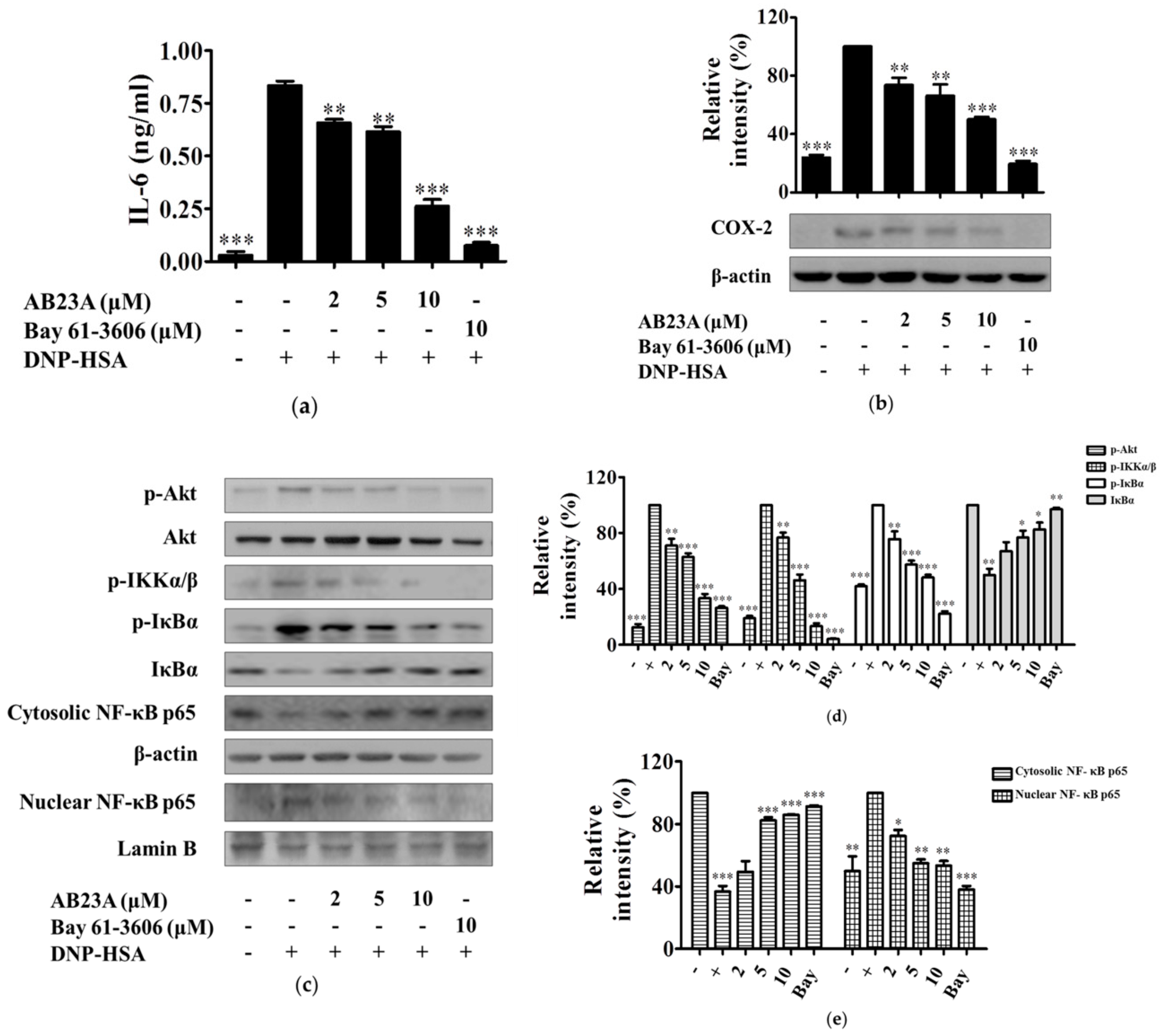
2.3. AB23A Decreases IL-6 and COX-2 Expression by Suppressing the Akt/IKK/NF-κB Pathway
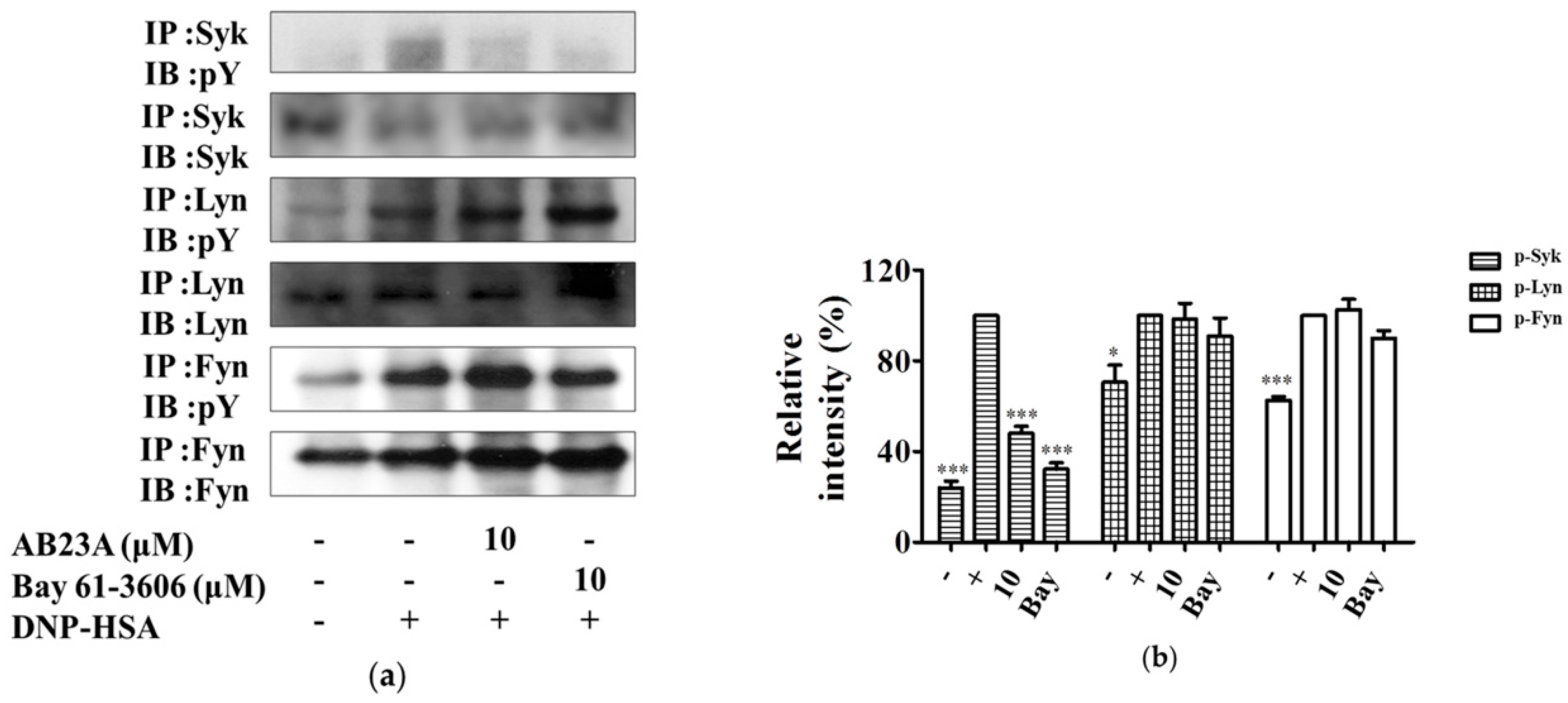
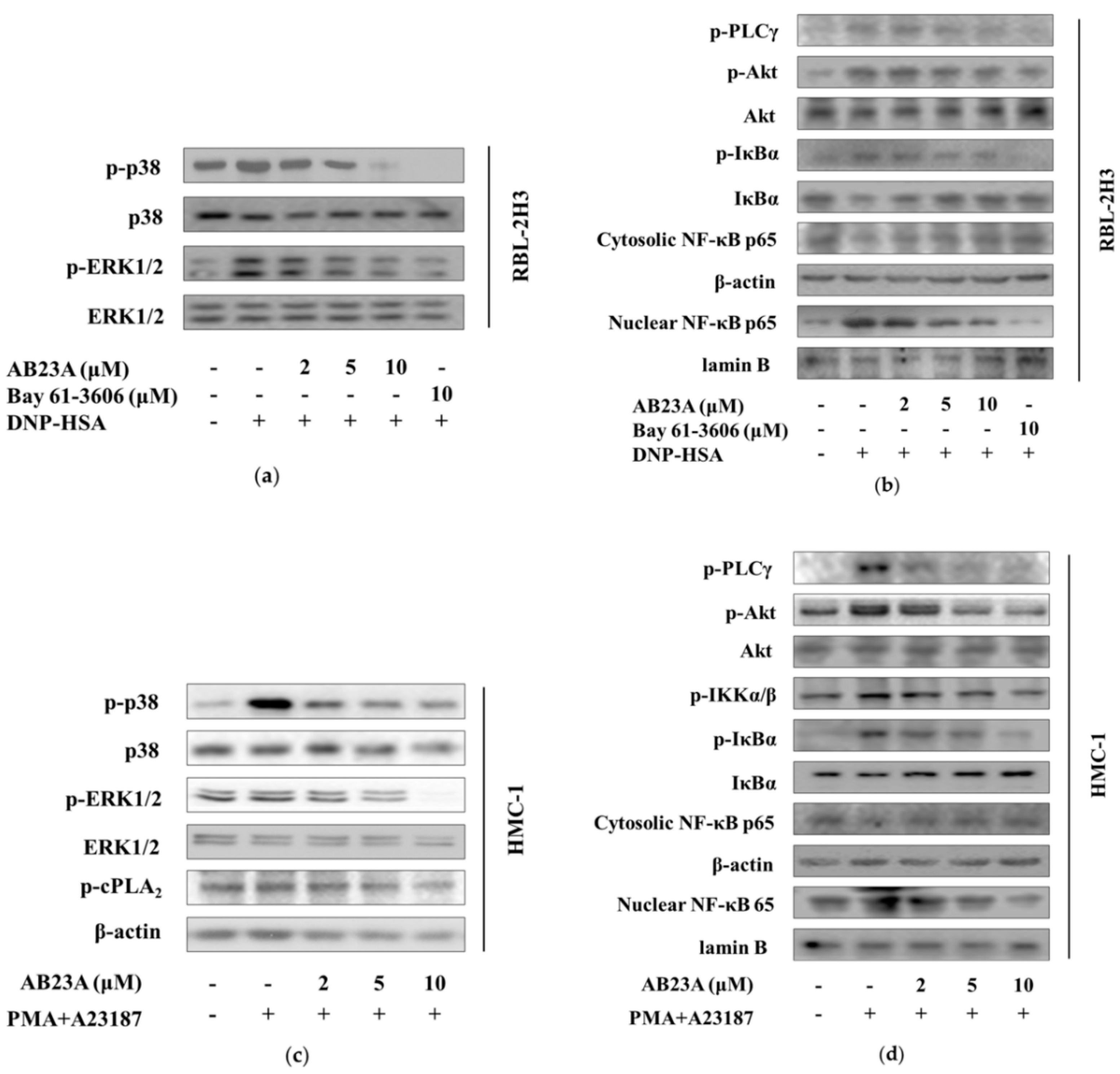
2.4. AB23A Inhibits Syk Phosphorylation Independent of Lyn and Fyn
2.5. AB23A Inhibits the Activation Signal of RBL-2H3 and HMC-1
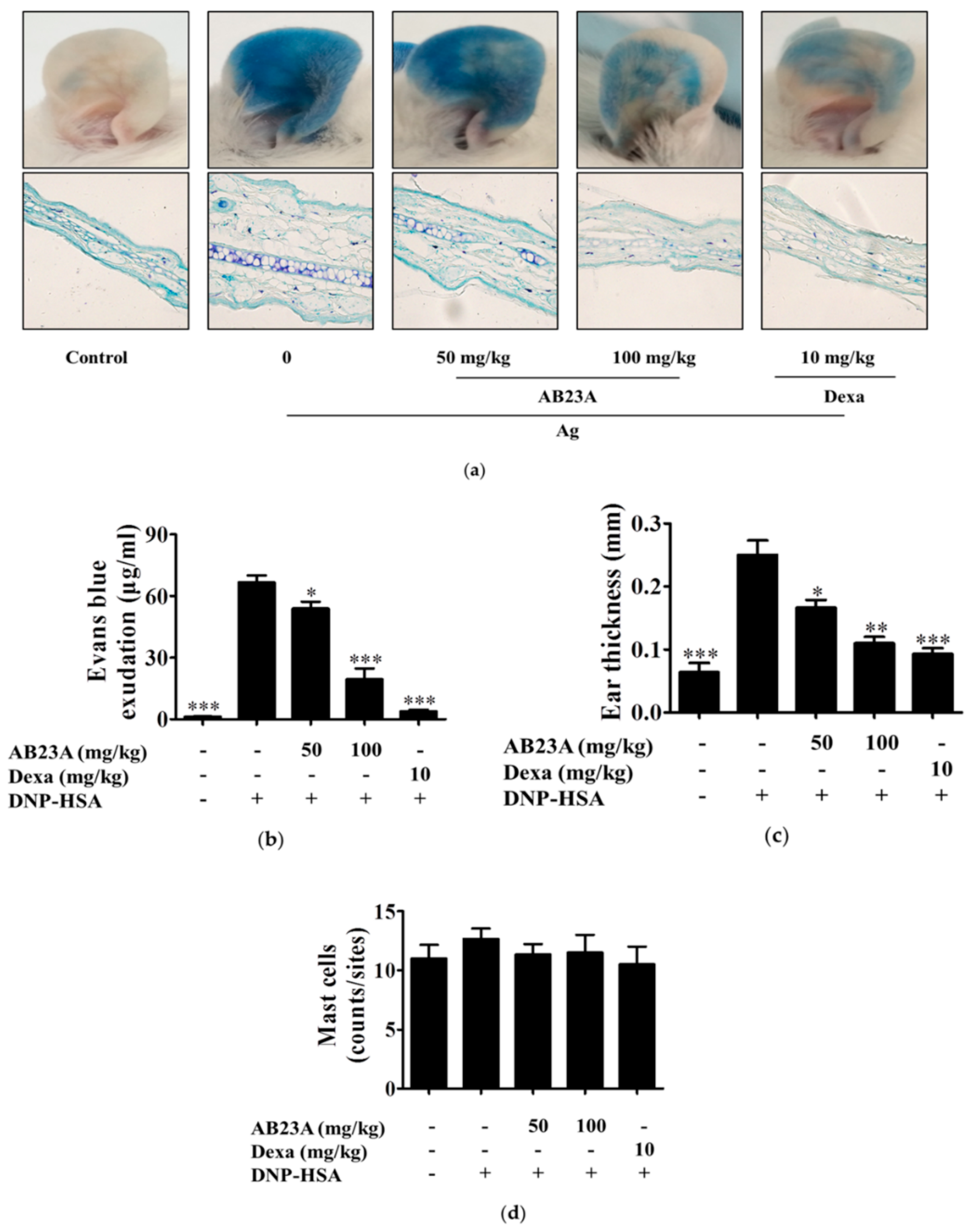
2.6. AB23A Attenuates IgE-Mediated PCA
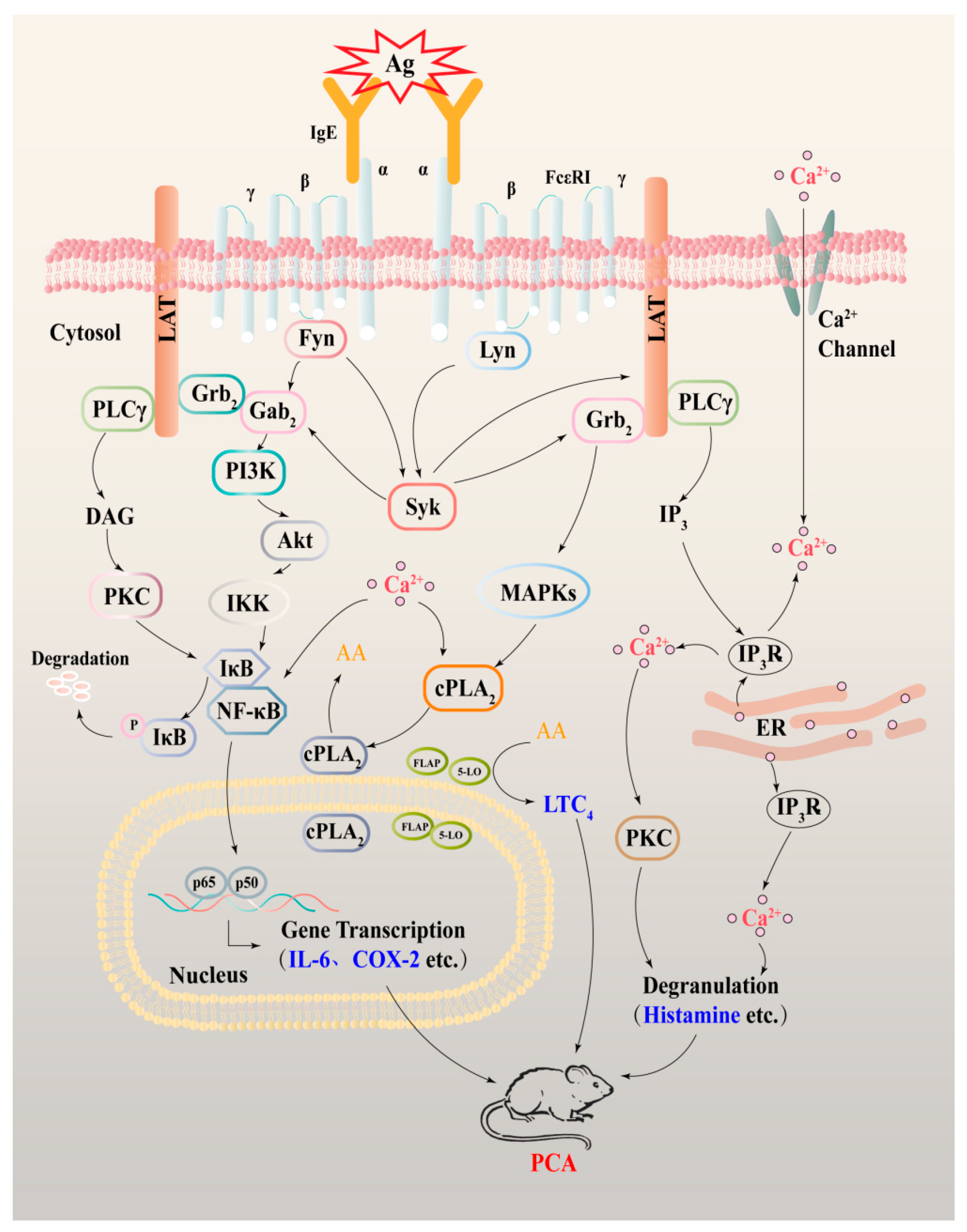
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Activation
4.3. Cell Viability
4.4. Measurement of Intracellular Ca2+ Level
4.5. Extraction of Nuclear Proteins
4.6. Western Blot Analysis
4.7. Immunoprecipitation (IP)
4.8. IgE-Mediated PCA in Mice
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AB23A | Alisol B 23-acetate |
| IgE | Immunoglobulin E |
| FcεRI | Immunoglobulin E receptor |
| LTC4 | Leukotriene C4 |
| IL-6 | Interlukin-6 |
| COX-2 | Cyclooxygenase-2 |
| BMMCs | Bone marrow-derived mast cells |
| RBL-2H3 | Rat basophilic leukemia |
| HMC-1 | Human mast cell line |
| Syk | Spleen tyrosine kinase |
| PLCγ | Phospholipase Cγ |
| MAPKs | Mitogen-activated protein kinases |
| cPLA2 | Cytosolic phospholipase A2 |
| PMA | Phorbol 12-myristate 13-acetate |
| A23187 | Calcium ionophore |
| PCA | Passive cutaneous anaphylaxis |
References
- Theoharides, T.C.; Kalogeromitros, D. The critical role of mast cells in allergy and inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–99. [Google Scholar] [CrossRef]
- Turner, H.; Kinet, J.P. Signalling through the high-affinity IgE receptor FC epsilonRI. Nature 1999, 402, B24–B30. [Google Scholar] [CrossRef]
- Reber, L.L.; Frossard, N. Targeting mast cells in inflammatory diseases. Pharmacol. Ther. 2014, 142, 416–435. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Peavy, R.D.; Gilfillan, A.M. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639–646. [Google Scholar] [CrossRef]
- Kopec, A.; Panaszek, B.; Fal, A.M. Intracellular signaling pathways in IgE-dependent mast cell activation. Archivum Immunologiae et Therapiae Experimentalis 2006, 54, 393–401. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, Y.L.; Tang, Z.H.; Xu, X.H.; Chen, X.; Li, T.; Ding, C.Y.; Huang, M.Q.; Chen, X.P.; Wang, Y.T.; et al. Effects of alisol B 23-acetate on ovarian cancer cells: G1 phase cell cycle arrest, apoptosis, migration and invasion inhibition. Phytomed. Int. J. Phytother. Phytopharmacol. 2016, 23, 800–809. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, X.; Wang, C.; Liu, Q.; Sun, H.; Sun, P.; Huo, X.; Liu, Z.; Liu, K. Protective effects of alisol B 23-acetate from edible botanical rhizoma alismatis against carbon tetrachloride-induced hepatotoxicity in mice. Food Funct. 2015, 6, 1241–1250. [Google Scholar] [CrossRef]
- Jin, H.G.; Jin, Q.; Ryun Kim, A.; Choi, H.; Lee, J.H.; Kim, Y.S.; Lee, D.G.; Woo, E.R. A new triterpenoid from alisma orientale and their antibacterial effect. Arch. Pharm. Res. 2012, 35, 1919–1926. [Google Scholar] [CrossRef]
- Hallgren, J.; Gurish, M.F. Granule maturation in mast cells: Histamine in control. Eur. J. Immunol. 2014, 44, 33–36. [Google Scholar] [CrossRef]
- Suzuki, R. The emerging picture of mast cell activation: The complex regulatory network of high-affinity receptor for immunoglobulin e signaling. Biol. Pharm. Bull. 2017, 40, 1828–1832. [Google Scholar] [CrossRef]
- Vig, M.; Kinet, J.P. Calcium signaling in immune cells. Nat. Immunol. 2009, 10, 21–27. [Google Scholar] [CrossRef]
- Rovati, G.E.; Capra, V. Cysteinyl-leukotriene receptors and cellular signals. Sci. World J. 2007, 7, 1375–1392. [Google Scholar] [CrossRef]
- Bair, A.M.; Turman, M.V.; Vaine, C.A.; Panettieri, R.A., Jr.; Soberman, R.J. The nuclear membrane leukotriene synthetic complex is a signal integrator and transducer. Mol. Biol. Cell 2012, 23, 4456–4464. [Google Scholar] [CrossRef]
- Leslie, C.C. Properties and regulation of cytosolic phospholipase A2. J. Biol. Chem. 1997, 272, 16709–16712. [Google Scholar] [CrossRef]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8, S3. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. Cox-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 2014, 35, 284–292. [Google Scholar] [CrossRef]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging roles for noncanonical NF-kappaB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm. Bowel Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef]
- Mohamed, M.R.; McFadden, G. Nfkb inhibitors: Strategies from poxviruses. Cell Cycle (Georgetown, Tex.) 2009, 8, 3125–3132. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Rivera, J. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 2009, 228, 149–169. [Google Scholar] [CrossRef]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef]
- Guilarte, M.; Sala-Cunill, A.; Luengo, O.; Labrador-Horrillo, M.; Cardona, V. The mast cell, contact, and coagulation system connection in anaphylaxis. Front. Immunol. 2017, 8, 846. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, O.S.; Jin, H.G.; Woo, E.R.; Kim, Y.S.; Kim, H.P. The rhizomes of Alisma orientale and alisol derivatives inhibit allergic response and experimental atopic dermatitis. Biol. Pharm. Bull. 2012, 35, 1581–1587. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Murayama, T.; Shimizu, T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol. Pharm. Bull. 2004, 27, 1168–1173. [Google Scholar] [CrossRef]
- Steinhilber, D.; Fischer, A.S.; Metzner, J.; Steinbrink, S.D.; Roos, J.; Ruthardt, M.; Maier, T.J. 5-lipoxygenase: Underappreciated role of a pro-inflammatory enzyme in tumorigenesis. Front. Pharmacol. 2010, 1, 143. [Google Scholar] [CrossRef]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex pollen: Phenolic pattern, colorimetric analysis and protective effects in immortalized prostate cells (PC3) and rat prostate challenged with LPS. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. PTR 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Ghosh, S.; Karin, M. Missing pieces in the nf-kappab puzzle. Cell 2002, 109, S81–S96. [Google Scholar] [CrossRef]
- Lin, T.H.; Pajarinen, J.; Lu, L.; Nabeshima, A.; Cordova, L.A.; Yao, Z.; Goodman, S.B. Nf-kappaB as a therapeutic target in inflammatory-associated bone diseases. Adv. Protein Chem. Struct. Biol. 2017, 107, 117–154. [Google Scholar]
- Sibilano, R.; Frossi, B.; Pucillo, C.E. Mast cell activation: A complex interplay of positive and negative signaling pathways. Eur. J. Immunol. 2014, 44, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
- Siraganian, R.P.; de Castro, R.O.; Barbu, E.A.; Zhang, J. Mast cell signaling: The role of protein tyrosine kinase syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010, 584, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Nagai, H. Analysis of the mechanism for the development of allergic skin inflammation and the application for its treatment: Mouse models for the development of remedies for human allergic dermatitis. J. Pharmacol. Sci. 2009, 110, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Kim, S.; Qin, N.; Chen, X.; Ji, N.; Tang, S.A.; Kong, D.; Lee, E.; Duan, H. 1,6-O,O-diacetylbritannilactone suppresses activation of mast cell and airway hyper-responsiveness. Immunopharmacol. Immunotoxicol. 2017, 39, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Wang, Y.; Tan, X.; Zhao, W.; Xing, X.; Qiu, Y.; Wang, R.; Jin, M.; Fan, G.; et al. Phosphatidylinositol 3-kinase beta and delta isoforms play key roles in metastasis of prostate cancer du145 cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018. [Google Scholar] [CrossRef]
- Ji, N.; Pan, S.; Shao, C.; Chen, Y.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Spinacetin suppresses the mast cell activation and passive cutaneous anaphylaxis in mouse model. Front. Pharmacol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, C.; Fu, B.; Ji, N.; Pan, S.; Zhao, X.; Zhang, Z.; Qiu, Y.; Wang, R.; Jin, M.; Wen, K.; et al. Alisol B 23-Acetate Inhibits IgE/Ag-Mediated Mast Cell Activation and Allergic Reaction. Int. J. Mol. Sci. 2018, 19, 4092. https://doi.org/10.3390/ijms19124092
Shao C, Fu B, Ji N, Pan S, Zhao X, Zhang Z, Qiu Y, Wang R, Jin M, Wen K, et al. Alisol B 23-Acetate Inhibits IgE/Ag-Mediated Mast Cell Activation and Allergic Reaction. International Journal of Molecular Sciences. 2018; 19(12):4092. https://doi.org/10.3390/ijms19124092
Chicago/Turabian StyleShao, Chen, Bingjie Fu, Ning Ji, Shunli Pan, Xiaoxia Zhao, Zhe Zhang, Yuling Qiu, Ran Wang, Meihua Jin, Ke Wen, and et al. 2018. "Alisol B 23-Acetate Inhibits IgE/Ag-Mediated Mast Cell Activation and Allergic Reaction" International Journal of Molecular Sciences 19, no. 12: 4092. https://doi.org/10.3390/ijms19124092
APA StyleShao, C., Fu, B., Ji, N., Pan, S., Zhao, X., Zhang, Z., Qiu, Y., Wang, R., Jin, M., Wen, K., & Kong, D. (2018). Alisol B 23-Acetate Inhibits IgE/Ag-Mediated Mast Cell Activation and Allergic Reaction. International Journal of Molecular Sciences, 19(12), 4092. https://doi.org/10.3390/ijms19124092



