Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy
Abstract
1. Introduction
2. The Vitreal Microenvironment in Proliferative Diabetic Retinopathy
2.1. Angiogenesis-Promoting Factors
2.2. Inflammation-Promoting Factors
2.3. Factors Involved in Lymphatic Vascular Formation
3. Physiological Lymphatic Vasculature in Ocular Structures
4. Lymphatic Vascular Formation in Ocular Diseases
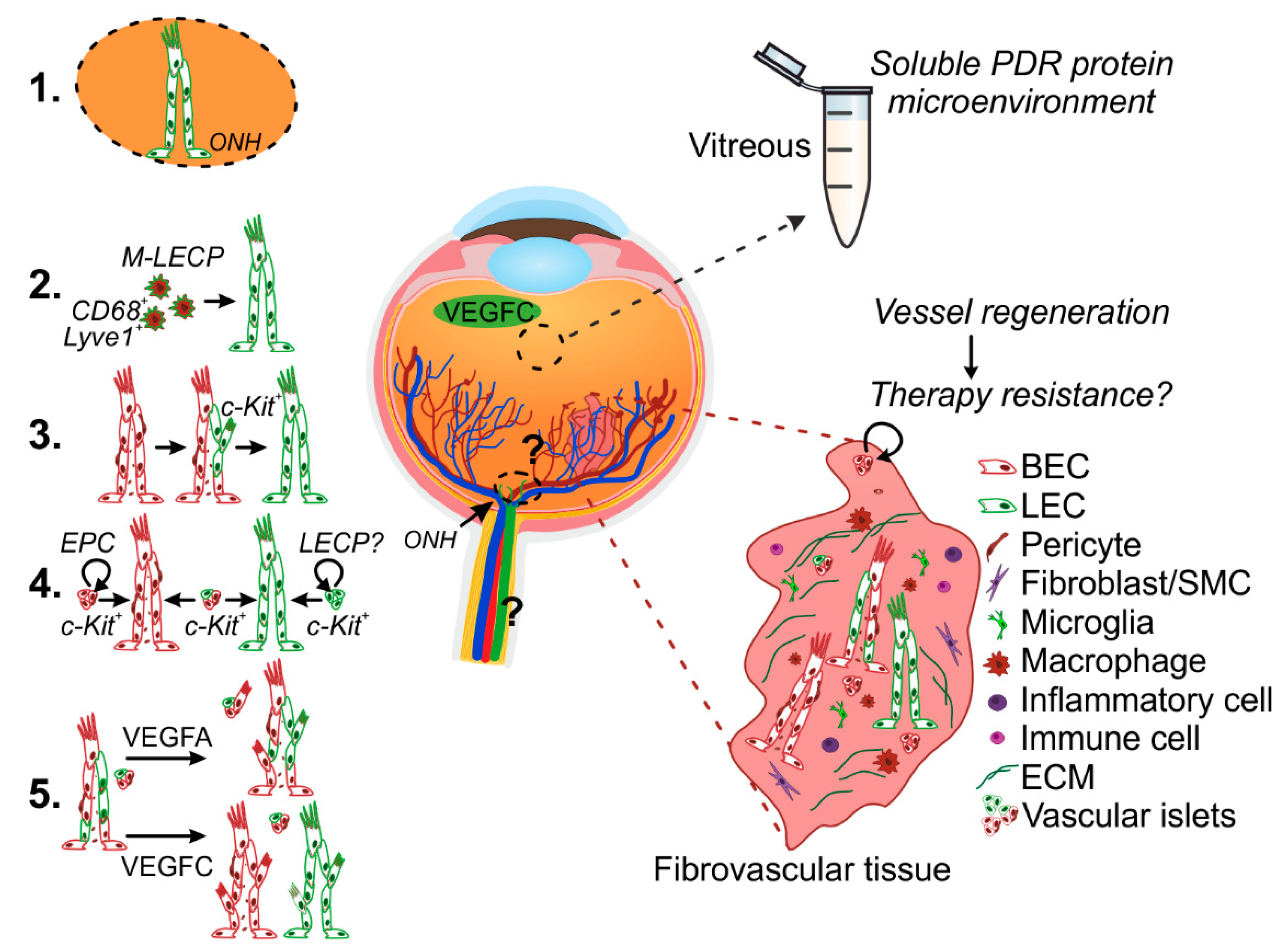
5. Mechanisms of Pathological Lymphatic Vascular Formation
5.1. Lymphatic Vascular Formation from Pre-Existing Vessels
5.2. De Novo Lymphatic Vascular Formation–Macrophages
5.3. De Novo Lymphatic Vessel Formation—Endothelial Progenitor Cells (EPCs)
6. Experimental Models of Diabetic Retinopathy
7. Multimodal Imaging and Improved Surgical Technology
8. Clinical Interventions and Potential Novel Drug Targets
9. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting Enzyme |
| BEC | Blood Endothelial Cell |
| bFGF | basic Fibroblast Growth Factor |
| CNV | Choroidal Neovascularization |
| CSF | Cerebrospinal Fluid |
| CTGF | Connective Tissue Growth Factor |
| CYR61 | Cysteine-rich 61 |
| DME | Diabetic Macular Edema |
| DR | Diabetic Retinopathy |
| EC | Endothelial Cell |
| ECM | Extracellular matrix |
| EPC | Endothelial Progenitor Cells |
| EPO | Erythropoietin |
| HA | Hyaluronic Acid |
| HMGB1 | High Mobility Group Box-1 |
| IL | Interleukin |
| LEC | Lymphatic Endothelial Cell |
| LECP | Lymphatic Endothelial Cell Precursor |
| LYVE1 | Lymphatic Vessel Endothelial Hyaluronan Receptor-1 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| M-CSF | Macrophage-colony Stimulating Factor |
| MIF | Macrophage Migration Inhibitory Factor |
| M-LECP | Macrophage-derived lymphatic endothelial cell precursor |
| MMP | Matrix Metalloproteinase |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nucleotide-binding oligomerization domain, leucine rich repeat and pyrin domain containing-3 |
| NOD | Non-obese Diabetic |
| NPDR | Non-Proliferative Diabetic Retinopathy |
| OCT | Optical Coherence Tomography |
| OIR | Oxygen-induced Retinopathy |
| ON | Optic nerve |
| OPC | Oligodendrocyte Precursor Cell |
| OPN | Osteopontin |
| PDGF | Platelet-derived Growth Factor |
| PDPN | Podoplanin |
| PDR | Proliferative Diabetic Retinopathy |
| PEDF | Pigment Epithelium-Derived Factor |
| PKC | Protein Kinase C |
| PROX1 | Prospero Homeobox Protein 1 |
| PRP | Panretinal Photocoagulation |
| RPE | Retinal Pigment Epithelium |
| RVO | Retinal Vein Occlusion |
| SDF1 | Stromal Cell-derived Factor-1 |
| TGFβ | Transforming Growth Factor-β |
| TNFα | Tumor Necrosis Factor-α |
| TRD | Tractional Retinal Detachment |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR | Vascular Endothelial Growth Factor Receptor |
| VESC | Vascular Endothelial Stem Cell |
| VH | Vitreous Hemorrhage |
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Loukovaara, S.; Gucciardo, E.; Repo, P.; Vihinen, H.; Lohi, J.; Jokitalo, E.; Salven, P.; Lehti, K. Indications of lymphatic endothelial differentiation and endothelial progenitor cell activation in the pathology of proliferative diabetic retinopathy. Acta Ophthalmol. 2015, 93, 512–523. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Roy, S.; Amin, S.; Roy, S. Retinal fibrosis in diabetic retinopathy. Exp. Eye Res. 2016, 142, 71–75. [Google Scholar] [CrossRef]
- Schroder, S.; Palinski, W.; Schmid-Schonbein, G.W. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol. 1991, 139, 81–100. [Google Scholar]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef]
- Loukovaara, S.; Nurkkala, H.; Tamene, F.; Gucciardo, E.; Liu, X.; Repo, P.; Lehti, K.; Varjosalo, M. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J. Proteome Res. 2015, 14, 5131–5143. [Google Scholar] [CrossRef]
- Nawaz, M.I.; Van Raemdonck, K.; Mohammad, G.; Kangave, D.; Van Damme, J.; Abu El-Asrar, A.M.; Struyf, S. Autocrine CCL2, CXCL4, CXCL9 and CXCL10 signal in retinal endothelial cells and are enhanced in diabetic retinopathy. Exp. Eye Res. 2013, 109, 67–76. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, J.X.; Guo, J.; Wang, J.; Zhu, M.; Chen, Y.; Le, Y.Z. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 2009, 219, 446–454. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, C.W.; Hsieh, M.C.; Wu, H.J.; Wu, W.S.; Wu, W.C.; Kao, Y.H. High mobility group B1 up-regulates angiogenic and fibrogenic factors in human retinal pigment epithelial ARPE-19 cells. Cell. Signal. 2017, 40, 248–257. [Google Scholar] [CrossRef]
- McGuire, P.G.; Rangasamy, S.; Maestas, J.; Das, A. Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e107–e115. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Liu, Y.; Luo, Y.; Ma, W.; Xiao, W.; Yu, Q. Neuronal-driven angiogenesis: Role of NGF in retinal neovascularization in an oxygen-induced retinopathy model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3749–3757. [Google Scholar] [CrossRef]
- Kovacs, K.; Marra, K.V.; Yu, G.; Wagley, S.; Ma, J.; Teague, G.C.; Nandakumar, N.; Lashkari, K.; Arroyo, J.G. Angiogenic and Inflammatory Vitreous Biomarkers Associated With Increasing Levels of Retinal Ischemia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6523–6530. [Google Scholar] [CrossRef]
- Dell’Omo, R.; Semeraro, F.; Bamonte, G.; Cifariello, F.; Romano, M.R.; Costagliola, C. Vitreous mediators in retinal hypoxic diseases. Mediators Inflamm. 2013, 2013, 935301. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, Y.; Yu, Y.; Lin, Q.; Yang, J.; Su, S.B.; Xu, G.T.; Yang, T. Involvement of IL-37 in the Pathogenesis of Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2955–2962. [Google Scholar] [CrossRef]
- Loukovaara, S.; Robciuc, A.; Holopainen, J.M.; Lehti, K.; Pessi, T.; Liinamaa, J.; Kukkonen, K.T.; Jauhiainen, M.; Koli, K.; Keski-Oja, J.; et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013, 91, 531–539. [Google Scholar] [CrossRef]
- Giebel, S.J.; Menicucci, G.; McGuire, P.G.; Das, A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab. Investig. 2005, 85, 597–607. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Nawaz, M.I.; Kangave, D.; Siddiquei, M.M.; Ola, M.S.; Opdenakker, G. Angiogenesis regulatory factors in the vitreous from patients with proliferative diabetic retinopathy. Acta Diabetol. 2013, 50, 545–551. [Google Scholar] [CrossRef]
- Adamis, A.P.; Miller, J.W.; Bernal, M.T.; D’Amico, D.J.; Folkman, J.; Yeo, T.K.; Yeo, K.T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am. J. Ophthalmol. 1994, 118, 445–450. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Bromberg-White, J.L.; Glazer, L.; Downer, R.; Furge, K.; Boguslawski, E.; Duesbery, N.S. Identification of VEGF-independent cytokines in proliferative diabetic retinopathy vitreous. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6472–6480. [Google Scholar] [CrossRef] [PubMed]
- Simo-Servat, O.; Hernandez, C.; Simo, R. Usefulness of the vitreous fluid analysis in the translational research of diabetic retinopathy. Mediators Inflamm. 2012, 2012, 872978. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Li, Y.M.; Gardiner, T.A.; Bucala, R.; Archer, D.B.; Vlassara, H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 1997, 150, 523–531. [Google Scholar]
- Kern, T.S. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 95103. [Google Scholar] [CrossRef] [PubMed]
- Adamis, A.P. Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 2002, 86, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Devi, T.S.; Lee, I.; Huttemann, M.; Kumar, A.; Nantwi, K.D.; Singh, L.P. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: Implications for diabetic retinopathy. Exp. Diabetes Res. 2012, 2012, 438238. [Google Scholar] [CrossRef]
- Loukovaara, S.; Sandholm, J.; Aalto, K.; Liukkonen, J.; Jalkanen, S.; Yegutkin, G.G. Deregulation of ocular nucleotide homeostasis in patients with diabetic retinopathy. J. Mol. Med. (Berl.) 2017, 95, 193–204. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef]
- Noda, K.; Nakao, S.; Ishida, S.; Ishibashi, T. Leukocyte adhesion molecules in diabetic retinopathy. J. Ophthalmol. 2012, 2012, 279037. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sonoda, K.H.; Sugahara, M.; Mochizuki, Y.; Enaida, H.; Oshima, Y.; Ueno, A.; Hata, Y.; Yoshida, H.; Ishibashi, T. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS ONE 2009, 4, e8158. [Google Scholar] [CrossRef] [PubMed]
- El-Asrar, A.M.; Nawaz, M.I.; Kangave, D.; Geboes, K.; Ola, M.S.; Ahmad, S.; Al-Shabrawey, M. High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol. Vis. 2011, 17, 1829–1838. [Google Scholar]
- Song, Z.; Sun, M.; Zhou, F.; Huang, F.; Qu, J.; Chen, D. Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1229–1234. [Google Scholar] [CrossRef]
- Loukovaara, S.; Piippo, N.; Kinnunen, K.; Hytti, M.; Kaarniranta, K.; Kauppinen, A. NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol. 2017, 95, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Capitao, M.; Soares, R. Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J. Cell. Biochem. 2016, 117, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Walia, A.; Huang, Y.H.; Han, K.Y.; Rosenblatt, M.I.; Azar, D.T.; Chang, J.H. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv. Ophthalmol. 2016, 61, 272–296. [Google Scholar] [CrossRef] [PubMed]
- Bautch, V.L.; Caron, K.M. Blood and lymphatic vessel formation. Cold Spring Harb. Perspect. Biol. 2015, 7, a008268. [Google Scholar] [CrossRef] [PubMed]
- Vaahtomeri, K.; Karaman, S.; Makinen, T.; Alitalo, K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev. 2017, 31, 1615–1634. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- Makinen, T.; Veikkola, T.; Mustjoki, S.; Karpanen, T.; Catimel, B.; Nice, E.C.; Wise, L.; Mercer, A.; Kowalski, H.; Kerjaschki, D.; et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001, 20, 4762–4773. [Google Scholar] [CrossRef]
- Joukov, V.; Sorsa, T.; Kumar, V.; Jeltsch, M.; Claesson-Welsh, L.; Cao, Y.; Saksela, O.; Kalkkinen, N.; Alitalo, K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997, 16, 3898–3911. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Stenvers, K.; Caesar, C.; Vitali, A.; Domagala, T.; Nice, E.; Roufail, S.; Simpson, R.J.; Moritz, R.; Karpanen, T.; et al. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J. Biol. Chem. 1999, 274, 32127–32136. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, H.; Falke, L.L.; Nguyen, T.Q.; Bovenschen, N.; Aten, J.; Leask, A.; Ito, Y.; Goldschmeding, R. Connective tissue growth factor regulates fibrosis-associated renal lymphangiogenesis. Kidney Int. 2017, 92, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Guc, E.; Briquez, P.S.; Foretay, D.; Fankhauser, M.A.; Hubbell, J.A.; Kilarski, W.W.; Swartz, M.A. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 2017, 131, 160–175. [Google Scholar] [CrossRef] [PubMed]
- Gucciardo, E.; Loukovaara, S.; Korhonen, A.; Repo, P.; Martins, B.; Vihinen, H.; Jokitalo, E.; Lehti, K. The microenvironment of proliferative diabetic retinopathy supports lymphatic neovascularization. J. Pathol. 2018, 245, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Tammela, T.; Antila, S.; Nurmi, H.; Leppanen, V.M.; Zarkada, G.; Stanczuk, L.; Francois, M.; Makinen, T.; Saharinen, P.; et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Investig. 2014, 124, 3975–3986. [Google Scholar] [CrossRef] [PubMed]
- Ristimaki, A.; Narko, K.; Enholm, B.; Joukov, V.; Alitalo, K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J. Biol. Chem. 1998, 273, 8413–8418. [Google Scholar] [CrossRef] [PubMed]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef]
- Jha, S.K.; Rauniyar, K.; Jeltsch, M. Key molecules in lymphatic development, function, and identification. Ann. Anat. 2018, 219, 25–34. [Google Scholar] [CrossRef]
- Schroedl, F.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; Lutty, G.A.; Maruyama, K.; Chen, L.; Lutjen-Drecoll, E.; et al. Consensus statement on the immunohistochemical detection of ocular lymphatic vessels. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6440–6442. [Google Scholar] [CrossRef]
- Nakao, S.; Hafezi-Moghadam, A.; Ishibashi, T. Lymphatics and lymphangiogenesis in the eye. J. Ophthalmol. 2012, 2012, 783163. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.H.; Johnston, M.G.; Ly, T.; Patel, M.; Drake, B.; Gumus, E.; Fraenkl, S.A.; Moore, S.; Tobbia, D.; Armstrong, D.; et al. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp. Eye Res. 2009, 89, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, S.L.; Neuser, B.; Herwig, M.C.; Muller, A.M.; Koch, K.R.; Reitsamer, H.A.; Schrodl, F.; Cursiefen, C.; Heindl, L.M. Absence of lymphatic vessels in the developing human sclera. Exp. Eye Res. 2014, 125, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, S.L.; Neuser, B.; Caramoy, A.; Grajewski, R.S.; Koch, K.R.; Schrodl, F.; Cursiefen, C.; Heindl, L.M. Enrichment of lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1)-positive macrophages around blood vessels in the normal human sclera. Investig. Ophthalmol. Vis. Sci. 2014, 55, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Koina, M.E.; Baxter, L.; Adamson, S.J.; Arfuso, F.; Hu, P.; Madigan, M.C.; Chan-Ling, T. Evidence for lymphatics in the developing and adult human choroid. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Schrodl, F.; Kaser-Eichberger, A.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Motloch, K.; Bruckner, D.; Laimer, M.; Heindl, L.M.; et al. Lymphatic Markers in the Adult Human Choroid. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7406–7416. [Google Scholar] [CrossRef] [PubMed]
- Schroedl, F.; Brehmer, A.; Neuhuber, W.L.; Kruse, F.E.; May, C.A.; Cursiefen, C. The normal human choroid is endowed with a significant number of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1)-positive macrophages. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5222–5229. [Google Scholar] [CrossRef]
- Heindl, L.M.; Kaser-Eichberger, A.; Schlereth, S.L.; Bock, F.; Regenfuss, B.; Reitsamer, H.A.; McMenamin, P.; Lutty, G.A.; Maruyama, K.; Chen, L.; et al. Sufficient Evidence for Lymphatics in the Developing and Adult Human Choroid? Investig. Ophthalmol. Vis. Sci. 2015, 56, 6709–6710. [Google Scholar] [CrossRef]
- Killer, H.E.; Jaggi, G.P.; Miller, N.R.; Flammer, J.; Meyer, P. Does immunohistochemistry allow easy detection of lymphatics in the optic nerve sheath? J. Histochem. Cytochem. 2008, 56, 1087–1092. [Google Scholar] [CrossRef]
- Killer, H.E.; Laeng, H.R.; Groscurth, P. Lymphatic capillaries in the meninges of the human optic nerve. J. Neuroophthalmol. 1999, 19, 222–228. [Google Scholar] [CrossRef]
- Herwig, M.C.; Munstermann, K.; Klarmann-Schulz, U.; Schlereth, S.L.; Heindl, L.M.; Loeffler, K.U.; Muller, A.M. Expression of the lymphatic marker podoplanin (D2-40) in human fetal eyes. Exp. Eye Res. 2014, 127, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Trost, A.; Runge, C.; Bruckner, D.; Kaser-Eichberger, A.; Bogner, B.; Strohmaier, C.; Reitsamer, H.A.; Schroedl, F. Lymphatic markers in the human optic nerve. Exp. Eye Res. 2018, 173, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gruntzig, J.; Hollmann, F. Lymphatic vessels of the eye—Old questions—New insights. Ann. Anat. 2018, 221, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Schlotzer-Schrehardt, U.; Kuchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2127–2135. [Google Scholar]
- Heindl, L.M.; Hofmann, T.N.; Schrodl, F.; Holbach, L.M.; Kruse, F.E.; Cursiefen, C. Intraocular lymphatics in ciliary body melanomas with extraocular extension: Functional for lymphatic spread? Arch. Ophthalmol. 2010, 128, 1001–1008. [Google Scholar] [CrossRef]
- Cursiefen, C.; Rummelt, C.; Junemann, A.; Vorwerk, C.; Neuhuber, W.; Kruse, F.E.; Schroedl, F. Absence of blood and lymphatic vessels in the developing human cornea. Cornea 2006, 25, 722–726. [Google Scholar] [CrossRef]
- Regina, M.; Zimmerman, R.; Malik, G.; Gausas, R. Lymphangiogenesis concurrent with haemangiogenesis in the human cornea. Clin. Exp. Ophthalmol. 2007, 35, 541–544. [Google Scholar] [CrossRef]
- Fogt, F.; Zimmerman, R.L.; Daly, T.; Gausas, R.E. Observation of lymphatic vessels in orbital fat of patients with inflammatory conditions: A form fruste of lymphangiogenesis? Int. J. Mol. Med. 2004, 13, 681–683. [Google Scholar] [CrossRef]
- Heindl, L.M.; Hofmann-Rummelt, C.; Adler, W.; Bosch, J.J.; Holbach, L.M.; Naumann, G.O.; Kruse, F.E.; Cursiefen, C. Tumor-associated lymphangiogenesis in the development of conjunctival melanoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7074–7083. [Google Scholar] [CrossRef]
- Gausas, R.E.; Daly, T.; Fogt, F. D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthalmic Plast Reconstr. Surg. 2007, 23, 32–36. [Google Scholar] [CrossRef]
- Birke, K.; Lutjen-Drecoll, E.; Kerjaschki, D.; Birke, M.T. Expression of podoplanin and other lymphatic markers in the human anterior eye segment. Investig. Ophthalmol. Vis. Sci. 2010, 51, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Kelley, P.M.; Steele, M.M.; Tempero, R.M. Regressed lymphatic vessels develop during corneal repair. Lab. Investig. 2011, 91, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.M.; Hofmann-Rummelt, C.; Kruse, F.E.; Cursiefen, C.; Heindl, L.M. Invasion of lymphatic vessels into the eye after open globe injuries. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3717–3725. [Google Scholar] [CrossRef] [PubMed]
- Kaser-Eichberger, A.; Schrodl, F.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Motloch, K.; Bruckner, D.; Laimer, M.; Schlereth, S.L.; et al. Topography of Lymphatic Markers in Human Iris and Ciliary Body. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4943–4953. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Hamanaka, T.; Takemura, T.; Murakami, A. Involvement of platelet coagulation and inflammation in the endothelium of Schlemm’s canal. Investig. Ophthalmol. Vis. Sci. 2010, 51, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, T.; Kasahara, K.; Takemura, T. Histopathology of the trabecular meshwork and Schlemm’s canal in primary angle-closure glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8849–8861. [Google Scholar] [CrossRef]
- Heindl, L.M.; Hofmann, T.N.; Knorr, H.L.; Rummelt, C.; Schrodl, F.; Schlotzer-Schrehardt, U.; Holbach, L.M.; Naumann, G.O.; Kruse, F.E.; Cursiefen, C. Intraocular lymphangiogenesis in malignant melanomas of the ciliary body with extraocular extension. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1988–1995. [Google Scholar] [CrossRef]
- Loukovaara, S.; Gucciardo, E.; Repo, P.; Lohi, J.; Salven, P.; Lehti, K. A Case of Abnormal Lymphatic-Like Differentiation and Endothelial Progenitor Cell Activation in Neovascularization Associated with Hemi-Retinal Vein Occlusion. Case Rep. Ophthalmol. 2015, 6, 228–238. [Google Scholar] [CrossRef]
- Chan-Ling, T.; Koina, M.E.; Arfuso, F.; Adamson, S.J.; Baxter, L.C.; Hu, P.; Madigan, M.C. Author Response: Sufficient Evidence for Lymphatics in the Developing and Adult Human Choroid? Investig. Ophthalmol. Vis. Sci. 2015, 56, 6711–6713. [Google Scholar] [CrossRef]
- Yucel, Y.; Gupta, N. Lymphatic drainage from the eye: A new target for therapy. Prog. Brain Res. 2015, 220, 185–198. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Investig. 2005, 115, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.L.; Gordon, E.J. Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vasc. Cell 2012, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Chinnery, H.R.; McMenamin, P.G.; Dando, S.J. Macrophage physiology in the eye. Pflugers Arch. 2017, 469, 501–515. [Google Scholar] [CrossRef]
- Yamagami, S.; Ebihara, N.; Usui, T.; Yokoo, S.; Amano, S. Bone marrow-derived cells in normal human corneal stroma. Arch. Ophthalmol. 2006, 124, 62–69. [Google Scholar] [CrossRef]
- Thill, M.; Schlagner, K.; Altenahr, S.; Ergun, S.; Faragher, R.G.; Kilic, N.; Bednarz, J.; Vohwinkel, G.; Rogiers, X.; Hossfeld, D.K.; et al. A novel population of repair cells identified in the stroma of the human cornea. Stem Cells Dev. 2007, 16, 733–745. [Google Scholar] [CrossRef]
- Maruyama, K.; Nakazawa, T.; Cursiefen, C.; Maruyama, Y.; Van Rooijen, N.; D’Amore, P.A.; Kinoshita, S. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3145–3153. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5997–6005. [Google Scholar] [CrossRef]
- Le Bras, B.; Barallobre, M.J.; Homman-Ludiye, J.; Ny, A.; Wyns, S.; Tammela, T.; Haiko, P.; Karkkainen, M.J.; Yuan, L.; Muriel, M.P.; et al. VEGF-C is a trophic factor for neural progenitors in the vertebrate embryonic brain. Nat. Neurosci. 2006, 9, 340–348. [Google Scholar] [CrossRef]
- Omri, S.; Behar-Cohen, F.; de Kozak, Y.; Sennlaub, F.; Verissimo, L.M.; Jonet, L.; Savoldelli, M.; Omri, B.; Crisanti, P. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: Role of PKCzeta in the Goto Kakizaki rat model. Am. J. Pathol. 2011, 179, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Zandi, S.; Kohno, R.; Sun, D.; Nakama, T.; Ishikawa, K.; Yoshida, S.; Enaida, H.; Ishibashi, T.; Hafezi-Moghadam, A. Lack of lymphatics and lymph node-mediated immunity in choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Corral, I.; Ulvmar, M.H.; Stanczuk, L.; Tatin, F.; Kizhatil, K.; John, S.W.; Alitalo, K.; Ortega, S.; Makinen, T. Nonvenous origin of dermal lymphatic vasculature. Circ. Res. 2015, 116, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Stanczuk, L.; Martinez-Corral, I.; Ulvmar, M.H.; Zhang, Y.; Lavina, B.; Fruttiger, M.; Adams, R.H.; Saur, D.; Betsholtz, C.; Ortega, S.; et al. cKit Lineage Hemogenic Endothelium-Derived Cells Contribute to Mesenteric Lymphatic Vessels. Cell Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wei, J.; Pentinmikko, N.; Leinonen, H.; Salven, P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012, 10, e1001407. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, J.Y.; Yoon, Y.S. Role of bone marrow-derived lymphatic endothelial progenitor cells for lymphatic neovascularization. Trends Cardiovasc. Med. 2011, 21, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Asrar, A.M.; Struyf, S.; Opdenakker, G.; Van Damme, J.; Geboes, K. Expression of stem cell factor/c-kit signaling pathway components in diabetic fibrovascular epiretinal membranes. Mol. Vis. 2010, 16, 1098–1107. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2545–2552. [Google Scholar] [CrossRef]
- Ogura, S.; Kurata, K.; Hattori, Y.; Takase, H.; Ishiguro-Oonuma, T.; Hwang, Y.; Ahn, S.; Park, I.; Ikeda, W.; Kusuhara, S.; et al. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight 2017, 2, e90905. [Google Scholar] [CrossRef]
- Kim, C.B.; D’Amore, P.A.; Connor, K.M. Revisiting the mouse model of oxygen-induced retinopathy. Eye Brain 2016, 8, 67–79. [Google Scholar] [CrossRef]
- Enge, M.; Bjarnegard, M.; Gerhardt, H.; Gustafsson, E.; Kalen, M.; Asker, N.; Hammes, H.P.; Shani, M.; Fassler, R.; Betsholtz, C. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002, 21, 4307–4316. [Google Scholar] [CrossRef]
- Kitahara, H.; Kajikawa, S.; Ishii, Y.; Yamamoto, S.; Hamashima, T.; Azuma, E.; Sato, H.; Matsushima, T.; Shibuya, M.; Shimada, Y.; et al. The Novel Pathogenesis of Retinopathy Mediated by Multiple RTK Signals is Uncovered in Newly Developed Mouse Model. EBioMedicine 2018, 31, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Mugisho, O.O.; Rupenthal, I.D.; Squirrell, D.M.; Bould, S.J.; Danesh-Meyer, H.V.; Zhang, J.; Green, C.R.; Acosta, M.L. Intravitreal pro-inflammatory cytokines in non-obese diabetic mice: Modelling signs of diabetic retinopathy. PLoS ONE 2018, 13, e0202156. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, A.; Herman, I.M. Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Transl. Res. 2014, 163, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gao, X.; Wang, S.; Han, K.; Ema, M.; Adams, S.; Adams, R.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Prox1-GFP/Flt1-DsRed transgenic mice: An animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 2017, 20, 581–598. [Google Scholar] [CrossRef]
- Ogata, F.; Azuma, R.; Kikuchi, M.; Koshima, I.; Morimoto, Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann. Plast. Surg. 2007, 58, 652–655. [Google Scholar] [CrossRef]
- Forbrich, A.; Heinmiller, A.; Zemp, R.J. Photoacoustic imaging of lymphatic pumping. J. Biomed. Opt. 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Louveau, A.; Plog, B.A.; Antila, S.; Alitalo, K.; Nedergaard, M.; Kipnis, J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Investig. 2017, 127, 3210–3219. [Google Scholar] [CrossRef]
- Absinta, M.; Ha, S.K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 2017, 6. [Google Scholar] [CrossRef]
- Lokmic, Z. Utilizing lymphatic cell markers to visualize human lymphatic abnormalities. J. Biophotonics 2018, 11, e201700117. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y. The challenge of modeling macular degeneration in mice. Trends Genet. 2007, 23, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.R.; Omri, S.; Gelize, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Lavine, K.J.; Randolph, G.J. Cardiac Lymphatic Vessels, Transport, and Healing of the Infarcted Heart. JACC Basic Transl. Sci. 2017, 2, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Akiyama, H.; Li, D.; Shimoda, Y.; Matsumoto, H.; Kishi, S. Observation of neovascularization of the disc associated with proliferative diabetic retinopathy using OCT angiography. Jpn. J. Ophthalmol. 2018, 62, 286–291. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Querques, G.; Lattanzio, R.; Querques, L.; Del Turco, C.; Forte, R.; Pierro, L.; Souied, E.H.; Bandello, F. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6017–6024. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Boyer, D.S.; Schmidt-Erfurth, U.; Heier, J.S.; Gordon, C.; Benz, M.S.; Marcus, D.M.; Sabates, N.R.; Vitti, R.; Kazmi, H.; et al. Microperimetric assessment of retinal sensitivity in eyes with diabetic macular edema from a phase 2 study of intravitreal aflibercept. Retina 2015, 35, 687–694. [Google Scholar] [CrossRef]
- Sharma, T.; Fong, A.; Lai, T.Y.; Lee, V.; Das, S.; Lam, D. Surgical treatment for diabetic vitreoretinal diseases: A review. Clin. Exp. Ophthalmol. 2016, 44, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, C.; Paulo, E.B. HEADS-UP SURGERY FOR VITREORETINAL PROCEDURES: An Experimental and Clinical Study. Retina 2016, 36, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Krick, T.W.; Bressler, N.M. Recent clinically relevant highlights from the Diabetic Retinopathy Clinical Research Network. Curr. Opin. Ophthalmol. 2018, 29, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.S.; Girach, A.; Boney, A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: A literature review. Retina 2007, 27, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Q.; Zhu, H.; Zhao, P.Q.; Hu, Y.Q. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br. J. Ophthalmol. 2011, 95, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharashi, A.; Galbinur, T.; Mandelcorn, E.D.; Muni, R.H.; Nabavi, M.; Kertes, P.J. The adjunctive use of pre-operative intravitreal bevacizumab in the setting of proliferative diabetic retinopathy. Saudi J. Ophthalmol. 2016, 30, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Sivaprasad, S.; Prevost, A.T.; Vasconcelos, J.C.; Riddell, A.; Murphy, C.; Kelly, J.; Bainbridge, J.; Tudor-Edwards, R.; Hopkins, D.; Hykin, P.; et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017, 389, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., 3rd; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs. Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Shima, C.; Wakabayashi, T.; Kusaka, S.; Shiraga, F.; Ohji, M.; Tano, Y. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009, 116, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Group, D.E.R. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Stratton, I.M.; Aldington, S.J.; Holman, R.R.; Kohner, E.M.; Group, U.K.P.D.S. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch. Ophthalmol. 2004, 122, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.; Andersson, D.K.; Dedorsson, I.; Svardsudd, K.; Jansson, S.P.; Stefansson, E. Early detection of type 2 diabetes mellitus and screening for retinopathy are associated with reduced prevalence and severity of retinopathy. Acta Ophthalmol. 2016, 94, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Park, S.W.; Kim, J.H.; Park, Y.J.; Cho, C.H.; Kim, J.H. Angiopoietin 2 induces astrocyte apoptosis via alphavbeta5-integrin signaling in diabetic retinopathy. Cell Death Dis. 2016, 7, e2101. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Yun, J.H.; Kim, J.H.; Kim, K.W.; Cho, C.H.; Kim, J.H. Angiopoietin 2 induces pericyte apoptosis via alpha3beta1 integrin signaling in diabetic retinopathy. Diabetes 2014, 63, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Kangas, J.; Saharinen, P. Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clin. Sci. (Lond.) 2017, 131, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Abdulaal, M.; Haddad, N.M.; Sun, J.K.; Silva, P.S. The Role of Plasma Kallikrein-Kinin Pathway in the Development of Diabetic Retinopathy: Pathophysiology and Therapeutic Approaches. Semin. Ophthalmol. 2016, 31, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Du, H.T.; Du, L.L.; Tang, X.L.; Ge, H.Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Fiori, A.; Terlizzi, V.; Kremer, H.; Gebauer, J.; Hammes, H.P.; Harmsen, M.C.; Bieback, K. Mesenchymal stromal/stem cells as potential therapy in diabetic retinopathy. Immunobiology 2018. [Google Scholar] [CrossRef] [PubMed]
- Mauer, M.; Zinman, B.; Gardiner, R.; Suissa, S.; Sinaiko, A.; Strand, T.; Drummond, K.; Donnelly, S.; Goodyer, P.; Gubler, M.C.; et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 2009, 361, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kramerov, A.A.; Ljubimov, A.V. Stem cell therapies in the treatment of diabetic retinopathy and keratopathy. Exp. Biol. Med. (Maywood) 2016, 241, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFj1–ORSFj10. [Google Scholar] [CrossRef] [PubMed]
- Patila, T.; Ikonen, T.; Rutanen, J.; Ahonen, A.; Lommi, J.; Lappalainen, K.; Krogerus, L.; Ihlberg, L.; Partanen, T.A.; Lahteenoja, L.; et al. Vascular endothelial growth factor C-induced collateral formation in a model of myocardial ischemia. J. Heart Lung Transplant. 2006, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Zarkada, G.; Wallgard, E.; Murtomaki, A.; Suchting, S.; Wirzenius, M.; Waltari, M.; Hellstrom, M.; Schomber, T.; Peltonen, R.; et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008, 454, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Goepferich, A. Nanoparticles for the treatment of ocular neovascularizations. Eur. J. Pharm. Biopharm. 2015, 95, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Wilber, A. Novel role of immature myeloid cells in formation of new lymphatic vessels associated with inflammation and tumors. J. Leukoc. Biol. 2017, 102, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; Montgomery, K.E. Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 2012, 4, 618–657. [Google Scholar] [CrossRef]
- Ji, R.C. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014, 346, 6–16. [Google Scholar] [CrossRef]
- Schetters, S.T.T.; Gomez-Nicola, D.; Garcia-Vallejo, J.J.; Van Kooyk, Y. Neuroinflammation: Microglia and T Cells Get Ready to Tango. Front. Immunol. 2017, 8, 1905. [Google Scholar] [CrossRef]
- Willermain, F.; Scifo, L.; Weber, C.; Caspers, L.; Perret, J.; Delporte, C. Potential Interplay between Hyperosmolarity and Inflammation on Retinal Pigmented Epithelium in Pathogenesis of Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, M.; Subramani, M.; Jayadev, C.; Shetty, R.; Das, D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017, 95, 126–135. [Google Scholar] [CrossRef] [PubMed]
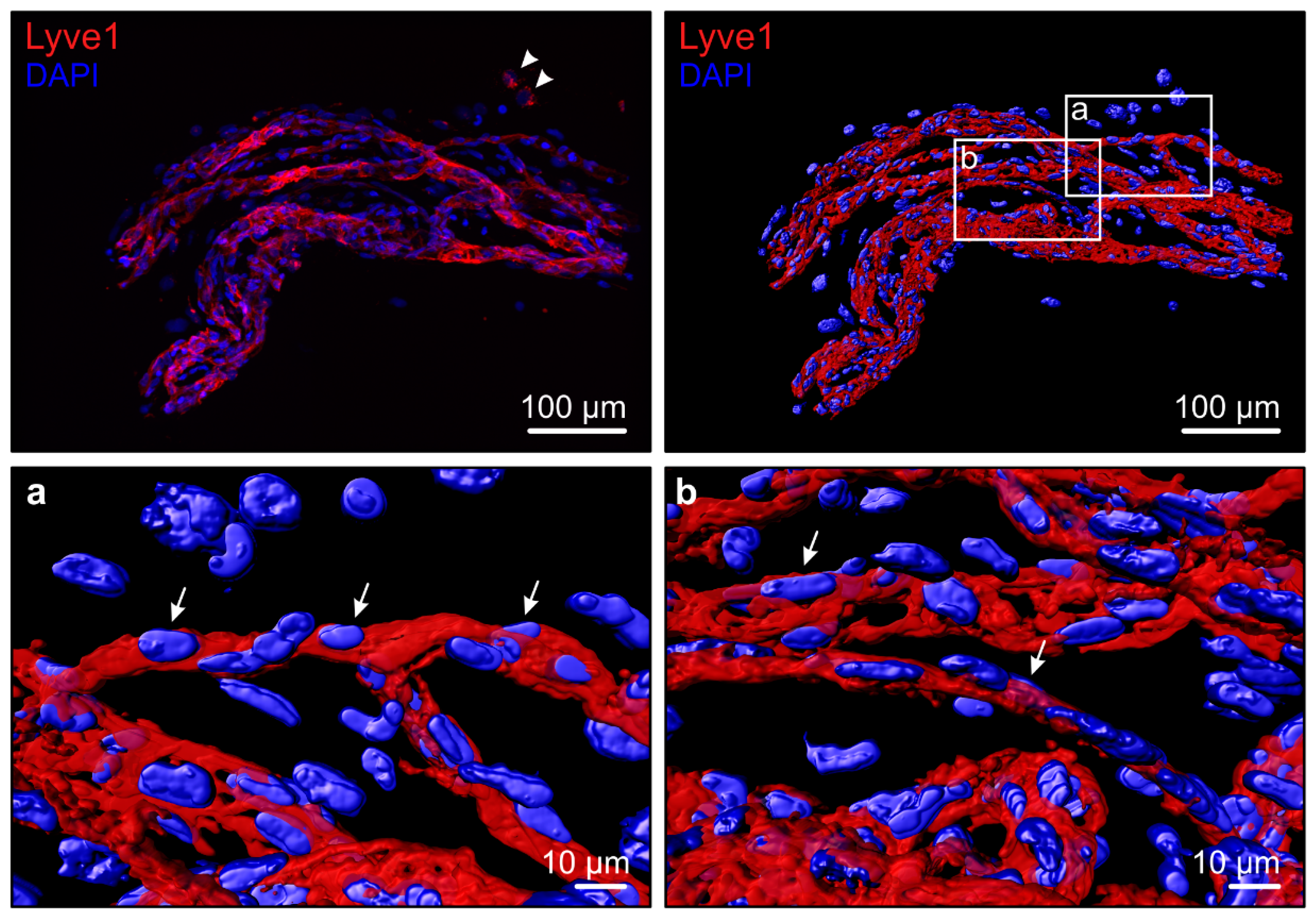
| Positive Findings in Physiological Conditions | Negative Findings in Physiological Conditions | Positive Findings in Pathological Conditions | |||||
|---|---|---|---|---|---|---|---|
| Marker | Reference | Marker | Reference | Marker | Reference | ||
| Conjunctiva | Vascular | Lyve1 Lyve1 * PDPN PDPN * VEGFR3 | [52,64,65] [53,61,66] [52,64,65,67,68] [53,61] [64] | Lyve1 PDPN † | [69] [70] | ||
| Extra-vascular | |||||||
| Cornea | Vascular | Lyve1 Lyve1 * PDPN VEGFR3 Prox1 | [64] [66] [64] [64,71] [71] | Lyve1 PDPN VEGFR3 | [64,72] [64,67,73] [64] | ||
| Extra-vascular | |||||||
| Lacrimal gland | Vascular | PDPN † | [70] | ||||
| Extra-vascular | |||||||
| Iris | Vascular | VEGFR3 | [74] | VEGFR3 Prox1 | [71] [71] | PDPN | [73] |
| Extra-vascular | Lyve1 ‡ PDPN VEGFR3 | [71], [74] ‡ [71,74] [74] | VEGFR3 Prox1 | [71] [71] | |||
| Trabecular meshwork | Vascular | ||||||
| Extra-vascular | Lyve1 Lyve1 * PDPN PDPN * | [71] [61] [71,75] [61] | VEGFR3 Prox1 | [71] [71] | PDPN | [76] | |
| Schlemm’s canal | Vascular | Prox1 | [46] | Lyve1 * PDPN * | [61] [61] | ||
| Extra-vascular | PDPN * | [61] | |||||
| Ciliary body | Vascular | Lyve1 PDPN immunoEM | [52] [52] [52] | Lyve1 PDPN PDPN * | [74] [74] [61] | Lyve1 PDPN | [77] [73,77] |
| Extra-vascular | Lyve1 PDPN VEGFR3 | [71,74] [71,74] [74] | PDPN * | [61] | |||
| Macula | Vascular | ||||||
| Extra-vascular | |||||||
| Retina | Vascular | Lyve1 | [57] | ||||
| Extra-vascular | PDPN * | [61] | Lyve1 | [57] | |||
| Choroid | Vascular | Lyve1 PDPN § VEGFR3 *,§ VEGFR 3,§ | [55] § [55] § [55] § [55] § | Lyve1 PDPN PDPN * VEGFR3 Prox1 | [56,57] [56] [61] [56] [56] | ||
| Extra-vascular | Lyve1 ‡ Lyve1 *,§ Lyve1 * PDPN PDPN *,§ VEGFR3 *,§ Prox1 *,§ | [57] ‡ [55] § [61] [57] [55] § [55] § [55] § | VEGFR3 Prox1 PDPN * | [56] [56] [61] | |||
| Sclera | Vascular | Lyve1 Lyve1 * PDPN PDPN * | [54,57] [53] [54] [53] | PDPN | [73] | ||
| Extra-vascular | Lyve1 ‡ | [54] ‡, [57] | PDPN | [54] | |||
| Optic nerve | Vascular | Histology Lyve1 PDPN | [60] [59] [59] | Lyve1 | [62] | PDPN † | [70] |
| Extra-vascular | Lyve1 ‡ Lyve1 ‡ Lyve 1 * PDPN * | [57], [62] ‡ [62] ‡ [61] [61] | PDPN † | [70] | |||
| Mechanism | Drug | Indication | Mode of Administration | Phase | Clinical Trial Identifier |
|---|---|---|---|---|---|
| Inhibitor of sodium-hydrogen exchanger NHE3, anti-angiogenic | Squalamine Lactate | PDR | Eye drop | Phase II | NCT01769183 |
| Soluble VEGFR1 (adenoviral) | rAAV.sFlt-1 | wet AMD | Subretinal injection | Phase I | NCT01494805 |
| β1-AR and β2-AR blocker | Propranolol | ROP | Eye drops | Phase II | NCT02504944 |
| 28-mer RNA aptamer against VEGFA-165 | Pegaptanib | IN, DR | Intravitreal injection | Phase I | NCT00295828 |
| Soluble VEGFR3 | OPT-302 | wet AMD | Intravitreal injection | Phase I | NCT02543229 |
| Analog of cortisol acetate | Anecortave Acetate | AMD | sub-tenon injection | Phase II | NCT00211484 |
| anti-VEGF/anti-angiopoietin-2 bispecific antibody | RO6867461/Faricimab | CNV/AMD | Intravitreal injection | Phase II | NCT02484690 |
| Chimeric protein against Tissue Factor | hI-con1 | CNV/AMD | Intravitreal injection | Phase II | NCT02358889 |
| human monoclonal antibody against TNFα | Adalimumab | CNV/AMD | Intravitreal injection | Phase II | NCT01136252 |
| human monoclonal antibody against IL-2R | Daclizumab | wet AMD | Intravenous | Phase II | NCT00304954 |
| humanized monoclonal antibody against TNFα | Infliximab | wet AMD | Intravenous | Phase II | NCT00304954 |
| mTOR inhibitor | Rapamycin | wet AMD | Oral | Phase II | NCT00304954 |
| siRNA against VEGFR1 | AGN211745 | CNV/AMD | Intravitreal injection | Phase I/II | NCT00363714 |
| Recombinant Human VEGF Receptor-Fc Fusion Protein | KH902 | wet AMD | Intravitreal injection | Phase III | NCT01436864 |
| siRNA against RTP801 | PF-04523655 | wet AMD | Intravitreal injection | Phase I | NCT00725686 |
| anti-VEGF aptamer | EYE001 | wet AMD | Intravitreal injection | Phase II/III | NCT00021736 |
| tetracycline antibiotic | Doxycycline monohydrate | PDR | Oral | Phase II | NCT00511875 |
| VEGFR inhibitor (inhibits also PDGFR, c-Kit and c-Fms) | PTK787 | wet AMD | Oral | Phase I/II | NCT00138632 |
| siRNA against CTGF | RXI-109 | wet AMD | Intravitreal injection | Phase I/II | NCT02599064 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gucciardo, E.; Loukovaara, S.; Salven, P.; Lehti, K. Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 4034. https://doi.org/10.3390/ijms19124034
Gucciardo E, Loukovaara S, Salven P, Lehti K. Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy. International Journal of Molecular Sciences. 2018; 19(12):4034. https://doi.org/10.3390/ijms19124034
Chicago/Turabian StyleGucciardo, Erika, Sirpa Loukovaara, Petri Salven, and Kaisa Lehti. 2018. "Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy" International Journal of Molecular Sciences 19, no. 12: 4034. https://doi.org/10.3390/ijms19124034
APA StyleGucciardo, E., Loukovaara, S., Salven, P., & Lehti, K. (2018). Lymphatic Vascular Structures: A New Aspect in Proliferative Diabetic Retinopathy. International Journal of Molecular Sciences, 19(12), 4034. https://doi.org/10.3390/ijms19124034





